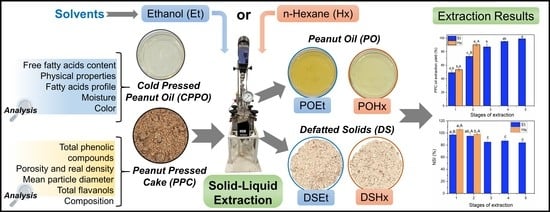
Experimental Comparison between Ethanol and Hexane as Solvents for Oil Extraction from Peanut Press Cake
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Peanut Press Cake Characterization
2.2.2. Cold-Pressed Peanut Oil Characterization
2.2.3. PPC Oil Extraction with Ethanol and Hexane
2.2.4. Characterization of Extract and Defatted Solid Phases
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. PPC Characterization
3.2. Single-Stage Batch Extractions of Oil from PPC Using Ethanol as Solvent
3.3. Sequential Oil Extraction from PPC Using Ethanol and Hexane
3.4. Peanut Oil Characterization
3.5. Peanut Defatted Solid Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as Functional Food: A Review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef] [Green Version]
- United States Department of Agriculture (USDA). Production, Supply and Distribution Online—Graphical Query: Stats by Commodity. Available online: https://apps.fas.usda.gov/psdonline/app/index.html#/app/statsByCommodity (accessed on 26 May 2023).
- Carrín, M.E.; Carelli, A.A. Peanut Oil: Compositional Data. Eur. J. Lipid Sci. Technol. 2010, 112, 697–707. [Google Scholar] [CrossRef]
- Davis, J.P.; Dean, L.O.; Faircloth, W.H.; Sanders, T.H. Physical and Chemical Characterizations of Normal and High-Oleic Oils from Nine Commercial Cultivars of Peanut. J. Am. Oil Chem. Soc. 2008, 85, 235–243. [Google Scholar] [CrossRef]
- Pattee, H.E. Peanut Oil. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; Wiley–Blackwell: Hoboken, NJ, USA, 2005; Volume 2, pp. 431–463. [Google Scholar]
- Akhtar, S.; Khalid, N.; Ahmed, I.; Shahzad, A.; Suleria, H.A.R. Physicochemical Characteristics, Functional Properties, and Nutritional Benefits of Peanut Oil: A Review. Crit. Rev. Food Sci. Nutr. 2014, 54, 1562–1575. [Google Scholar] [CrossRef]
- Li, Y.; Fine, F.; Fabiano-Tixier, A.S.; Abert-Vian, M.; Carre, P.; Pages, X.; Chemat, F. Evaluation of alternative solvents for improvement of oil extraction from rapeseeds. Comptes Rendus Chim. 2014, 17, 242–251. [Google Scholar] [CrossRef]
- Cravotto, C.; Fabiano-Tixier, A.S.; Claux, O.; Abert-Vian, M.; Tabasso, S.; Cravotto, G.; Chemat, F. Towards Substitution of Hexane as Extraction Solvent of Food Products and Ingredients with No Regrets. Foods 2022, 11, 3412–3444. [Google Scholar] [CrossRef]
- de Jesus, S.S.; Filho, R.M. Recent Advances in Lipid Extraction Using Green Solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289–110323. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). U.S. Department of Health and Human Services Food and Drug Administration. Q3C-Tables and List Guidance for Industry 1. 2017. Available online: https://www.fda.gov/downloads/drugs/guidances/ucm073395.pdf (accessed on 26 May 2023).
- U. S. Department of Energy (DOE). Alternative Fuels Data Center Maps and Data—Global Ethanol Production by Country or Region. Available online: https://afdc.energy.gov/data/10331#:~:text=The%20United%20States%20is%20the,while%20Brazil%20primarily%20useu%20sugarcane (accessed on 26 May 2023).
