Chemical and Bioactive Screening of Green Polyphenol-Rich Extracts from Chestnut By-Products: An Approach to Guide the Sustainable Production of High-Added Value Ingredients
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Plant Material
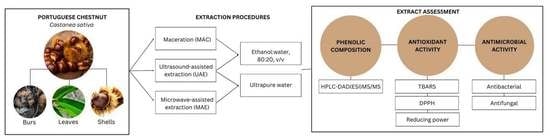
2.3. Extraction of Phenolic Compounds
2.3.1. Maceration (MAC)
2.3.2. Ultrasound-Assisted Extraction (UAE)
2.3.3. Microwave-Assisted Extraction (MAE)
2.4. Composition in Phenolic Compounds
2.5. In Vitro Bioactivity Assays
2.5.1. Antioxidant Capacity
TBARS Assay
DPPH• Radical Scavenging Assay
Reducing Power Assay
2.5.2. Hepatotoxicity
2.5.3. Antimicrobial Activity
2.6. Statistical Analyses
3. Results and Discussion
3.1. Composition of Polyphenols in Extracts of Chestnut By-Products
3.1.1. Phenolic Compound Identification
3.1.2. Quantification of Phenolic Compounds
3.2. Phenolic Compound Classes (Relative Percentage) in the Different Extracts
3.3. Phenolic Compound Classes and Total Phenolic Compounds (Absolute Values) in the Different Extracts
3.4. Bioactivities
3.4.1. Antioxidant Capacity
3.4.2. Hepatotoxicity
3.4.3. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization. Technical Platform on the Measurement and Reduction of Food Loss and Waste. 2021. Available online: https://www.fao.org/platform-food-loss-waste/ (accessed on 7 April 2023).
- Ballesteros-Vivas, D.; Socas-Rodríguez, B.; Mendiola, J.A.; Ibáñez, E.; Cifuentes, A. Green food analysis: Current trends and perspectives. Curr. Opin. Green Sustain. Chem. 2021, 31, 100522. [Google Scholar] [CrossRef]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making sense of the “clean label” trends: A review of consumer food choice behavior and discussion of industry implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Saulais, L.; Corcuff, R.; Boonefaes, E. Natural and healthy? Consumers knowledge, understanding and preferences regarding naturalness and healthiness of processed foods. Int. J. Gastron. Food Sci. 2023, 31, 100662. [Google Scholar] [CrossRef]
- Witkowski, M.; Grajeta, H.; Gomułka, K. Hypersensitivity reactions to food additives-preservatives, antioxidants, flavor enhancers. Int. J. Environ. Res. Public Health 2022, 19, 11493. [Google Scholar] [CrossRef]
- Alexandri, M.; Kachrimanidou, V.; Papapostolou, H.; Papadaki, A.; Kopsahelis, N. Sustainable food systems: The case of functional compounds towards the development of clean label food products. Foods 2022, 11, 2796. [Google Scholar] [CrossRef]
- Braga, N.; Rodrigues, F.; Oliveira, M.B.P.P. Castanea sativa by-products: A review on added value and sustainable application. Nat. Prod. Res. 2015, 29, 1–18. [Google Scholar] [CrossRef]
- Pinto, D.; Cádiz-Gurrea, M.D.L.L.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Castanea sativa shells: A review on phytochemical composition, bioactivity and waste management approaches for industrial valorization. Food Res. Int. 2021, 144, 110364. [Google Scholar] [CrossRef]
- Instituto Nacional de Estatística. Estatísticas Agrícolas; Instituto Nacional de Estatística: Lisbon, Portugal, 2018; ISBN 978-989-25-04957. [Google Scholar]
- Borges, O.; Gonçalves, B.; de Carvalho, J.L.S.; Correia, P.; Silva, A.P. Nutritional quality of chestnut (Castanea sativa Mill.) cultivars from Portugal. Food Chem. 2008, 106, 976–984. [Google Scholar] [CrossRef]
- Costa-Trigo, I.; Otero-Penedo, P.; Outeiriño, D.; Paz, A.; Domínguez, J.M. Valorization of chestnut (Castanea sativa) residues: Characterization of different materials and optimization of the acid-hydrolysis of chestnut burrs for the elaboration of culture broths. Waste Manag. 2019, 87, 472–484. [Google Scholar] [CrossRef]
- Romano, A.; Aponte, M. Chestnut as Source of Novel Ingredients for Celiac People. In Encyclopedia of Food Security and Sustainability; Ferranti, P., Berry, E.M., Anderson, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 364–368. [Google Scholar] [CrossRef]
- FAO; UNEP. Food and Food Waste. 2010. Available online: https://www.unep.org/explore-topics/resource-efficiency/what-we-do/sustainable-lifestyles/food-and-food-waste (accessed on 7 April 2023).
