Comparing Different Packaging Conditions on Quality Stability of High-Pressure Treated Serra da Estrela Cheeses during Cold Storage †
Abstract
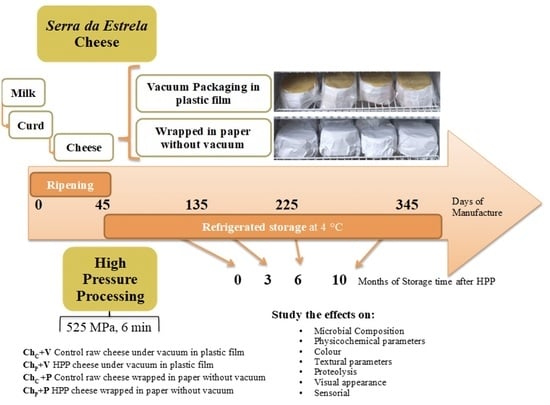
:1. Introduction
2. Materials and Methods
2.1. Milk Supply and Cheese Manufacture
2.2. High-Pressure Processing
2.3. Samples Identification and Sampling
2.4. Microbiological Analyses
2.5. Physicochemical Analyses
2.6. Color
2.7. Proteolysis
2.8. Instrumental Texture Profile Analysis (TPA)
2.9. Sensorial Evaluation
2.10. Statistical Analyses
3. Results and Discussion
3.1. Microbial Composition of Milk and Fresh Curd
3.2. Changes in Microbial Composition Induced by HPP and Packaging System
3.3. Changes in Physicochemical Characteristics
3.4. Changes in Proteolytic Indexes
3.5. Color
3.6. Changes in Textural Properties
3.7. Changes in Sensorial Attributes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Inácio, R.S.; Gomes, A.M.P.; Saraiva, J.A. Serra da Estrela cheese: A review. J. Food Process. Preserv. 2020, 44, e14412. [Google Scholar] [CrossRef]
- Macedo, A.C.; Malcata, F.X.; Oliveira, J.C. The technology, chemistry, and microbiology of Serra cheese: A review. J. Dairy Sci. 1993, 76, 1725–1739. [Google Scholar] [CrossRef]
- Macedo, A.C.; Malcata, F.X.; Hogg, T.A. Microbiological profile in Serra ewes’ cheese during ripening. J. Appl. Microbiol. 1995, 79, 1–11. [Google Scholar] [CrossRef]
- Dhineshkumar, V.; Ramasamy, D.; Siddharth, M. High pressure processing technology in dairy processing: A review. Asian J. Dairy Food Res. 2016, 35, 87–95. [Google Scholar] [CrossRef]
- Rodríguez-Pinilla, J.; Márquez, G.; Tabla, R.; Ramírez, R.; Delgado, F.J. Microbiological and lipolytic changes in high-pressure-treated raw milk cheeses during refrigerated storage. Dairy Sci. Technol. 2015, 95, 425–436. [Google Scholar] [CrossRef]
- Delgado, F.J.; Rodríguez-Pinilla, J.; Márquez, G.; Roa, I.; Ramírez, R. Physicochemical, proteolysis and texture changes during the storage of a mature soft cheese treated by high-pressure hydrostatic. Eur. Food Res. Technol. 2015, 240, 1167–1176. [Google Scholar] [CrossRef]
- Miles, A.A.; Misra, S.S.; Irwin, J.O. The estimation of the bactericidal power of the blood. J. Pathol. Bacteriol. 1938, 38, 732–749. [Google Scholar] [CrossRef]
- AOAC Official Method 920.124 Acidity of Cheese—Titrimetric Method 2002; AOAC: Rockville, ML, USA, 2002.
- AOAC Official Method 2001.14 Determination of Nitrogen (Total) in Cheese 2002; AOAC: Rockville, ML, USA, 2002.
- ISO 8968-4:2001; International IDF Standard 20D Milk: Determination of Nitrogen Content: 436 Inspection by Attributes. ISO: Geneva, Switzerland, 1993.
