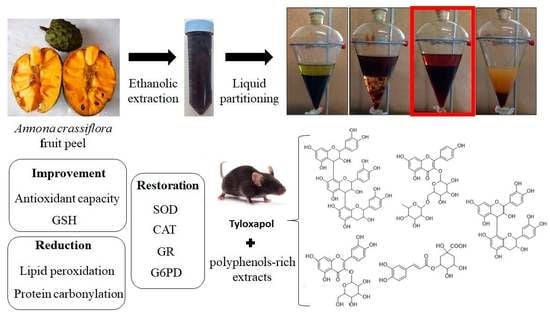
Annona crassiflora Mart. Fruit Peel Polyphenols Preserve Cardiac Antioxidant Defense and Reduce Oxidative Damage in Hyperlipidemic Mice
Abstract
:1. Introduction
2. Material and Methods
2.1. Preparation and Extraction of ACM Polyphenols
2.2. Phytochemical Prospection
2.3. Animals and Experimental Design
2.4. Tissue Preparation
2.5. Oxidative Stress Markers Analysis
2.5.1. Analysis of Oxidative Damage
2.5.2. Analysis of Enzymatic and Non-Enzymatic Antioxidants
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Csonka, C.; Sárközy, M.; Pipicz, M.; Dux, L.; Csont, T. Modulation of Hypercholesterolemia-Induced Oxidative/Nitrative Stress in the Heart. Oxidative Med. Cell. Longev. 2016, 2016, 3863726. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free. Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef]
- van der Pol, A.; van Gilst, W.H.; Voors, A.A.; van der Meer, P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019, 21, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Bungau, S.; Kumar, K.; Zengin, G.; Khan, F.; Kumar, A.; Kaur, R.; Venkatachalam, T.; Tit, D.M.; Vesa, C.M.; et al. Pleotropic Effects of Polyphenols in Cardiovascular System. Biomed. Pharmacother. 2020, 130, 110714. [Google Scholar] [CrossRef] [PubMed]
- Al-Numair, K.S.; Chandramohan, G.; Veeramani, C.; Alsaif, M.A. Ameliorative effect of kaempferol, a flavonoid, on oxidative stress in streptozotocin-induced diabetic rats. Redox Rep. 2015, 20, 198–209. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pastore, G.M. Araticum (Annona crassiflora Mart.) as a source of nutrients and bioactive compounds for food and non-food purposes: A comprehensive review. Food Res. Int. 2019, 123, 450–480. [Google Scholar] [CrossRef]
- Justino, A.B.; Pereira, M.N.; Vilela, D.D.; Peixoto, L.G.; Martins, M.M.; Teixeira, R.R.; Miranda, N.C.; da Silva, N.M.; de Sousa, R.M.; de Oliveira, A.; et al. Peel of araticum fruit (Annona crassiflora Mart.) as a source of antioxidant compounds with α-amylase, α-glucosidase and glycation inhibitory activities. Bioorganic Chem. 2016, 69, 167–182. [Google Scholar] [CrossRef]
- Ramos, L.P.A.; Justino, A.B.; Tavernelli, N.; Saraiva, A.L.; Franco, R.R.; de Souza, A.V.; Silva, H.C.G.; de Moura, F.B.R.; Botelho, F.V.; Espindola, F.S. Antioxidant compounds from Annona crassiflora fruit peel reduce lipid levels and oxidative damage and maintain the glutathione defense in hepatic tissue of Triton WR-1339-induced hyperlipidemic mice. Biomed. Pharmacother. 2021, 142, 112049. [Google Scholar] [CrossRef] [PubMed]
- Zarzecki, M.S.; Araujo, S.M.; Bortolotto, V.C.; de Paula, M.T.; Jesse, C.R.; Prigol, M. Hypolipidemic action of chrysin on Triton WR-1339-induced hyperlipidemia in female C57BL/6 mice. Toxicol. Rep. 2014, 1, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yagi, K. Assay For Serum Lipid Peroxide Level And Its Clinical Significance. In Lipid Peroxides in Biology and Medicine; Yagi, K., Ed.; Academic Press: Cambridge, MA, USA, 1982; pp. 223–242. [Google Scholar]
- Reznick, A.Z.; Packer, L. Oxidative damage to proteins: Spectrophotometric method for carbonyl assay. Methods Enzymol. 1994, 233, 357–363. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the auto-oxidation of pyrogallol and convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Justino, A.B.; Pereira, M.N.; Peixoto, L.G.; Vilela, D.D.; Caixeta, D.C.; de Souza, A.V.; Teixeira, R.R.; Silva, H.C.G.; de Moura, F.B.R.; Moraes, I.B.; et al. Hepatoprotective properties of a polyphenols-enriched fraction from Annona crassiflora Mart. fruit peel against diabetes-induced oxidative and nitrosative stress. J. Agric. Food Chem. 2017, 65, 4428–4438. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Mannervik, B. Glutathione Peroxidase, Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1985; pp. 490–495. [Google Scholar]