- Aires, A.; Carvalho, R.; Saavedra, M.J. Valorization of solid wastes from chestnut industry processing: Extraction and optimization of polyphenols, tannins and ellagitannins and its potential for adhesives, cosmetic and pharmaceutical industry. Waste Manag. 2016, 48, 457–464. [Google Scholar] [CrossRef]
- Squillaci, G.; Apone, F.; Sena, L.M.; Carola, A.; Tito, A.; Bimonte, M.; De Lucia, A.; Colucci, G.; La Cara, F.; Morana, A. Chestnut (Castanea sativa Mill.) industrial wastes as a valued bioresource for the production of active ingredients. Process. Biochem. 2018, 64, 228–236. [Google Scholar] [CrossRef]
- Chiocchio, I.; Prata, C.; Mandrone, M.; Ricciardiello, F.; Marrazzo, P.; Tomasi, P.; Angeloni, C.; Fiorentini, D.; Malaguti, M.; Poli, F.; et al. Leaves and spiny burs of Castanea sativa from an experimental chestnut grove: Metabolomic analysis and anti-neuroinflammatory activity. Metabolites 2020, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Piazza, S.; Martinelli, G.; Fumagalli, M.; Pozzoli, C.; Maranta, N.; Giavarini, F.; Colombo, L.; Nicotra, G.; Vicentini, S.F.; Genova, F.; et al. Ellagitannins from Castanea sativa Mill. Leaf Extracts Impair H. pylori Viability and Infection-Induced Inflammation in Human Gastric Epithelial Cells. Nutrients 2023, 15, 1504. [Google Scholar] [CrossRef]
- Pinto, D.; Cádiz-Gurrea, M.d.l.L.; Garcia, J.; Saavedra, M.J.; Freitas, V.; Costa, P.; Sarmento, B.; Delerue-Matos, C.; Rodrigues, F. From soil to cosmetic industry: Validation of a new cosmetic ingredient extracted from chestnut shells. Sustain. Mater. Technol. 2021, 29, e00309. [Google Scholar] [CrossRef]
- Pinto, D.; Almeida, A.; López-Yerena, A.; Pinto, S.; Sarmento, B.; Lamuela-Raventós, R.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Appraisal of a new potential antioxidants-rich nutraceutical ingredient from chestnut shells through in-vivo assays—A targeted metabolomic approach in phenolic compounds. Food Chem. 2023, 404, 134546. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Piazza, S.; Vrhovsek, U.; Fumagalli, M.; Khalilpour, S.; Masuero, D.; Di Lorenzo, C.; Colombo, L.; Mattivi, F.; De Fabiani, E.; et al. A bio-guided approach for the development of a chestnut-based proanthocyanidin-enriched nutraceutical with potential anti-gastritis properties. Pharmacol. Res. 2018, 134, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Sorice, A.; Siano, F.; Capone, F.; Guerriero, E.; Picariello, G.; Budillon, A.; Ciliberto, G.; Paolucci, M.; Costantini, S.; Volpe, M.G. Potential anticancer effects of polyphenols from chestnut shell extracts: Modulation of cell growth, and cytokinomic and metabolomic profiles. Molecules 2016, 21, 1411. [Google Scholar] [CrossRef] [Green Version]
- ben Alaya, I.; Pereira, E.; Dias, M.I.; Pinela, J.; Calhelha, R.C.; Soković, M.; Kostić, M.; Prieto, M.A.; Essid, F.; Caleja, C.; et al. Development of a natural preservative from chestnut flowers: Ultrasound-assisted extraction optimization and functionality assessment. Chemosensors 2021, 9, 141. [Google Scholar] [CrossRef]
- Caleja, C.; Barros, L.; Prieto, M.A.; Bento, A.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Development of a natural preservative obtained from male chestnut flowers: Optimization of a heat-assisted extraction technique. Food Funct. 2019, 10, 1352–1363. [Google Scholar] [CrossRef]
- Carocho, M.; Barreira, J.C.M.; Bento, A.; Fernández-Ruiz, V.; Morales, P.; Ferreira, I.C.F.R. Chestnut and lemon balm-based ingredients as natural preserving agents of the nutritional profile in matured “serra da Estrela” cheese. Food Chem. 2016, 204, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Caleja, C.; Barros, L.; Barreira, J.C.M.; Soković, M.; Calhelha, R.C.; Bento, A.; Oliveira, M.B.P.P.; Ferreira, I.C.F.R. Castanea sativa male flower extracts as an alternative additive in the Portuguese pastry delicacy “pastel de nata”. Food Funct. 2020, 11, 2208–2217. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, I.C.F.R.; Paiva, F.M.P.; Bento, A.A. Processo de Produção de Vinho, Utilizando Flores de Castanea sativa Mill como Conservantes Naturais em Alternativa à Adição de Sulfitos. WIPO WO2017212351A12020, 14 December 2017. Available online: https://patents.google.com/patent/WO2017212351A1/pt (accessed on 17 January 2023).
