Use of Tuna Visceral Pepsin in Combination with Trypsin as Digestion Aid: Enhanced Protein Hydrolysis and Bioavailability
Abstract
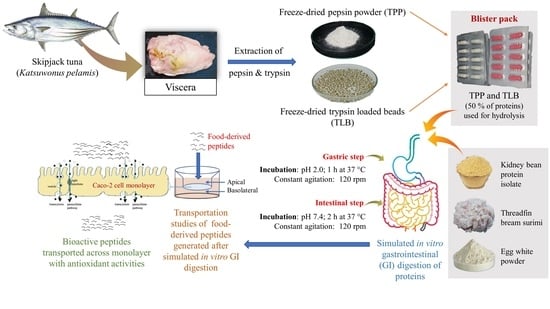
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Skipjack Tuna Pepsin Powder
2.3. Preparation of Skipjack Tuna Trypsin-Loaded Beads
2.4. Storage Stability of TPP and TLB Capsules in Blister Pack at Various Temperatures
2.5. Simulated In Vitro Gastrointestinal (GI) Digestion of Some Proteins by TPP and TLB
2.5.1. Degree of Hydrolysis (DH) of Digests
2.5.2. SDS-PAGE of Digests
2.6. Measurement of Cytotoxicity
2.7. Transport Studies
2.7.1. Cell Culture
2.7.2. Apical-to-Basolateral Transport Studies
Percentage of Permeability
Size Distribution of Digests
Antioxidant Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Storage Stability of TPP and TLB Capsules in Blister Pack at Various Temperatures
3.2. Simulated In Vitro Gastrointestinal (GI) Digestion of Some Proteins by TPP and TLB
3.2.1. Degree of Hydrolysis (DH)
3.2.2. Protein Patterns
3.3. Cell Cytotoxicity
3.4. Transport Study of Digested Proteins
3.4.1. Percentage of Permeability or Bioavailability
3.4.2. Size Distribution
3.4.3. Antioxidant Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moraes, G.; de Almeida, L.C. Nutrition and functional aspects of digestion in fish. In Biology and Physiology of Freshwater Neotropical Fish; Elsevier: Amsterdam, The Netherlands, 2020; pp. 251–271. [Google Scholar]
- Feldman, M.; Cryer, B.; McArthur, K.E.; Huet, B.A.; Lee, E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: A prospective study. Gastroenterology 1996, 110, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Buamard, N.; Aluko, R.E.; Benjakul, S. Stability of tuna trypsin-loaded alginate—chitosan beads in acidic stomach fluid and the release of active enzyme in a simulated intestinal tract environment. J. Food Biochem. 2020, 44, e13455. [Google Scholar] [CrossRef] [PubMed]
- Jurado, E.; Vicari, J.; Lechug, M.; Moya-Ramirez, I. Pepsin extraction process from swine wastes. Procedia Eng. 2012, 42, 1346–1350. [Google Scholar] [CrossRef] [Green Version]
- Imelio, N.; Marini, A.; Spelzini, D.; Picó, G.; Farruggia, B. Pepsin extraction from bovine stomach using aqueous two-phase systems: Molecular mechanism and influence of homogenate mass and phase volume ratio. J. Chromatogr. B 2008, 873, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.; Nikoo, M.; Zhang, B.; Benjakul, S. Freeze-dried tuna pepsin powder stabilized by some cryoprotectants: In vitro simulated gastric digestion toward different proteins and its storage stability. Foods 2022, 11, 2292. [Google Scholar] [CrossRef]
- Patil, U.; Nagarajarao, R.C.; Balange, A.K.; Zhang, B.; Benjakul, S. Proteolytic activity and characteristics of skipjack tuna trypsin loaded alginate–chitosan beads as affected by drying methods and trehalose/glycerol. Int. J. Food Sci. 2022. [Google Scholar] [CrossRef]
- Hernandez-Gordillo, V.; Koppes, A.N.; Griffith, L.G.; Breault, D.T.; Carrier, R.L. Engineering the Niche for Intestinal Regeneration. In Biology and Engineering of Stem Cell Niches; Elsevier: Amsterdam, The Netherlands, 2017; pp. 601–615. [Google Scholar]