- Macedo, A.C.; Malcata, F.X. Secondary proteolysis in Serra cheese during ripening and throughout the cheese-making season. Eur. Food Res. Technol. 1997, 204, 173–179. [Google Scholar] [CrossRef]
- Bourne, M.C. Texture profile analysis. Food Technol. 1978, 32, 62–66. [Google Scholar]
- Inácio, R.S.; Fidalgo, L.G.; Santos, M.D.; Queirós, R.P.; Saraiva, J.A. Effect of high-pressure treatments on microbial loads and physicochemical characteristics during refrigerated storage of raw milk Serra da Estrela cheese samples. Int. J. Food Sci. Technol. 2014, 49, 1272–1278. [Google Scholar] [CrossRef]
- Tavaria, F.K.; Malcata, F.X. On the microbiology of Serra da Estrela cheese: Geographical and chronological considerations. Food Microbiol. 2000, 17, 293–304. [Google Scholar] [CrossRef]
- Calzada, J.; Del Olmo, A.; Picon, A.; Gaya, P.; Nuñez, M. Reducing biogenic-amine-producing bacteria, decarboxylase activity, and biogenic amines in raw milk cheese by high-pressure treatments. Appl. Environ. Microbiol. 2013, 79, 1277–1283. [Google Scholar] [CrossRef]
- Macedo, A.C.; Costa, M.L.; Malcata, F.X. Changes in the microflora of Serra cheese: Evolution throughout ripening time, lactation period and axial location. Int. Dairy J. 1996, 6, 79–94. [Google Scholar] [CrossRef]
- Arqués, J.L.; Garde, S.; Gaya, P.; Medina, M.; Nuñez, M. Short Communication: Inactivation of Microbial Contaminants in Raw Milk La Serena Cheese by High-Pressure Treatments. J. Dairy Res. 2006, 89, 888–891. [Google Scholar] [CrossRef]
- European Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; European Commission: Brussels, Belgium, 2005; Volume L338, pp. 1–19.
- Correia, P.; Vítor, A.; Tenreiro, M.; Correia, A.C.; Madanelo, J.; Guiné, R. Effect of different thistle flower ecotypes as milk-clotting in Serra da Estrela cheese. Nutr. Food Sci. 2016, 46, 458–475. [Google Scholar] [CrossRef]
- Macedo, A.C.; Tavares, T.G.; Malcata, F.X. Influence of native lactic acid bacteria on the microbiological, biochemical and sensory profiles of Serra da Estrela cheese. Food Microbiol. 2004, 21, 233–240. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Tenreiro, M.I.C.; Correia, A.C.; Correia, P.M.R.; Barracosa, P. Analysis of factors influencing the physical, chemical and sensorial properties of Serra da Estrela cheeses. J. Food Meas. Charact. 2016, 10, 643–657. [Google Scholar] [CrossRef]