- Carocho, M.; Barreira, J.C.M.; Barros, L.; Bento, A.; Cámara, M.; Morales, P.; Ferreira, I.C. Traditional pastry with chestnut flowers as natural ingredients: An approach of the effects on nutritional value and chemical composition. J. Food Compos. Anal. 2015, 44, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Cacciola, N.A.; Squillaci, G.; D’apolito, M.; Petillo, O.; Veraldi, F.; La Cara, F.; Peluso, G.; Margarucci, S.; Morana, A. Castanea sativa Mill. Shells aqueous extract exhibits anticancer properties inducing cytotoxic and pro-apoptotic effects. Molecules 2019, 24, 3401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carocho, M.; Barros, L.; Bento, A.; Santos-Buelga, C.; Morales, P.; Ferreira, I.C.F.R. Castanea sativa mill. Flowers amongst the most powerful antioxidant matrices: A phytochemical approach in decoctions and infusions. BioMed Res. Int. 2014, 2014, 232956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerulli, A.; Masullo, M.; Mari, A.; Balato, A.; Filosa, R.; Lembo, S.; Napolitano, A.; Piacente, S. Phenolics from Castanea sativa leaves and their effects on UVB-induced damage. Nat. Prod. Res. 2018, 32, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Micucci, M.; Malaguti, M.; Budriesi, R.; Ioan, P.; Lenzi, M.; Fimognari, C.; Toschi, T.G.; Comandini, P.; Hrelia, S. Sweet chestnut (Castanea sativa Mill.) bark extract: Cardiovascular activity and myocyte protection against oxidative damage. Oxidative Med. Cell. Longev. 2013, 2013, 471790. [Google Scholar] [CrossRef] [Green Version]
- Genovese, C.; Addamo, A.; Malfa, G.A.; Acquaviva, R.; Di Giacomo, C.; Tomasello, B.; La Mantia, A.; Ragusa, M.; Toscano, M.A.; Lupo, G.; et al. Antioxidant, antimicrobial and anticancer activities of Castanea sativa (Fagaceae) extract: New therapeutic perspectives. Plant Biosyst. 2021, 155, 1032–1040. [Google Scholar] [CrossRef]
- Marrazzo, P.; Mandrone, M.; Chiocchio, I.; Zambonin, L.; Barbalace, M.C.; Zalambani, C.; Angeloni, C.; Malaguti, M.; Prata, C.; Poli, F.; et al. By-Product Extracts from Castanea sativa Counteract Hallmarks of Neuroinflammation in a Microglial Model. Antioxidants 2023, 12, 808. [Google Scholar] [CrossRef]
- Echegaray, N.; Gómez, B.; Barba, F.J.; Franco, D.; Estévez, M.; Carballo, J.; Marszałek, K.; Lorenzo, J.M. Chestnuts and by-products as source of natural antioxidants in meat and meat products: A review. Trends Food Sci. Technol. 2018, 82, 110–121. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Barros, L.; Alves, M.J.; Oliveira, M.B.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant and antimicrobial properties of dried Portuguese apple variety (Malus domestica Borkh. cv Bravo de Esmolfe). Food Chem. 2018, 240, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Zamuz, S.; López-Pedrouso, M.; Barba, F.J.; Lorenzo, J.M.; Domínguez, H.; Franco, D. Application of hull, bur and leaf chestnut extracts on the shelf-life of beef patties stored under MAP: Evaluation of their impact on physicochemical properties, lipid oxidation, antioxidant, and antimicrobial potential. Food Res. Int. 2018, 112, 263–273. [Google Scholar] [CrossRef]
- Herrero, M.; Ibañez, E. Green extraction processes, biorefineries and sustainability: Recovery of high added-value products from natural sources. J. Supercrit. Fluids 2018, 134, 252–259. [Google Scholar] [CrossRef] [Green Version]
- Barro Losada, F.; Bartomeus, I.; Carbonell, A.; Carrera, M.; Castañeda del Álamo, C.; Gómez Aparicio, L.; Gómez Guillén, M.C.; Herrero, M.; Molina Alcaide, E.; Navarro, G.; et al. Sustainable primary production. In CSIC Scientific. Challenges towards 2030; Consejo Superior de Investigaciones Científicas (España): Madrid, Spain, 2020; Volume 6, ISBN 978-984-00-10748-2. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant activities of the extracts from chestnut flower, leaf, skins and fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant potential of chestnut (Castanea sativa L.) and almond (Prunus dulcis L.) by-products. Food Sci. Technol. Int. 2010, 16, 209–216. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Falco, V.; Dias, M.I.; Barros, L.; Silva, A.; Capita, R.; Alonso-Calleja, C.; Amaral, J.S.; Igrejas, G.; Ferreira, I.C.F.R.; et al. Evaluation of the phenolic profile of Castanea sativa Mill. By-products and their antioxidant and antimicrobial activity against multiresistant bacteria. Antioxidants 2020, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vella, F.M.; Laratta, B.; La Cara, F.; Morana, A. Recovery of bioactive molecules from chestnut (Castanea sativa Mill.) by-products through extraction by different solvents. Nat. Prod. Res. 2018, 32, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Phenolic composition and bioactivity of Lavandula pedunculata (Mill.) Cav. samples from different geographical origin. Molecules 2018, 23, 1037. [Google Scholar] [CrossRef] [Green Version]