- Justino, A.B.; Franco, R.R.; Silva, H.C.G.; Saraiva, A.L.; Sousa, R.M.F.; Espindola, F.S. B procyanidins of Annona crassiflora fruit peel inhibited glycation, lipid peroxidation and protein-bound carbonyls, with protective effects on glycated catalase. Sci. Rep. 2019, 9, 19183. [Google Scholar] [CrossRef]
- Justino, A.B.; Florentino, R.M.; França, A.; Filho, A.C.M.L.; Franco, R.R.; Saraiva, A.L.; Fonseca, M.C.; Leite, M.F.; Espindola, F.S. Alkaloid and acetogenin-rich fraction from Annona crassiflora fruit peel inhibits proliferation and migration of human liver cancer HepG2 cells. PLoS ONE 2021, 16, e0250394. [Google Scholar] [CrossRef]
- Zhou, Q.; Yin, Z.P.; Ma, L.; Zhao, W.; Hao, H.W.; Li, H.L. Free radical-scavenging activities of oligomeric proanthocyanidin from Rhodiola rosea L. and its antioxidant effects in vivo. Nat. Prod. Res. 2014, 28, 2301–2303. [Google Scholar] [CrossRef]
- Fernández-Martínez, E.; Lira-Islas, I.G.; Cariño-Cortés, R.; Soria-Jasso, L.E.; Pérez-Hernández, E.; Pérez-Hernández, N. Dietary chia seeds (Salvia hispanica) improve acute dyslipidemia and steatohepatitis in rats. J. Food Biochem. 2019, 43, e12986. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Lipid peroxidation products as oxidative stress biomarkers. BioFactors 2008, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Carini, M.; Butterfield, D.A. Protein carbonylation. Antioxid. Redox Signal. 2010, 12, 323–325. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Giustarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonylation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Justino, A.B.; Costa, M.S.; Saraiva, A.L.; Silva, P.H.; Vieira, T.N.; Dias, P.; Linhares, C.R.B.; Dechichi, P.; Avila, V.D.M.R.; Espindola, F.S.; et al. Protective effects of a polyphenol-enriched fraction of the fruit peel of Annona crassiflora Mart. on acute and persistent inflammatory pain. Inflammopharmacology 2020, 28, 759–771. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komino, E.A.; Ramos, L.P.A.; de Souza, A.V.; Caixeta, D.C.; Bittar, V.P.; Borges, A.L.; Botelho, F.V.; Espindola, F.S.; Justino, A.B. Annona crassiflora Mart. Fruit Peel Polyphenols Preserve Cardiac Antioxidant Defense and Reduce Oxidative Damage in Hyperlipidemic Mice. Foods 2023, 12, 2097. https://doi.org/10.3390/foods12112097
Komino EA, Ramos LPA, de Souza AV, Caixeta DC, Bittar VP, Borges AL, Botelho FV, Espindola FS, Justino AB. Annona crassiflora Mart. Fruit Peel Polyphenols Preserve Cardiac Antioxidant Defense and Reduce Oxidative Damage in Hyperlipidemic Mice. Foods. 2023; 12(11):2097. https://doi.org/10.3390/foods12112097
Chicago/Turabian StyleKomino, Eliana Akemi, Letícia Pereira Afonso Ramos, Adriele Vieira de Souza, Douglas Carvalho Caixeta, Vinicius Prado Bittar, Ana Luiza Borges, Françoise Vasconcelos Botelho, Foued Salmen Espindola, and Allisson Benatti Justino. 2023. "Annona crassiflora Mart. Fruit Peel Polyphenols Preserve Cardiac Antioxidant Defense and Reduce Oxidative Damage in Hyperlipidemic Mice" Foods 12, no. 11: 2097. https://doi.org/10.3390/foods12112097
APA StyleKomino, E. A., Ramos, L. P. A., de Souza, A. V., Caixeta, D. C., Bittar, V. P., Borges, A. L., Botelho, F. V., Espindola, F. S., & Justino, A. B. (2023). Annona crassiflora Mart. Fruit Peel Polyphenols Preserve Cardiac Antioxidant Defense and Reduce Oxidative Damage in Hyperlipidemic Mice. Foods, 12(11), 2097. https://doi.org/10.3390/foods12112097