- Sousa, M.J.; Malcata, F.X. Comparison of Plant and Animal Rennets in Terms of Microbiological, Chemical, and Proteolysis Characteristics of Ovine Cheese. J. Agric. Food Chem. 1997, 45, 74–81. [Google Scholar] [CrossRef]
- Tavaria, F.K.; Franco, I.; Carballo, J.F.; Malcata, F.X. Amino acid and soluble nitrogen evolution throughout ripening of Serra da Estrela cheese. Int. Dairy J. 2003, 13, 537–545. [Google Scholar] [CrossRef]
- Garde, S.; Arqués, J.L.; Gaya, P.; Medina, M.; Nuñez, M. Effect of high-pressure treatments on proteolysis and texture of ewes’ raw milk La Serena cheese. Int. Dairy J. 2007, 17, 1424–1433. [Google Scholar] [CrossRef]
- Delgado, F.J.; Delgado, J.; González-Crespo, J.; Cava, R.; Ramírez, R. High-pressure processing of a raw milk cheese improved its food safety maintaining the sensory quality. Food Sci. Technol. Int. 2013, 19, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Voigt, D.D.; Chevalier, F.; Qian, M.C.; Kelly, A.L. Effect of high-pressure treatment on microbiology, proteolysis, lipolysis and levels of flavour compounds in mature blue-veined cheese. Innov. Food Sci. Emerg. Technol. 2010, 11, 68–77. [Google Scholar] [CrossRef]
- Calzada, J.; Del Olmo, A.; Picon, A.; Gaya, P.; Nuñez, M. High-Pressure Processing for the Control of Lipolysis, Volatile Compounds and Off-odours in Raw Milk Cheese. Food Bioprocess Technol. 2014, 7, 2207–2217. [Google Scholar] [CrossRef]
- Calzada, J.; Del Olmo, A.; Picon, A. Using high-pressure processing for reduction of proteolysis and prevention of over-ripening of raw milk cheese. Food Bioprocess Technol. 2014, 7, 1404–1413. [Google Scholar] [CrossRef]
- Calzada, J.; Del Olmo, A.; Picon, A.; Gaya, P.; Nuñez, M. Effect of High-Pressure Processing on the Microbiology, Proteolysis, Biogenic Amines and Flavour of Cheese Made from Unpasteurized Milk. Food Bioprocess Technol. 2014, 8, 319–332. [Google Scholar] [CrossRef]



| ChC+V | ChP+V | ChC+P | ChP+P | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moisture Content | ||||||||||||||||
| 0 | 46.0 | ± | 2.43 | a,A | 46.1 | ± | 0.93 | a,A | 46.4 | ± | 0.39 | a,A | 43.3 | ± | 1.36 | b,A |
| 3 | 41.7 | ± | 0.65 | a,B | 41.8 | ± | 1.88 | a,B | 43.3 | ± | 0.39 | a,B | 42.9 | ± | 0.29 | a,A,B |
| 6 | 41.7 | ± | 0.73 | b,B | 40.4 | ± | 0.65 | c,B | 43.8 | ± | 0.77 | a,B | 41.8 | ± | 0.56 | b,B |
| 10 | 40.8 | ± | 1.47 | a,B | 40.9 | ± | 0.40 | a,B | ||||||||
| Protein Content | ||||||||||||||||
| 0 | 21.9 | ± | 1.83 | a,A | 22.2 | ± | 1.43 | a,A | 24.0 | ± | 0.73 | a,A | 22.1 | ± | 0.47 | a,A |