- Pinela, J.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant activity, ascorbic acid, phenolic compounds and sugars of wild and commercial Tuberaria lignosa samples: Effects of drying and oral preparation methods. Food Chem. 2012, 135, 1028–1035. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Heleno, S.A.; Ferreira, I.C.F.R.; Esteves, A.P.; Ćirić, A.; Glamočlija, J.; Martins, A.; Soković, M.; Queiroz, M.J.R.P. Antimicrobial and demelanizing activity of Ganoderma lucidum extract, p-hydroxybenzoic and cinnamic acids and their synthetic acetylated glucuronide methyl esters. Food Chem. Toxicol. 2013, 58, 95–100. [Google Scholar] [CrossRef]
- Bowers, J.J.; Gunawardena, H.P.; Cornu, A.; Narvekar, A.S.; Richieu, A.; Deffieux, D.; Quideau, S.; Tharayil, N. Rapid screening of ellagitannins in natural sources via Targeted Reporter Ion Triggered Tandem Mass Spectrometry. Sci. Rep. 2018, 8, 10399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clifford, M.N.; Scalbert, A. Ellagitannins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Esposito, T.; Celano, R.; Pane, C.; Piccinelli, A.L.; Sansone, F.; Picerno, P.; Zaccardelli, M.; Aquino, R.P.; Mencherini, T. Chestnut (Castanea sativa Miller.) burs extracts and functional compounds: UHPLC-UV-HRMS profiling, antioxidant activity, and inhibitory effects on phytopathogenic fungi. Molecules 2019, 24, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Formato, M.; Vastolo, A.; Piccolella, S.; Calabrò, S.; Cutrignelli, M.I.; Zidorn, C.; Pacifico, S. Castanea sativa Mill. Leaf: UHPLC-HR MS/MS Analysis and Effects on In Vitro Rumen Fermentation and Methanogenesis. Molecules 2022, 27, 8662. [Google Scholar] [CrossRef]
- Ozawa, T.; Kobayashi, D.; Takino, Y. Structures of the New Phenolic Glycosides MP-2 and MP-10 from Chestnut Galls. Agric. Biol. Chem. 1977, 41, 1257–1262. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, T.; Arai, N.; Takino, Y. Structure of a new phenolic glycoside, chesnatin, from chestnut galls. Agric. Biol. Chem. 1978, 42, 1907–1910. [Google Scholar] [CrossRef]
- Ferrara, E.; Pecoraro, M.T.; Cice, D.; Piccolella, S.; Formato, M.; Esposito, A.; Petriccione, M.; Pacifico, S. A Joint Approach of Morphological and UHPLC-HRMS Analyses to Throw Light on the Autochthonous ‘Verdole’ Chestnut for Nutraceutical Innovation of Its Waste. Molecules 2022, 27, 8924. [Google Scholar] [CrossRef]
- Cerulli, A.; Napolitano, A.; Hošek, J.; Masullo, M.; Pizza, C.; Piacente, S. Antioxidant and in vitro preliminary anti-inflammatory activity of Castanea sativa (Italian cultivar “Marrone di Roccadaspide” PGI) burs, leaves and chestnuts extracts and their metabolite profiles by LC-ESI/LTQOrbitrap/MS/MS. Antioxidants 2021, 10, 278. [Google Scholar] [CrossRef]
- Feng, H.; Nonaka, G.; Nishroka, I. Hydrolysable tannins and related compounds from Castanea mollissima. Phytochemistry 1988, 27, 1185–1189. [Google Scholar] [CrossRef]
- Ozawa, T.; Haga, K.; Kobayashi, D.; Kamiyama, T.; Takino, Y. Isolation and characteristics of new phenolic glycosides of chestnut galls. Agric. Biol. Chem. 1977, 41, 1249–1256. [Google Scholar] [CrossRef] [Green Version]
- Moilanen, J.; Sinkkonen, J.; Salminen, J.-P. Characterization of bioactive plant ellagitannins by chromatographic, spectroscopic and mass spectrometric methods. Chemoecology 2013, 23, 165–179. [Google Scholar] [CrossRef]