- Ahlin, G.; Hilgendorf, C.; Karlsson, J.; Szigyarto, C.A.-K.; Uhlén, M.; Artursson, P. Endogenous gene and protein expression of drug-transporting proteins in cell lines routinely used in drug discovery programs. Drug Metab. Dispos. 2009, 37, 2275–2283. [Google Scholar] [CrossRef] [Green Version]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Ding, L.; Wang, L.; Zhang, T.; Yu, Z.; Liu, J. Hydrolysis and transepithelial transport of two corn gluten derived bioactive peptides in human Caco-2 cell monolayers. Food Res. Int. 2018, 106, 475–480. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Chen, H.; Xue, J.; Guo, X.; Liang, M.; Chen, M. BioPepDB: An integrated data platform for food-derived bioactive peptides. Int. J. Food Sci. Nutr. 2018, 69, 963–968. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.; Kitts, D.D.; Li-Chan, E.C. Antioxidative and angiotensin-I-converting enzyme inhibitory potential of a Pacific hake (Merluccius productus) fish protein hydrolysate subjected to simulated gastrointestinal digestion and Caco-2 cell permeation. J. Agric. Food Chem. 2010, 58, 1535–1542. [Google Scholar] [CrossRef]
- Shi, Y.; Kovacs-Nolan, J.; Jiang, B.; Tsao, R.; Mine, Y. Antioxidant activity of enzymatic hydrolysates from eggshell membrane proteins and its protective capacity in human intestinal epithelial Caco-2 cells. J. Funct. Foods 2014, 10, 35–45. [Google Scholar] [CrossRef]
- Ao, J.; Li, B. Stability and antioxidative activities of casein peptide fractions during simulated gastrointestinal digestion in vitro: Charge properties of peptides affect digestive stability. Food Res. Int. 2013, 52, 334–341. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Qi, B.; Wang, Z.; Li, Y.; Sui, X.; Jiang, L. Changes in antioxidant activity of Alcalase-hydrolyzed soybean hydrolysate under simulated gastrointestinal digestion and transepithelial transport. J. Funct. Foods 2018, 42, 298–305. [Google Scholar] [CrossRef]
- Singh, A.; Benjakul, S. Proteolysis and its control using protease inhibitors in fish and fish products: A review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 496–509. [Google Scholar] [CrossRef] [Green Version]
- Gulzar, S.; Balange, A.K.; Nagarajarao, R.C.; Zhao, Q.; Benjakul, S. Microcapsules of shrimp oil using kidney bean protein isolate and κ-carrageenan as wall materials with the aid of ultrasonication or high-pressure microfluidization: Characteristics and oxidative stability. Foods 2022, 11, 1431. [Google Scholar] [CrossRef]
- Patil, U.; Benjakul, S. Comparative study on extraction of virgin coconut oil with the aid of partially purified protease from seabass pyloric caeca and commercial trypsin. J. Food Biochem. 2019, 43, e13024. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Robinson, H.W.; Hogden, C.G. The biuret reaction in the determination of serum proteins. 1. A study of the conditions necessary for the production of a stable color which bears a quantitative relationship to the protein concentration. J. Biol. Chem. 1940, 135, 707–725. [Google Scholar] [CrossRef]
- Yang, Y.-J.; He, H.-Y.; Wang, F.-Z.; Ju, X.-R.; Yuan, J.; Wang, L.-F.; Aluko, R.E.; He, R. Transport of angiotensin converting enzyme and renin dual inhibitory peptides LY, RALP and TF across Caco-2 cell monolayers. J. Funct. Foods 2017, 35, 303–314. [Google Scholar] [CrossRef]
- Benjakul, S.; Morrissey, M.T. Protein hydrolysates from Pacific whiting solid wastes. J. Agric. Food Chem. 1997, 45, 3423–3430. [Google Scholar] [CrossRef]
- Benjakul, S.; Karnjanapratum, S.; Visessanguan, W. Hydrolysed collagen from Lates calcarifer skin: Its acute toxicity and impact on cell proliferation and collagen production of fibroblasts. Int. J. Food Sci. 2018, 53, 1871–1879. [Google Scholar] [CrossRef]