| 6 | 24.9 | ± | 1.20 | a,B | 24.7 | ± | 1.39 | a,A,B | 23.8 | ± | 1.29 | a,A | 24.6 | ± | 1.12 | a,B |
| 10 | 24.8 | ± | 0.48 | a,B | 25.3 | ± | 1.30 | a,B | ||||||||
| pH values | ||||||||||||||||
| 0 | 5.24 | ± | 0.01 | a,c,B | 5.19 | ± | 0.01 | b,A | 5.25 | ± | 0.02 | a,C | 5.19 | ± | 0.05 | b,B |
| 3 | 5.17 | ± | 0.05 | b,C | 5.18 | ± | 0.01 | b,A,B | 5.82 | ± | 0.05 | a,B | 5.19 | ± | 0.02 | b,B |
| 6 | 5.28 | ± | 0.05 | c,B | 5.13 | ± | 0.03 | b,B | 6.69 | ± | 0.09 | a,A | 5.71 | ± | 0.07 | b,A |
| 10 | 5.45 | ± | 0.03 | a,A | 5.27 | ± | 0.05 | b,A,B | ||||||||
| TA | ||||||||||||||||
| 0 | 1.43 | ± | 0.10 | a,b,B | 1.39 | ± | 0.04 | a,b,B | 1.30 | ± | 0.02 | b,A | 1.19 | ± | 0.04 | c,B |
| 3 | 1.69 | ± | 0.14 | a,A | 1.38 | ± | 0.11 | b,B | 1.20 | ± | 0.07 | c,B | 1.31 | ± | 0.08 | b,c,A |
| 6 | 1.58 | ± | 0.06 | a,A,B | 1.25 | ± | 0.11 | c,B | 1.39 | ± | 0.06 | b,A | 1.22 | ± | 0.10 | c,A,B |
| 10 | 1.72 | ± | 0.15 | a,A | 1.90 | ± | 0.05 | b,A | ||||||||
| ChC+V | ChP+V | ChC+P | ChP+P | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Storage Time (Months) | ||||||||||||||||||
| Cheese Surface Colour | L* | 0 | 71.9 | ± | 2.04 | a,C | 73.6 | ± | 2.02 | a,B | 70.6 | ± | 2.24 | a,B | 86.7 | ± | 0.92 | a,B |
| 3 | 77.3 | ± | 1.26 | a,B | 78.7 | ± | 2.04 | a,A | 78.7 | ± | 2.04 | a,A | 77.4 | ± | 1.93 | a,A | ||
| 6 | 79.8 | ± | 0.35 | a,A | 78.5 | ± | 2.05 | a,A | ||||||||||
| a* | 0 | −0.12 | ± | 1.20 | a,A | −0.43 | ± | 1.31 | a,A | 0.92 | ± | 0.93 | a,A | −2.93 | ± | 0.14 | a,B | |
| 3 | −0.29 | ± | 1.08 | a,b.A | 0.57 | ± | 1.38 | b,A | 0.57 | ± | 1.38 | a,A | 0.04 | ± | 2.27 | a,b,A | ||
| 6 | −0.29 | ± | 0.58 | a,A | −0.88 | ± | 1.05 | a,A | ||||||||||
| b* | 0 | 24.4 | ± | 1.20 | c,A | 28.0 | ± | 1.40 | b,A | 25.7 | ± | 1.69 | c,A | 22.3 | ± | 0.74 | a,B | |
| 3 | 22.1 | ± | 1.08 | b,B | 24.6 | ± | 1.78 | a,B | 24.6 | ± | 1.78 | a,A | 26.2 | ± | 1.02 | a,A | ||
| 6 | 20.9 | ± | 0.58 | b,B | 24.3 | ± | 2.05 | a,B | ||||||||||
| Cheese Core Colour | L* | 0 | 85.1 | ± | 2.04 | a,b,A | 85.0 | ± | 1.37 | b,A | 86.3 | ± | 1.83 | a,b,A | 86.7 | ± | 0.92 | a,A |
| 3 | 83.7 | ± | 1.26 | b,A,B | 85.6 | ± | 1.14 | a,A | 85.6 | ± | 1.78 | a,b,A,B | 84.3 | ± | 1.55 | a,b,B | ||
| 6 | 82.3 | ± | 0.35 | b,B | 82.9 | ± | 1.14 | b,B | 84.4 | ± | 1.32 | a,B | 84.9 | ± | 0.82 | a,B | ||
| a* | 0 | −1.54 | ± | 1.20 | a,A | −1.88 | ± | 0.16 | b,A | −2.97 | ± | 0.22 | c,A | 0.12 | ± | 1.32 | c,A | |