- Romani, A.; Campo, M.; Pinelli, P. HPLC/DAD/ESI-MS analyses and anti-radical activity of hydrolyzable tannins from different vegetal species. Food Chem. 2012, 130, 214–221. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumar, B. Profiling of Gallic and Ellagic Acid Derivatives in Different Plant Parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellström, J.; Sinkkonen, J.; Karonen, A.M.; Mattila, P. Isolation and structure elucidation of procyanidin oligomers from saskatoon berries (Amelanchier alnifolia). J. Agric. Food Chem. 2007, 55, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Loponen, J.; Ossipov, V.; Pihlaja, K. Analysis of procyanidins in pine bark with reversed-phase and normal-phase high-performance liquid chromatography–electrospray ionization mass spectrometry. Anal. Chim. Acta 2004, 522, 105–112. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Oliveira, M.M.; Almeida, J.; Costa, R.; Gomes-Laranjo, J.; Peixoto, F. Antioxidant activities of chestnut nut of Castanea sativa Mill. (cultivar ‘Judia’) as function of origin ecosystem. Food Chem. 2012, 132, 1–8. [Google Scholar] [CrossRef]
- Pinto, D.; Silva, A.M.; Freitas, V.; Vallverdú-Queralt, A.; Delerue-Matos, C.; Rodrigues, F. Microwave-Assisted Extraction as a Green Technology Approach to Recover Polyphenols from Castanea sativa Shells. ACS Food Sci. Technol. 2021, 1, 229–241. [Google Scholar] [CrossRef]
- Lameirão, F.; Pinto, D.; Vieira, E.F.; Peixoto, A.F.; Freire, C.; Sut, S.; Dall’acqua, S.; Costa, P.; Delerue-Matos, C.; Rodrigues, F. Green-sustainable recovery of phenolic and antioxidant compounds from industrial chestnut shells using ultrasound-assisted extraction: Optimization and evaluation of biological activities in vitro. Antioxidants 2020, 9, 267. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [Green Version]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative Structure–Activity Relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Agulló, A.; Freire, M.S.; Antorrena, G.; Pereira, J.A.; González-Álvarez, J. Effect of the Extraction Technique and Operational Conditions on the Recovery of Bioactive Compounds from Chestnut (Castanea sativa) Bur and Shell. Sep. Sci. Technol. 2014, 49, 267–277. [Google Scholar] [CrossRef]






| Peaks a | Tentative Identification | Rt (min) b | Λmax (nm) c | [M-H]− (m/z) | MS2 Fragments (m/z) d | Phenolic Compound Class | Chestnut By-Products | ||
|---|---|---|---|---|---|---|---|---|---|
| L | S | B | |||||||
| 1 | Gallic acid | 3.9 | 270 | 169 | 125 (100) | Phenolic acid | |||
| 2 | Bis-HHDP-glucose (pedunculagin I) (isomer 1) | 4.38 | 278 | 783 | 481 (15), 301 (87) | Hydrolysable tannin | |||
| 3 | Galloyl-bis-HHDP-glucose (isomer 1) | 4.76 | 288 | 935 | 633 (100), 301 (18) | Hydrolysable tannin | |||
| 4 | Chesnatin (isomer 1) | 4.91 | 270 | 637 | 467 (100), 305 (23) | Hydrolysable tannin | |||
| 5 | Digalloyl-HHDP-glucose (isomer 1) | 5.09 | 280 | 785 | 633 (100), 483 (24), 301 (38) | Hydrolysable tannin | |||
| 6 | Trigalloyl-HHDP-glucose | 5.11 | 274 | 937 | 937 (100), 637 (20), 301 (12) | Hydrolysable tannin | |||
| 7 | Galloyl-bis-HHDP-glucose (isomer 2) | 5.45 | 275 | 935 | 633 (100), 301 (18) | Hydrolysable tannin | |||
| 8 | Digalloyl-HHDP-glucose (isomer 1) | 5.46 | 280 | 785 | 633 (100), 483 (24), 301 (38) | Hydrolysable tannin | |||
| 9 | Bis-HHDP-glucose (pedunculagin I) (isomer 2) | 5.56 | 278 | 783 | 481 (13), 301 (85) | Hydrolysable tannin | |||
| 10 | Procyanidin tetramer | 5.92 | 281 | 1153 | 865 (22), 713 (4), 577 (33), 575 (16), 561 (20), 289 (100) | Condensed tannin | |||
| 11 | Chesnatin (isomer 2) | 5.95 | 272 | 637 | 467 (100), 305 (23) | Hydrolysable tannin | |||
| 12 | (-)-Epicatechin | 6.21 | 280 | 289 | 245 (100) | Flavonoid | |||
| 13 | Procyanidin trimer | 6.55 | 281 | 865 | 451 (44), 425 (59), 407 (97), 289 (65) | Condensed tannin | |||
| 14 | Galloyl-bis-HHDP-glucose (isomer 3) | 6.92 | 281 | 935 | 633 (100), 301 (18) | Hydrolysable tannin | |||
| 15 | Galloyl-bis-HHDP-glucose (isomer 4) | 8.22 | 281 | 935 | 633 (100), 301 (18) | Hydrolysable tannin | |||
| 16 | Cretanin | 8.38 | 274 | 469 | 169 (100) | Hydrolysable tannin | |||
| 17 | Chestanin (isomer 1) | 10.47 | 274 | 937 | 637 (6), 467 (100), 305 (7), 169 (17) | Hydrolysable tannin | |||
| 18 | Isorhamnetin-O-hexoside | 12.34 | 353 | 477 | 315 (100) | Glycosylated flavonoid | |||
| 19 | Ellagic acid | 12.53 | 366 | 301 | 135 (100) | Phenolic acid | |||
| 20 | Quercetin-3-O-rutinoside | 12.67 | 347 | 609 | 301 (100) | Glycosylated flavonoid | |||
| 21 | Quercetin-deoxyhexosyl-hexoside | 13.08 | 346 | 609 | 301 (100) | Glycosylated flavonoid | |||
| 22 | Galloyl-bis-HHDP-glucose (isomer 5) | 13.27 | 281 | 935 | 633 (100), 301 (18) | Hydrolysable tannin | |||
| 23 | Quercetin-3-O-glucuronide | 13.77 | 352 | 477 | 301 (100) | Glycosylated flavonoid | |||