- Potrich, E.; Miyoshi, S.C.; Machado, P.F.S.; Furlan, F.F.; Ribeiro, M.P.A.; Tardioli, P.W.; Giordano, R.L.C.; Cruz, A.J.G.; Giordano, R.C. Replacing Hexane by Ethanol for Soybean Oil Extraction: Modeling, Simulation, and Techno-Economic-Environmental Analysis. J. Clean. Prod. 2020, 244, 118660–118671. [Google Scholar] [CrossRef]
- Javed, F.; Ahmad, S.W.; Rehman, A.; Zafar, S.; Malik, S.R. Recovery of Rice Bran Oil Using Solid-Liquid Extraction Technique. J. Food Process. Eng. 2015, 38, 357–362. [Google Scholar] [CrossRef]
- Sampaio Neto, O.Z.; Batista, E.A.C.; Meirelles, A.J.A. The Employment of Ethanol as Solvent to Extract Brazil Nut Oil. J. Clean. Prod. 2018, 180, 866–875. [Google Scholar] [CrossRef]
- Sampaio Neto, O.Z.; Gonçalves, D.; Bergara, S.d.F.; Batista, E.A.C.; Meirelles, A.J.A. Oil Extraction from Semi-Defatted Babassu Bagasse with Ethanol: Liquid-Liquid Equilibrium and Solid-Liquid Extraction in a Single Stage. J. Food Eng. 2020, 276, 109845–109852. [Google Scholar] [CrossRef]
- Aracava, K.K.; Capellini, M.C.; Gonçalves, D.; Soares, I.D.; Margoto, C.M.; Rodrigues, C.E.C. Valorization of the Baru (Dipteryx alata Vog.) Processing Chain: Technological Properties of Defatted Nut Flour and Oil Solubility in Ethanol and Isopropanol. Food Chem. 2022, 383, 132587–132597. [Google Scholar] [CrossRef]
- Scharlack, N.K.; Aracava, K.K.; Rodrigues, C.E.C. Effect of the Type and Level of Hydration of Alcoholic Solvents on the Simultaneous Extraction of Oil and Chlorogenic Acids from Sunflower Seed Press Cake. J. Sci. Food Agric. 2017, 97, 4612–4620. [Google Scholar] [CrossRef]
- Navarro, S.L.B.; Rodrigues, C.E.C. Macadamia Oil Extraction with Alcoholic Solvents: Yield and Composition of Macadamia Oil and Production of Protein Concentrates from Defatted Meal. Eur. J. Lipid Sci. Technol. 2018, 120, 1800092–1800100. [Google Scholar] [CrossRef]
- Morejón Caraballo, S.; Rohm, H.; Struck, S. Green Solvents for Deoiling Pumpkin and Sunflower Press Cakes: Impact on Composition and Technofunctional Properties. Int. J. Food Sci. Technol. 2023, 58, 1931–1939. [Google Scholar] [CrossRef]
- Arnold, L.K.; Choudhury, R.B.R. Hexane and Ethanol as Peanut Oil Solvents. J. Am. Oil Chem. Soc. 1962, 39, 296–297. [Google Scholar] [CrossRef]
- Desmarina, D.; Bahri, S.; Zulnazri, Z. Ekstraksi Minyak Kacang Tanah (peanut oil) Dengan Pelarut Etanol dan n-Heksan. Chem. Eng. J. Storage 2021, 1, 29–41. [Google Scholar] [CrossRef]
- Gandh, A.P.; Joshi, K.C.; Krishna, J.; Parihar, V.S.; Srivastav, D.C.; Raghunadh, P.; Kawalkar, J.; Jain, S.K.; Tripathi, R.N. Studies on alternative solvents for the extraction of peanut oil. J. Food Sci. Technol. Mysore 2005, 42, 352–355. [Google Scholar]
- Fonseca, H.; Regitano-d’Arce, M.A.B. Aflatoxin removal of peanut meals with aqueous ethanol. Sci. Agric. 1993, 50, 154–156. [Google Scholar] [CrossRef] [Green Version]
- Tate, P.V.; Chavan, J.K.; Patil, P.B.; Kadam, S.S. Processing of commercial peanut cake into food-grade meal and its utilization in preparation of cookies. Plant Foods Hum. Nutr. 1990, 40, 115–121. [Google Scholar] [CrossRef]
- ASAE. Method of Determining and Expressing Fineness of Feed Materials by Sieving, S319.2. 1995. Available online: https://dokumen.tips/documents/asae-s3193-method-of-determining-and-expressing-fineness-of-feed-materials-by-sieving.html?page=1 (accessed on 28 June 2023).