| 3 | −1.43 | ± | 1.08 | a,A | −1.34 | ± | 0.10 | a,A | −3.03 | ± | 0.20 | c,A | −2.81 | ± | 0.25 | b,A | ||
| 6 | −1.49 | ± | 0.58 | a,A | −1.30 | ± | 0.47 | a,A | −2.77 | ± | 0.26 | b,A | −2.47 | ± | 0.10 | b,A | ||
| b* | 0 | 18.8 | ± | 1.18 | b,B | 22.1 | ± | 1.08 | a,A | 22.6 | ± | 0.98 | a,A | 30.2 | ± | 0.94 | a,B | |
| 3 | 19.7 | ± | 1.64 | b,A,B | 20.4 | ± | 1.75 | b,B | 23.5 | ± | 1.08 | a,A | 24.4 | ± | 0.69 | a,A | ||
| 6 | 21.0 | ± | 1.37 | b,A | 22.2 | ± | 1.44 | b,A | 23.9 | ± | 0.75 | a,A | 24.0 | ± | 0.94 | a,A | ||
| Property | Storage Time (Months) | ChC+V | ChP+V | ChC+P | ChP+P | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hardness | 0 | 0.27 | ± | 0.14 | b,B | 0.18 | ± | 0.04 | b,C | 0.58 | ± | 0.14 | a,A | 0.56 | ± | 0.11 | a,A |
| (N) | 3 | 0.47 | ± | 0.093 | a,A | 0.37 | ± | 0.041 | a,b,B | 0.28 | ± | 0.14 | b,B | 0.39 | ± | 0.046 | a,b,B |
| 6 | 0.49 | ± | 0.085 | a,A | 0.59 | ± | 0.13 | a,A | 0.28 | ± | 0.079 | b,B | 0.59 | ± | 0.10 | a,A | |
| 10 | 0.30 | ± | 0.047 | a,B | 0.35 | ± | 0.084 | a,B | |||||||||
| Consistency | 0 | 1.7 | ± | 0.41 | b,B | 1.4 | ± | 0.34 | b,C | 4.8 | ± | 1.36 | a,A | 5.1 | ± | 0.87 | a,A |
| (N s−1) | 3 | 3.6 | ± | 0.67 | a,A | 2.9 | ± | 0.61 | a,B | 1.3 | ± | 0.47 | b,C | 3.1 | ± | 0.65 | a,B,B |
| 6 | 4.1 | ± | 0.79 | a,A | 5.1 | ± | 0.67 | a,A | 2.8 | ± | 0.91 | b,B | 5.0 | ± | 0.92 | a,A | |
| 10 | 2.4 | ± | 0.33 | a,B | 2.7 | ± | 0.33 | a,B | |||||||||
| Adhesiveness | 0 | 0.6 | ± | 0.19 | a,A | 0.3 | ± | 0.07 | a,A | 1.3 | ± | 0.61 | b,A,B | 1.4 | ± | 0.09 | b,A |
| (N s−1) | 3 | 1.5 | ± | 0.44 | b,B | 1.1 | ± | 0.41 | a,b,B | 0.7 | ± | 0.12 | a,A | 1.4 | ± | 0.37 | b,A |
| 6 | 2.4 | ± | 0.58 | a,b,C | 2.3 | ± | 0.47 | a,b,C | 1.6 | ± | 0.69 | a,B | 2.8 | ± | 0.61 | b,B | |
| 10 | 1.8 | ± | 0.39 | a,B,C | 1.8 | ± | 0.37 | a,C | |||||||||
| Cohesiveness (dimensionless) | 0 | 5.5 | ± | 2.2 | a,A | 6.6 | ± | 2.0 | a,A | 2.7 | ± | 0.56 | b,B | 2.6 | ± | 0.49 | b,B |
| 3 | 3.2 | ± | 0.65 | a,B | 4.2 | ± | 0.63 | a,C | 4.4 | ± | 1.8 | a,A,B | 3.9 | ± | 0.46 | a,A | |
| 6 | 4.2 | ± | 0.72 | a,A,B | 2.3 | ± | 0.68 | b,B | 5.6 | ± | 1.8 | a,A | 2.6 | ± | 0.32 | b,B | |
| 10 | 4.7 | ± | 1.7 | a,A,B | 4.2 | ± | 0.71 | a,B | |||||||||
| Gumminess | 0 | 1.4 | ± | 0.33 | a,b,C | 1.1 | ± | 0.16 | b,B | 1.6 | ± | 0.16 | a,A | 1.4 | ± | 0.28 | a,b,A |
| (N) | 3 | 1.5 | ± | 0.052 | a,B,C | 1.5 | ± | 0.082 | a,A | 1.6 | ± | 0.13 | a,A | 1.5 | ± | 0.043 | a,A |
| 6 | 2.0 | ± | 0.054 | a,A | 1.5 | ± | 0.085 | b,c,A | 1.3 | ± | 0.12 | c,B | 1.6 | ± | 0.16 | b,A | |