| 24 | 3-O-galloylpunicalin | 14.21 | 288 | 933 | 753 (45), 631 (26), 597 (18), 451 (39), 425 (11), 301 (100) | Hydrolysable tannin | |||
| 25 | Quercetin-O-hexoside | 14.71 | 354 | 463 | 301 (100) | Glycosylated flavonoid | |||
| 26 | Chestanin (isomer 2) | 15.12 | 274 | 937 | 637 (6), 467 (100), 305 (7), 169 (17) | Hydrolysable tannin | |||
| 27 | Quercetin dirhamnoside | 15.95 | 334 | 593 | 301 (100) | Glycosylated flavonoid | |||
| 28 | Quercetin-O-pentoside | 16.14 | 345 | 433 | 301 (100) | Glycosylated flavonoid | |||
| 29 | Isorhamnetin-3-O-rutinoside | 16.62 | 355 | 623 | 315 (100) | Glycosylated flavonoid | |||
| 30 | Kaempferol-3-O-glucoside | 17.23 | 348 | 447 | 285 (100) | Glycosylated flavonoid | |||
| 31 | Quercetin-O-deoxyhexoside | 17.87 | 349 | 447 | 301 (100) | Glycosylated flavonoid | |||
| 32 | Procyanidin trimer | 18.02 | 280 | 867 | 715 (46), 577 (100), 409 (58), 287 (63) | Condensed tannin | |||
| 33 | Isorhamnetin-O-hexoside (isomer 1) | 18.17 | 353 | 477 | 315 (100) | Glycosylated flavonoid | |||
| 34 | Methyl ellagic acid hexoside | 18.46 | 362 | 477 | 301 (100) | Glycosylate Phenolic acid | |||
| 35 | Ellagic acid trimethyl-glucoside (isomer 1) | 18.6 | 355 | 551 | 343 (100) | Glycosylate Phenolic acid | |||
| 36 | Isorhamnetin-O-hexoside (isomer 2) | 18.75 | 353 | 477 | 315 (100) | Glycosylated flavonoid | |||
| 37 | Methyl ellagic acid deoxyhexoside (isomer 1) | 18.88 | 368 | 461 | 315 (100), 301 (32) | Glycosylate Phenolic acid | |||
| 38 | Methyl ellagic acid deoxyhexoside (isomer 2) | 19.89 | 367 | 461 | 315 (100), 301 (32) | Glycosylate Phenolic acid | |||
| 39 | Ellagic acid trimethyl-glucoside (isomer 2) | 23.97 | 355 | 551 | 343 (100) | Glycosylate Phenolic acid | |||
| Phenolic Compound 1 | Individual Quantification (mg.g−1) 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaves | Shells | Burs | ||||||||||
| MAC-HE | UAE-HE | UAE-W | MAE-W | MAC-HE | UAE-HE | UAE-W | MAE-W | MAC-HE | UAE-HE | UAE-W | MAE-W | |
| Gallic acid | 7.91 ± 0.010 | 7.24 ± 0.17 | 9.82 ± 0.25 | 10.86 ± 0.092 | 13.75 ± 0.075 | 3.78 ± 0.056 | 14.84 ± 0.02 | 4.89 ± 0.062 | 17.55 ± 0.067 | 7.58 ± 0.012 | 10.31 ± 0.093 | 4.28 ± 0.03 |
| Bis-HHDP-glucose (pedunculagin I) (isomer 1) | 13.4 ± 0.77 | 2.39 ± 0.15 | nd | nd | nd | nd | nd | nd | 2.04 ± 0.02 | 5.67 ± 0.14 | 2.23 ± 0.03 | 0.5 ± 0.02 |
| Galloyl-bis-HHDP-glucose (isomer 1) | 2.4 ± 0.11 | 3.77 ± 0.2 | nd | nd | 0.40 ± 0.01 | 0.23 ± 0.002 | 0.90 ± 0.02 | 0.44 ± 0.01 | nd | nd | nd | nd |
| Chesnatin (isomer 1) | nd | nd | 2.04 ± 0.13 | 2.93 ± 0.12 | nd | nd | nd | nd | nd | nd | nd | nd |
| Digalloyl-HHDP-glucose (isomer 1) | nd | nd | nd | nd | 0.32 ± 0.02 | 0.196 ± 0.001 | 0.49 ± 0.02 | 0.31 ± 0.01 | nd | nd | nd | nd |
| Trigalloyl-HHDP-glucose | nd | nd | nd | nd | nd | nd | nd | nd | 0.18 ± 0.003 | 1.26 ± 0.05 | 1.55 ± 0.05 | 0.47 ± 0.02 |
| Galloyl-bis-HHDP-glucose (isomer 2) | nd | nd | nd | nd | nd | nd | nd | nd | 0.065 ± 0.004 | 1.71 ± 0.11 | nd | 0.53 ± 0.03 |
| Digalloyl-HHDP-glucose (isomer 1) | nd | nd | nd | nd | 0.194 ± 0.001 | 0.119 ± 0.001 | 0.33 ± 0.01 | 0.178 ± 0.001 | nd | nd | nd | nd |
| Bis-HHDP-glucose (pedunculagin I) (isomer 2) | 11.12 ± 0.31 | 2.87 ± 0.12 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Procyanidin tetramer | nd | nd | nd | nd | nd | 0.45 ± 0.01 | 1.01 ± 0.04 | 0.52 ± 0.002 | nd | nd | nd | nd |
| Chesnatin (isomer 2) | 2.01 ± 0.09 | 2.89 ± 0.14 | 1.19 ± 0.09 | 2.02 ± 0.14 | nd | nd | nd | nd | nd | nd | nd | nd |
| (-)-Epicatechin | nd | nd | nd | nd | nd | nd | nd | 0.50 ± 0.004 | nd | nd | nd | nd |
| Procyanidin trimer | nd | nd | nd | nd | 0.59 ± 0.01 | 0.426 ± 0.004 | 1.35 ± 0.05 | 0.62 ± 0.005 | nd | nd | nd | nd |
| Galloyl-bis-HHDP-glucose (isomer 3) | nd | nd | nd | nd | nd | 0.193 ± 0.001 | 0.59 ± 0.01 | 0.21 ± 0.001 | nd | nd | nd | nd |
| Galloyl-bis-HHDP-glucose (isomer 4) | nd | nd | nd | nd | 0.28 ± 0.01 | 0.152 ± 0.001 | 0.71 ± 0.01 | 0.21 ± 0.002 | nd | nd | nd | nd |
| Cretanin | 1.84 ± 0.12 | 1.87 ± 0.13 | 1.01 ± 0.05 | 1.28 ± 0.1 | nd | nd | nd | nd | 0.186 ± 0.002 | 3.66 ± 0.17 | 0.79 ± 0.03 | 0.52 ± 0.02 |
| Chestanin (isomer 1) | 7.47 ± 0.43 | 10.8 ± 0.31 | 0.66 ± 0.02 | 2.82 ± 0.15 | nd | nd | nd | nd | 0.077 ± 0.004 | 7.53 ± 0.31 | 0.26 ± 0 | 0.65 ± 0.03 |
| Isorhamnetin-O-hexoside | 0.38 ± 0.04 | 0.41 ± 0.02 | 0.3 ± 0.05 | 0.27 ± 0.04 | nd | nd | nd | nd | nd | nd | nd | 0.14 ± 0.01 |