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 3rd ed.; AOCS: Champaign, IL, USA, 1998. [Google Scholar]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society, 6th ed.; AOCS: Champaign, IL, USA, 2009. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Washington, DC, USA, 2007. [Google Scholar]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC: Rockville, MD, USA, 2005. [Google Scholar]
- Sulyok, M.; Krska, R.; Schuhmacher, R. A Liquid Chromatography/Tandem Mass Spectrometric Multi-Mycotoxin Method for the Quantification of 87 Analytes and Its Application to Semi-Quantitative Screening of Moldy Food Samples. Anal. Bioanal. Chem. 2007, 389, 1505–1523. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.T.; Petta, T.; Vendrametto, M.E.; Oliveira, C.A.F. Performance and Application of a “Dilute-and-Shoot” LC-MS/MS Method for Determination of Mycotoxins in Food Products in São Paulo, Brazil. Int. J. Food Eng. 2018, 4, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Ortega, N.; Romero, M.P.; Macià, A.; Reguant, J.; Anglès, N.; Morelló, J.R.; Motilva, M.J. Comparative Study of UPLC-MS/MS and HPLC-MS/MS to Determine Procyanidins and Alkaloids in Cocoa Samples. J. Food Compos. Anal. 2010, 23, 298–305. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Okiyama, D.C.G.; Soares, I.D.; Cuevas, M.S.; Crevelin, E.J.; Moraes, L.A.B.; Melo, M.P.; Oliveira, A.L.; Rodrigues, C.E.C. Pressurized Liquid Extraction of Flavanols and Alkaloids from Cocoa Bean Shell Using Ethanol as Solvent. Food Res. Int. 2018, 114, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Sawada, M.M.; Venâncio, L.L.; Toda, T.A.; Rodrigues, C.E.C. Effects of Different Alcoholic Extraction Conditions on Soybean Oil Yield, Fatty Acid Composition and Protein Solubility of Defatted Meal. Food Res. Int. 2014, 62, 662–670. [Google Scholar] [CrossRef]
- Antoniosi Filho, N.R.; Mendes, O.L.; Lanças, F.M. Computer Prediction of Triacylglycerol Composition of Vegetable Oils by HRGC. Chromatographia 1995, 40, 557–562. [Google Scholar] [CrossRef]
- IUPAC. Standard Methods for the Analysis of Oils, Fats and Derivatives (Part 1, Sections I and II), 6th ed.; IUPAC: Oxford, U.K, 1979. [Google Scholar]
- McGuire, R.G. Reporting of Objective Color Measurements. Hortscience 1992, 27, 1254–1255. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Long, D.; Peng, J.; Ming, J.; Zhao, G. A Novel In-Situ Enhanced Blasting Extrusion Technique—Extrudate Analysis and Optimization of Processing Conditions with Okara. Innov. Food Sci. Emerg. Technol. 2012, 16, 80–88. [Google Scholar] [CrossRef]
- Oliveira, R.; Oliveira, V.; Aracava, K.K.; Rodrigues, C.E.D.C. Effects of the Extraction Conditions on the Yield and Composition of Rice Bran Oil Extracted with Ethanol—A Response Surface Approach. Food Bioprod. Process. 2012, 90, 22–31. [Google Scholar] [CrossRef]
- Morr, C.V.; German, B.; Kinsella, J.E.; Regenstein, J.M.; Van Buren, J.P.; Kilara, A.; Mangino, M.E. A Collaborative Study to Develop a Standardized Food Protein Solubility Procedure. J. Food Sci. 1985, 50, 1715–1718. [Google Scholar] [CrossRef]
- Toda, T.A.; Santana, A.J.M.; Ferreira, J.A.; de Jesus Agnolon Pallone, E.M.; de Aguiar, C.L.; da Costa Rodrigues, C.E. Evaluation of Techniques for Intensifying the Process of the Alcoholic Extraction of Coffee Ground Oil Using Ultrasound and a Pressurized Solvent. Foods 2022, 11, 584–603. [Google Scholar] [CrossRef] [PubMed]
- Przybylski, R.; Mag, T.; Eskin, N.A.M.; McDonald, B.E. Canola Oil. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 2, pp. 61–121. [Google Scholar]
- Campos-Mondragón, M.G.; De La Barca, A.M.C.; Durán-Prado, A.; Campos-Reyes, L.C.; Oliart-Ros, R.M.; Ortega-García, J.; Medina-Juárez, L.A.; Angulo, O. Nutritional Composition of New Peanut (Arachis hypogaea L.) Cultivars. Grasas Aceites 2009, 60, 161–167. [Google Scholar] [CrossRef]
- Jonnala, R.S.; Dunford, N.T.; Dashiell, K.E. New High-Oleic Peanut Cultivars Grown in the Southwestern United States. J. Am. Oil Chem. Soc. 2005, 82, 125–128. [Google Scholar] [CrossRef]