- Mittal, A.; Singh, A.; Zhang, B.; Visessanguan, W.; Benjakul, S. Chitooligosaccharide conjugates prepared using several phenolic compounds via ascorbic acid/H2O2 -free radical grafting: Characteristics, antioxidant, antidiabetic, and antimicrobial activities. Foods 2022, 11, 920. [Google Scholar] [CrossRef] [PubMed]
- Steel, R.; Torrie, J. Principles and Practices of Statistics; McGraw Book Coy Inc.: New York, NY, USA, 1980. [Google Scholar]
- Goradia, D.; Cooney, J.; Hodnett, B.; Magner, E. The adsorption characteristics, activity and stability of trypsin onto mesoporous silicates. J. Mol. Catal. B Enzym. 2005, 32, 231–239. [Google Scholar] [CrossRef]
- Wen, S.; Zhou, G.; Song, S.; Xu, X.; Voglmeir, J.; Liu, L.; Zhao, F.; Li, M.; Li, L.; Yu, X. Discrimination of in vitro and in vivo digestion products of meat proteins from pork, beef, chicken, and fish. Proteomics 2015, 15, 3688–3698. [Google Scholar] [CrossRef] [Green Version]
- Nalinanon, S.; Benjakul, S.; Kishimura, H. Purification and biochemical properties of pepsins from the stomach of skipjack tuna (Katsuwonus pelamis). Eur. Food Res. Technol. 2010, 231, 259–269. [Google Scholar] [CrossRef]
- Yildirim-Elikoglu, S.; Vural, H.; Erdem, Y.K. Effect of phenolic compounds on the activity of proteolytic enzymes during rennet induced coagulation of milk and ripening of miniature cheese. LWT 2021, 136, 110337. [Google Scholar] [CrossRef]
- Kaewudom, P.; Benjakul, S.; Kijroongrojana, K. Effect of bovine and fish gelatin in combination with microbial transglutaminase on gel properties of threadfin bream surimi. Int. Aquat. Res. 2012, 4, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kuan, Y.-H.; Bhat, R.; Karim, A.A. Emulsifying and foaming properties of ultraviolet-irradiated egg white protein and sodium caseinate. J. Agric. Food Chem. 2011, 59, 4111–4118. [Google Scholar] [CrossRef]
- Amigo, L.; Hernández-Ledesma, B. Current evidence on the bioavailability of food bioactive peptides. Molecules 2020, 25, 4479. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, Y.; Jiang, Y.; Wang, L.; Liu, B.; Liu, J. Transport of egg white ACE-inhibitory peptide, Gln-Ile-Gly-Leu-Phe, in human intestinal Caco-2 cell monolayers with cytoprotective effect. J. Agric. Food Chem. 2014, 62, 3177–3182. [Google Scholar] [CrossRef]
- Xu, Z.; Bai, H.; Ma, X.; Wu, Y.; Wu, Z.; Yang, A.; Mao, W.; Li, X.; Chen, H. Cytological evaluation by Caco-2 and KU812 of non-allergenic peptides from simulated digestion of infant formula in vitro. Food Sci. Hum. Wellness 2023, 12, 817–824. [Google Scholar] [CrossRef]
- Linnankoski, J.; Mäkelä, J.; Palmgren, J.; Mauriala, T.; Vedin, C.; Ungell, A.L.; Lazorova, L.; Artursson, P.; Urtti, A.; Yliperttula, M. Paracellular porosity and pore size of the human intestinal epithelium in tissue and cell culture models. J. Pharm. Sci. 2010, 99, 2166–2175. [Google Scholar] [CrossRef]
- Shimizu, K.; Sato, M.; Zhang, Y.; Kouguchi, T.; Takahata, Y.; Morimatsu, F.; Shimizu, M. Molecular size of collagen peptide reverses the permeability of Caco-2 cells. Biosci. Biotechnol. Biochem. 2010, 74, 1123–1125. [Google Scholar] [CrossRef] [Green Version]
- Bruno, B.J.; Miller, G.D.; Lim, C.S. Basics and recent advances in peptide and protein drug delivery. Ther. Deliv. 2013, 4, 1443–1467. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Ding, L.; Du, Z.; Liu, J. Effects of hydrophobicity and molecular weight on the transport permeability of oligopeptides across Caco-2 cell monolayers. J. Food Biochem. 2020, 44, e13188. [Google Scholar] [CrossRef]