| Ellagic acid | nd | nd | nd | nd | 1.81 ± 0.04 | 0.33 ± 0.003 | 1.27 ± 0.02 | 0.74 ± 0.03 | nd | nd | nd | nd |
| Quercetin-3-O-rutinoside | 0.33 ± 0.01 | 0.58 ± 0.09 | 1.54 ± 0.07 | 0.41 ± 0.02 | nd | nd | nd | nd | 0.18 ± 0.004 | 2.67 ± 0.12 | 1.24 ± 0.07 | 0.61 ± 0.02 |
| Quercetin-deoxyhexosyl-hexoside | 4.09 ± 0.17 | 4.62 ± 0.38 | 0.92 ± 0.06 | 1.87 ± 0.15 | nd | nd | nd | nd | nd | nd | nd | nd |
| Galloyl-bis-HHDP-glucose (isomer 5) | nd | nd | nd | nd | 0.47 ± 0.02 | nd | nd | 0.29 ± 0.01 | nd | nd | nd | nd |
| Quercetin-3-O-glucuronide | 3.49 ± 0.02 | 4.23 ± 0.32 | 1.12 ± 0.05 | 2.61 ± 0.13 | nd | nd | nd | nd | 0.36 ± 0.004 | 1.66 ± 0.15 | 0.75 ± 0.02 | 0.57 ± 0.03 |
| 3-O-galloylpunicalin | nd | nd | nd | nd | nd | 0.10 ± 0.002 | 0.29 ± 0.01 | 0.24 ± 0.001 | nd | nd | nd | nd |
| Quercetin-O-hexoside | 2.68 ± 0.1 | 3.03 ± 0.12 | 0.25 ± 0.04 | 1.29 ± 0.15 | nd | nd | nd | nd | 0.063 ± 0.001 | 1.18 ± 0.05 | nd | 0.4 ± 0.01 |
| Chestanin (isomer 2) | 1.21 ± 0.08 | 1.65 ± 0.14 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Quercetin dirhamnoside | 0.62 ± 0.07 | 0.62 ± 0.05 | 0.33 ± 0.01 | 0.93 ± 0.1 | nd | nd | nd | nd | 0.62 ± 0.004 | 0.41 ± 0.05 | nd | 0.16 ± 0.01 |
| Quercetin-O-pentoside | 0.8 ± 0.01 | 0.85 ± 0.04 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Isorhamnetin-3-O-rutinoside | 2.63 ± 0.17 | 3.08 ± 0.2 | 0.93 ± 0.05 | 1.33 ± 0.05 | nd | nd | nd | nd | 0.10 ± 0.005 | 0.83 ± 0.07 | 0.29 ± 0.04 | 0.27 ± 0.01 |
| Kaempferol-3-O-glucoside | 6.98 ± 0.1 | 1.51 ± 0.09 | 0.39 ± 0.04 | 0.89 ± 0.1 | nd | nd | nd | nd | 0.1 ± 0.002 | 0.41 ± 0.01 | nd | 0.15 ± 0.01 |
| Quercetin-O-deoxyhexoside | 0.48 ± 0.05 | 0.49 ± 0.04 | nd | 0.28 ± 0.04 | nd | nd | nd | nd | nd | 0.404 | nd | 0.123 |
| Procyanidin trimer | nd | nd | nd | nd | 0.43 ± 0.005 | 0.29 ± 0.005 | 1.20 ± 0.04 | 0.52 ± 0.003 | nd | nd | nd | nd |
| Isorhamnetin-O-hexoside (isomer 1) | 0.31 ± 0.01 | 0.4 ± 0.02 | nd | 0.25 ± 0.01 | nd | nd | nd | nd | nd | nd | nd | nd |
| Methyl ellagic acid hexoside | nd | nd | nd | nd | nd | nd | nd | nd | 0.11 ± 0.004 | 0.4 ± 0.01 | nd | 0.12 ± 0.01 |
| Ellagic acid trimethyl-glucoside (isomer 1) | 0.34 ± 0.001 | 0.33 ± 0.01 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Isorhamnetin-O-hexoside (isomer 2) | nd | nd | nd | nd | nd | nd | nd | nd | 0.10 ± 0.004 | 0.23 ± 0.04 | nd | nd |
| Methyl ellagic acid deoxyhexoside (isomer 1) | 0.45 ± 0.02 | 0.35 ± 0.02 | nd | 0.56 ± 0.02 | nd | nd | nd | nd | 0.11 ± 0.003 | 0.32 ± 0.01 | nd | 0.17 ± 0 |
| Methyl ellagic acid deoxyhexoside (isomer 2) | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.34 ± 0.01 | nd | nd |
| Ellagic acid trimethyl glucoside (isomer 2) | nd | nd | nd | nd | nd | nd | nd | nd | nd | 1.01 ± 0.02 | nd | nd |
| Total quantification (mg.g−1) 2 | ||||||||||||
| Leaves | Shells | Burs | ||||||||||
| MAC-HE | UAE-HE | UAE-W | MAE-W | MAC-HE | UAE-HE | UAE-W | MAE-W | MAC-HE | UAE-HE | UAE-W | MAE-W | |
| Total phenolic acids (TPA) | 8.71 ± 0.04 | 7.93 ± 0.15 | 9.82 ± 0.25 | 11.42 ± 0.07 | 15.56 ± 0.04 | 4.11 ± 0.06 | 16.11 ± 0.04 | 5.64 ± 0.03 | 17.78 ± 0.06 | 9.25 ± 0.06 | 10.31 ± 0.09 | 4.45 ± 0.04 |
| Total flavonoids (TF) | 22.78 ± 0.77 | 19.81 ± 1.37 | 5.77 ± 0.38 | 10.12 ± 0.77 | nd | nd | nd | 0.50 ± 0.004 | 1.52 ± 0.02 | 7.80 ± 0.26 | 2.28 ± 0.14 | 2.43 ± 0.06 |
| Total hydrolysable tannins (THT) | 39.44 ± 1.91 | 26.23 ± 1.19 | 4.90 ± 0.29 | 9.06 ± 0.51 | 1.66 ± 0.06 | 1.00 ± 0.009 | 3.33 ± 0.08 | 1.89 ± 0.04 | 2.55 ± 0.04 | 19.82 ± 0.77 | 4.84 ± 0.11 | 2.68 ± 0.1 |
| Total condensed tannins (TCT) | nd | nd | nd | nd | 1.02 ± 0.01 | 1.17 ± 0.019 | 3.58 ± 0.14 | 1.67 ± 0.01 | nd | nd | nd | nd |
| Total phenolic compounds (TPC) | 70.92 ± 2.72 a | 53.97 ± 2.41 b | 20.49 ± 0.92 ef | 30.59 ± 1.2 d | 18.24 ± 0.03 f | 6.28 ± 0.09 g | 23.03 ± 0.26 e | 9.69 ± 0.03 g | 21.85 ± 0.01 ef | 36.87 ± 1.09 c | 17.42 ± 0.16 f | 9.56 ± 0.13 g |
| Antioxidant Activity (EC50, mg.mL−1) 1 | Extraction Method | Chestnut By-Products | Positive Control Trolox | ||
|---|---|---|---|---|---|
| Leaves | Shells | Burs | |||
| TBARS | MAC-HE | 0.23 ± 0.05 b,c | 0.56 ± 0.03 d | 0.002 ± 0.0001 a | 0.0058 ± 0.0006 |
| UAE-HE | 2.0 ± 0.2 e | 0.2 ± 0.01 b,c | 0.002 ± 0.0001 a | ||
| UAE-W | 0.36 ± 0.16 c | 0.018 ± 0.0005 a | 0.004 ± 0.0001 a | ||
| MAE-W | 0.08 ± 0.02 a,b | 0.071 ± 0.012 a,b | 0.008 ± 0.0002 a | ||