| 10 | 1.7 | ± | 0.042 | a,A,B | 1.6 | ± | 0.074 | b,A | |||||||||
| Storage Time (Months) | 0 | 3 | 6 | 10 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ChP+V Vs. ChC+V | ChP+P Vs. ChC+P | ChP+V Vs. ChC+V | ChP+P Vs. ChC+P | ChP+V Vs. ChC+V | ChP+P Vs. ChC+P | ChP+V Vs. ChC+V | ||||||||
| Rind Appearance | ||||||||||||||
| Tonality | −0.59 | −0.86 | 2.41 | * | 4.22 | * | 1.86 | * | −1.55 | * | 2.37 | * | ||
| Homogeneity | −1.38 | 1.61 | −4.58 | * | −0.38 | 3.36 | * | 0.62 | −0.86 | |||||
| Defects | 0.38 | −2.59 | * | 1.98 | −4.85 | * | −2.67 | * | 0.01 | −0.17 | ||||
| Paste Appearance | ||||||||||||||
| Color | 0.23 | −1.48 | * | 2.80 | * | −0.71 | 1.63 | * | −0.99 | * | 2.38 | * | ||
| Consistency | −1.24 | −2.35 | * | 3.67 | * | 2.32 | * | 1.53 | 1.49 | * | −0.12 | |||
| Odour | ||||||||||||||
| Lactic | −0.53 | −0.92 | −1.60 | 3.52 | * | −2.52 | * | −2.04 | * | 0.63 | ||||
| Acid | −0.01 | 0.36 | −1.63 | 0.02 | −1.50 | −0.27 | 1.16 | |||||||
| Animal | −0.55 | 0.42 | −0.66 | −0.46 | −0.30 | −2.07 | * | 0.88 | ||||||
| SCFA | −0.26 | −0.24 | −1.11 | −0.42 | 0.69 | * | −0.23 | 1.15 | ||||||
| Texture | ||||||||||||||
| Consistency | 3.87 | * | 2.60 | * | 1.00 | 2.62 | * | 2.52 | * | |||||
| Friability | 3.00 | * | 0.27 | −0.49 | 0.56 | −0.02 | ||||||||
| Unctuosity | 3.00 | * | −2.66 | * | −0.86 | −3.97 | * | −0.03 | ||||||
| Flavor | ||||||||||||||
| Salty | −2.47 | * | −0.39 | −1.25 | −3.45 | * | −0.17 | |||||||
| Acid | 3.72 | * | 2.67 | 2.06 | * | 3.74 | * | 1.23 | ||||||
| Bitter | −1.90 | 1.31 | 0.13 | −0.85 | −0.77 | |||||||||
| After-taste | 3.43 | * | 0.06 | −0.61 | 0.01 | −0.88 | ||||||||
| Storage Time (Months) | 0 | 3 | 6 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ChC+V | ChP+V | ChC+P | ChP+P | ChC+V | ChP+V | ChC+P | ChP+P | ChC+V | ChP+V | ChC+P | ChP+P | |
| Rind Appearance | ||||||||||||
| Tonality | 2.81 a,b | 4.36 a,b | 4.36 a | 2.53 b | 1.13 c | 5.00 b | 7.96 a | 3.68 b | 0.91 d | 2.61 c | 9.1 a | 6.65 b |
| Homogeneity | 4.94 a | 2.26 b | 3.01 a,b | 4.31 a,b | 7.36 a | 7.66 a | 0.94 b | 6.66 a | 6.02 a | 6.11 a | 3.08 b | 3.33 b |
| Defects | 1.38 b | 4.20 a | 3.54 a | 1.20 b | 0.71 b | 0.69 b | 6.26 a | 1.04 b | 3.50 c | 1.72 d | 5.64 b | 8.15 a |
| Paste Appearance | ||||||||||||
| Color | 4.65 a | 2.44 b | 3.83 a,b | 2.24 b | 3.72 b | 4.78 a,b | 6.34 a | 5.10 a,b | 3.90 a | 4.39 a | 5.44 a | 5.91 a |