- Sibilla, S.; Godfrey, M.; Brewer, S.; Budh-Raja, A.; Genovese, L. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: Scientific background and clinical studies. Open Nutraceuticals J. 2015, 8, 29–42. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Guo, Z.; Wei, J.; Han, L.; Yu, Q.-l.; Chen, H.; Chen, Y.; Zhang, W. Extraction of low molecular weight peptides from bovine bone using ultrasound-assisted double enzyme hydrolysis: Impact on the antioxidant activities of the extracted peptides. LWT 2021, 146, 111470. [Google Scholar] [CrossRef]
- Gu, R.-Z.; Li, C.-Y.; Liu, W.-Y.; Yi, W.-X.; Cai, M.-Y. Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from Atlantic salmon (Salmo salar L.) skin. Food Res. Int. 2011, 44, 1536–1540. [Google Scholar] [CrossRef]
- Lee, J.-M.; You, S.-G.; Kim, S.-M. Functional activities of low molecular weight peptides purified from enzymatic hydrolysates of seaweeds. J. Korean Soc. Food Sci. Nutr. 2005, 34, 1124–1129. [Google Scholar]
- Fillería, S.G.; Tironi, V. Intracellular antioxidant activity and intestinal absorption of amaranth peptides released using simulated gastrointestinal digestion with Caco-2 TC7 cells. Food Biosci. 2021, 41, 101086. [Google Scholar] [CrossRef]
- Nalinanon, S.; Benjakul, S.; Kishimura, H.; Shahidi, F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 2011, 124, 1354–1362. [Google Scholar] [CrossRef]
- Wiriyaphan, C.; Xiao, H.; Decker, E.A.; Yongsawatdigul, J. Chemical and cellular antioxidative properties of threadfin bream (Nemipterus spp.) surimi byproduct hydrolysates fractionated by ultrafiltration. Food Chem. 2015, 167, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y. An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Nicoli, M.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Reddy, C.V.K.; Sreeramulu, D.; Raghunath, M. Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Res. Int. 2010, 43, 285–288. [Google Scholar] [CrossRef]









| Treatments | Enzyme Level (w/w, Based on Protein) | Degree of Hydrolysis (%) | ||
|---|---|---|---|---|
| Red Kidney Bean Isolate (RKB) | Threadfin Bream Surimi (TBS) | Egg White Proteins (EWP) | ||
| Absence of TPP | 25 | 24.79 ± 0.64 cF | 32.98 ± 1.65 aF | 28.82 ± 1.49 bF |
| 50 | 48.36 ± 1.77 cD | 60.04 ± 1.10 aD | 51.64 ± 1.04 bD | |
| Absence of TLB | 25 | 29.15 ± 1.65 cE | 36.75 ± 0.14 aE | 31.37 ± 0.57 bE |
| 50 | 55.41 ± 1.30 cC | 66.94 ± 1.08 aC | 59.98 ± 0.30 bC | |
| Presence of both TPP and TLB | 25 | 68.27 ± 1.53 cB | 81.41 ± 1.17 aB | 70.86 ± 1.41 bB |
| 50 | 76.82 ± 0.82 cA | 89.13 ± 1.15 aA | 74.16 ± 0.99 bA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, U.; Saetang, J.; Zhang, B.; Benjakul, S. Use of Tuna Visceral Pepsin in Combination with Trypsin as Digestion Aid: Enhanced Protein Hydrolysis and Bioavailability. Foods 2023, 12, 125. https://doi.org/10.3390/foods12010125
Patil U, Saetang J, Zhang B, Benjakul S. Use of Tuna Visceral Pepsin in Combination with Trypsin as Digestion Aid: Enhanced Protein Hydrolysis and Bioavailability. Foods. 2023; 12(1):125. https://doi.org/10.3390/foods12010125
Chicago/Turabian StylePatil, Umesh, Jirakrit Saetang, Bin Zhang, and Soottawat Benjakul. 2023. "Use of Tuna Visceral Pepsin in Combination with Trypsin as Digestion Aid: Enhanced Protein Hydrolysis and Bioavailability" Foods 12, no. 1: 125. https://doi.org/10.3390/foods12010125
APA StylePatil, U., Saetang, J., Zhang, B., & Benjakul, S. (2023). Use of Tuna Visceral Pepsin in Combination with Trypsin as Digestion Aid: Enhanced Protein Hydrolysis and Bioavailability. Foods, 12(1), 125. https://doi.org/10.3390/foods12010125