| DPPH | MAC-HE | 0.22 ± 0.07 b | 0.12 ± 0.02 c | 0.33 ± 0.01 f | 0.043 ± 0.002 |
| UAE-HE | 0.27 ± 0.02 b | 0.16 ± 0.01 a | 0.14 ± 0.02 c,d | ||
| UAE-W | 0.20 ± 0.12 a,b | 0.90 ± 0.08 i | 0.28 ± 0.02 e,f | ||
| MAE-W | 0.18 ± 0.01 a,b | 0.07 ± 0.01 h | 0.32 ± 0.01 f,g | ||
| Reducing Power | MAC-HE | 0.43 ± 0.06 e | 0.07 ± 0.01 d | 0.21 ± 0.02 a | 0.029 ± 0.003 |
| UAE-HE | 0.14 ± 0.03 b | 0.17 ± 0.05 a,b | 0.28 ± 0.02 c | ||
| UAE-W | 0.17 ± 0.04 a,b | 1.13 ± 0.03 f | 1.34 ± 0.05 g | ||
| MAE-W | 0.18 ± 0.03 a,b | 0.20 ± 0.15 a | 0.32 ± 0.01 c | ||
| Hepatotoxicity (GI50, μg.mL−1) 2 | Elipticine | ||||
| PLP2 cells | MAC-HE | >400 a | >400 a | >400 a | 1.4 ± 0.1 |
| UAE-HE | >400 a | >400 a | >400 a | ||
| UAE-W | >400 a | >400 a | >400 a | ||
| MAE-W | 209 ± 19 c | 228 ± 10 b | 227 ± 5 a | ||
| Leaves | Shells | Burs | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAC-HE | UAE-HE | UAE-W | MAE-W | MAC-HE | UAE-HE | UAE-W | MAE-W | MAC-HE | UAE-HE | UAE-W | MAE-W | |
| MIC/MBC | MIC/MBC | MIC/MBC | MIC/MBC | MIC/MBC | MIC/MBC | MIC/MBC | MIC/MBC | MIC/MBC | MIC/MBC | MIC/MBC | MIC/MBC | |
| Gram-negative bacteria | ||||||||||||
| Enterobacter cloacae | 2.5/10 | 5/>10 | 2.5/>10 | 1.25/>10 | 2.5/>10 | 2.5/>10 | 1.25/>10 | 5/>10 | >10/>10 | >10/>10 | 5/>10 | 2.5/5 |
| Escherichia coli | 10/>10 | 10/>10 | 5/>10 | 10/>10 | 10/>10 | 10/>10 | 5/>10 | 10/>10 | >10/>10 | >10/>10 | 5/>10 | 10/>10 |
| Pseudomonas aeruginosa | 10/>10 | 10/>10 | 10/>10 | 10/>10 | >10/>10 | >10/>10 | 5/>10 | 10/>10 | >10/>10 | >10/>10 | >10/>10 | 2.5/5 |
| Salmonella enterocolitica | 5/10 | 5/>10 | 1.25/>10 | 1.25/>10 | 5/>10 | 5/>10 | 2.5/>10 | 5/>10 | 10/>10 | 10/>10 | 5/>10 | 1.25/10 |
| Yersinia enterocolitica | 1.25/10 | 1.25/10 | 5/>10 | 5/>10 | 1.25/>10 | 1.25/>10 | 10/>10 | 5/>10 | >10/>10 | >10/>10 | >10/>10 | >10/>10 |
| Gram-positive bacteria | ||||||||||||
| Bacillus cereus | 5/>10 | 5/>10 | 1.25/>10 | 2.5/>10 | 2.5/>10 | 2.5/>10 | 10/>10 | 5/>10 | 2.5/>10 | 2.5/>10 | 5/>10 | 1.25/>10 |
| Listeria monocytogenes | 2.5/10 | 5/>10 | 2.5/>10 | 1.25/>10 | 1.25/>10 | 1.25/>10 | 2.5/>10 | 5>10 | 10/>10 | 5/>10 | 1.25/>10 | 0.6/>10 |
| Staphylococcus aureus | 1.25/10 | 1.25/>10 | 2.5/>10 | 0.3/>10 | 0.6/>10 | 0.6>10 | 2.5/>10 | 5/>10 | 2.5/>10 | 1.25/>10 | 0.6/10 | 0.3/5 |
| Fungi | MIC/MFC | MIC/MFC | MIC/MFC | MIC/MFC | MIC/MFC | MIC/MFC | MIC/MFC | MIC/MFC | MIC/MFC | MIC/MFC | MIC/MFC | MIC/MFC |
| Aspergillus brasiliensis | >10/>10 | >10/>10 | 10/>10 | >10/>10 | >10/>10 | >10/>10 | >10/>10 | >10/>10 | 10/>10 | 10/>10 | 10/>10 | 10/>10 |
| Aspergillus fumigatus | >10/>10 | >10/>10 | 10/>10 | >10/>10 | >10/>10 | >10/>10 | >10/>10 | >10/>10 | 10/>10 | 10/>10 | 10/>10 | 10/>10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, D.B.; Veríssimo, L.; Finimundy, T.; Rodrigues, J.; Oliveira, I.; Gonçalves, J.; Fernandes, I.P.; Barros, L.; Heleno, S.A.; Calhelha, R.C. Chemical and Bioactive Screening of Green Polyphenol-Rich Extracts from Chestnut By-Products: An Approach to Guide the Sustainable Production of High-Added Value Ingredients. Foods 2023, 12, 2596. https://doi.org/10.3390/foods12132596
Rodrigues DB, Veríssimo L, Finimundy T, Rodrigues J, Oliveira I, Gonçalves J, Fernandes IP, Barros L, Heleno SA, Calhelha RC. Chemical and Bioactive Screening of Green Polyphenol-Rich Extracts from Chestnut By-Products: An Approach to Guide the Sustainable Production of High-Added Value Ingredients. Foods. 2023; 12(13):2596. https://doi.org/10.3390/foods12132596
Chicago/Turabian StyleRodrigues, Daniele Bobrowski, Lavínia Veríssimo, Tiane Finimundy, Joana Rodrigues, Izamara Oliveira, João Gonçalves, Isabel P. Fernandes, Lillian Barros, Sandrina A. Heleno, and Ricardo C. Calhelha. 2023. "Chemical and Bioactive Screening of Green Polyphenol-Rich Extracts from Chestnut By-Products: An Approach to Guide the Sustainable Production of High-Added Value Ingredients" Foods 12, no. 13: 2596. https://doi.org/10.3390/foods12132596
APA StyleRodrigues, D. B., Veríssimo, L., Finimundy, T., Rodrigues, J., Oliveira, I., Gonçalves, J., Fernandes, I. P., Barros, L., Heleno, S. A., & Calhelha, R. C. (2023). Chemical and Bioactive Screening of Green Polyphenol-Rich Extracts from Chestnut By-Products: An Approach to Guide the Sustainable Production of High-Added Value Ingredients. Foods, 12(13), 2596. https://doi.org/10.3390/foods12132596