| Consistency | 5.09 a | 2.53 b | 4.95 a | 2.15 b | 4.42 b | 6.90 b | 3.56 a | 5.48 a,b | 4.38 a | 6.07 a | 5.30 a | 6.66 a |
| Odour | ||||||||||||
| Lactic | 3.66 a | 2.69 a | 3.45 a | 2.28 a | 3.83 a | 4.08 a | 3.48 a | 2.99 a | 4.96 a | 3.63 a | 3.22 a | 2.60 a |
| Acid | 3.41 a | 2.19 a | 3.13 a | 2.32 a | 3.30 a | 3.59 a | 2.13 a | 2.74 a | 4.35 a | 3.24 a,b | 1.86 b | 1.90 b |
| Animal | 2.74 a | 2.56 a | 2.52 a | 2.73 a | 3.22 a | 2.22 a | 3.31 a | 2.97 a | 1.40 a | 0.94 a | 2.98 a | 2.33 a |
| SCFA | 2.59 a | 1.82 a | 2.16 a | 1.88 a | 2.61 a | 1.90 a | 1.59 a | 3.48 a | 1.06 a | 1.67 a | 1.43 a | 0.81 a |
| Texture | ||||||||||||
| Consistency | 2.50 b | 5.64 a | 1.49 b | 4.61 a | 2.94 b | 3.96 a,b | 3.60 a,b | 5.19 a | ||||
| Friability | 1.78 a,b | 3.60 a | 0.19 b | 2.04 a,b | 1.32 a | 1.32 a | 1.42 a | 1.28 a | ||||
| Unctuosity | 6.66 a | 3.52 b | 6.31 a | 3.82 b | 5.77 a | 5.3 a | 5.31 a | 2.16 b | ||||
| Flavor | ||||||||||||
| Salty | 4.67 a | 4.49 a | 4.20 a | 3.59 a | 4.58 a | 3.60 a | 3.97 a | 2.26 a | ||||
| Acid | 4.40 a | 2.84 a | 2.84 a | 2.64 a | 5.65 a | 3.75 a,b | 3.16 a,b | 1.40 b | ||||
| Bitter | 3.93 a | 2.49 a | 3.59 a | 2.97 a | 3.00 a | 2.94 a | 3.62 a | 2.98 a | ||||
| After-taste | 5.56 a | 3.70 a | 5.14 a | 3.33 a | 5.52 a | 4.31 a | 4.21 a | 2.57 a | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inácio, R.S.; Monteiro, M.J.P.; Lopes-da-Silva, J.A.; Gomes, A.M.P.; Saraiva, J.A. Comparing Different Packaging Conditions on Quality Stability of High-Pressure Treated Serra da Estrela Cheeses during Cold Storage. Foods 2023, 12, 1935. https://doi.org/10.3390/foods12101935
Inácio RS, Monteiro MJP, Lopes-da-Silva JA, Gomes AMP, Saraiva JA. Comparing Different Packaging Conditions on Quality Stability of High-Pressure Treated Serra da Estrela Cheeses during Cold Storage. Foods. 2023; 12(10):1935. https://doi.org/10.3390/foods12101935
Chicago/Turabian StyleInácio, Rita S., Maria J. P. Monteiro, José A. Lopes-da-Silva, Ana M. P. Gomes, and Jorge A. Saraiva. 2023. "Comparing Different Packaging Conditions on Quality Stability of High-Pressure Treated Serra da Estrela Cheeses during Cold Storage" Foods 12, no. 10: 1935. https://doi.org/10.3390/foods12101935
APA StyleInácio, R. S., Monteiro, M. J. P., Lopes-da-Silva, J. A., Gomes, A. M. P., & Saraiva, J. A. (2023). Comparing Different Packaging Conditions on Quality Stability of High-Pressure Treated Serra da Estrela Cheeses during Cold Storage. Foods, 12(10), 1935. https://doi.org/10.3390/foods12101935