- Attree, R.; Du, B.; Xu, B. Distribution of Phenolic Compounds in Seed Coat and Cotyledon, and Their Contribution to Antioxidant Capacities of Red and Black Seed Coat Peanuts (Arachis hypogaea L.). Ind. Crops Prod. 2015, 67, 448–456. [Google Scholar] [CrossRef]
- Rao, R.K.; Krishna, M.G.; Zaheer, S.H.; Arnold, L.K. Alcoholic Extraction of Vegetable Oils. I. Solubilities of Cottonseed, Peanut, Sesame and Soybean Oils in Aqueous Ethanol. J. Am. Oil Chem. Soc. 1955, 32, 420–423. [Google Scholar] [CrossRef]
- Bessa, L.C.B.A.; Ferreira, M.C.; Rodrigues, C.E.C.; Batista, E.A.C.; Meirelles, A.J.A. Simulation and Process Design of Continuous Countercurrent Ethanolic Extraction of Rice Bran Oil. J. Food Eng. 2017, 202, 99–113. [Google Scholar] [CrossRef]
- Johnson, L.A.; Lusas, E.W. Comparison of Alternative Solvents for Oils Extraction. J. Am. Oil Chem. Soc. 1983, 60, 229–242. [Google Scholar] [CrossRef]
- Wohlfarth, C. Permittivity (dielectric constant) of liquids. In CRC Handbook of Chemistry and Physics, 95th ed.; Haynes, W.M., Ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Cheryan, M.; Shane, P.; Arana, F. Germ Oil Extraction with Ethanol. E.E.U.U Invention Patent n. US US20120141644A1, 7 June 2012. [Google Scholar]
- Araújo, A.C.M.A.; Oliveira, E.R.; Menezes, E.G.T.; Dias, B.O.; Terra, A.W.C.; Queiroz, F. Solvent Effect on the Extraction of Soluble Solids from Murici and Pequi Seeds. J. Food Process. Eng. 2018, 41, e12813. [Google Scholar] [CrossRef]
- Rittner, H. Extraction of Vegetable Oils with Ethyl Alcohol. Oleagineux 1992, 47, 39–42. [Google Scholar]
- Dong, X.Y.; Zhong, J.; Wei, F.; Lv, X.; Wu, L.; Lei, Y.; Liao, B.S.; Quek, S.Y.; Chen, H. Triacylglycerol Composition Profiling and Comparison of High-Oleic and Normal Peanut Oils. J. Am. Oil Chem. Soc. 2015, 92, 233–242. [Google Scholar] [CrossRef]
- Davis, J.P.; Sweigart, D.S.; Price, K.M.; Dean, L.L.; Sanders, T.H. Refractive Index and Density Measurements of Peanut Oil for Determining Oleic and Linoleic Acid Contents. J. Am. Oil Chem. Soc. 2013, 90, 199–206. [Google Scholar] [CrossRef]
- Zhang, F.; Rhee, K.C.; Koseoglu, S.S. Isopropyl Alcohol Extraction of Cottonseed Collets: Efficiency and Performance. J. Food Lipids 2002, 9, 147–160. [Google Scholar] [CrossRef]
- Saravanan, M.; Bhosle, B.M.; Subramanian, R. Processing Hexane-Oil Miscella Using a Nonporous Polymeric Composite Membrane. J. Food Eng. 2006, 74, 529–535. [Google Scholar] [CrossRef]
- Sahasrabudhe, S.N.; Rodriguez-Martinez, V.; O’Meara, M.; Farkas, B.E. Density, Viscosity, and Surface Tension of Five Vegetable Oils at Elevated Temperatures: Measurement and Modeling. Int. J. Food Prop. 2017, 20, 1965–1981. [Google Scholar] [CrossRef] [Green Version]
- Gandova, V.; Ivanova, P.; Kalaydzhiev, H.; Perifanova-Nemska, M.; Chalova, V.I. Dissolution and Surface Tension Properties of Ethanol-Wash Solute Obtained from Industrial Sunflower Meal. Biointerface Res. Appl. Chem. 2021, 11, 11284–11292. [Google Scholar] [CrossRef]
- Bodoira, R.; Cecilia Cittadini, M.; Velez, A.; Rossi, Y.; Montenegro, M.; Martínez, M.; Maestri, D. An Overview on Extraction, Composition, Bioactivity and Food Applications of Peanut Phenolics. Food Chem. 2022, 381, 132250–132261. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of Extraction of Phenolic Compounds from Wheat Using Response Surface Methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]



| Temperature (°C) | Y (%) | RO (Mass%) | Protein (Mass%) | NSI (%) |
|---|---|---|---|---|
| 60 | 28 ± 1 C | 28 ± 1 A | 31 ± 1 C | 91 ± 2 B |
| 75 | 53 ± 3 B | 20 ± 1 B | 37 ± 1 B | 97 ± 1 A |
| 90 | 69 ± 2 A | 14 ± 1 C | 43 ± 2 A | 83 ± 3 C |
| Fatty Acid | Cx:y * | CPPO | POEt | POHx | Davis et al. [4] | Dong et al. [54] |
|---|---|---|---|---|---|---|
| Palmitic (P) | (C16:0) | 5.5 ± 0.1 C | 6.23 ± 0.01 A | 5.9 ± 0.1 B | 4.64–5.65 | 6.19–6.73 |
| Stearic (S) | (C18:0) | 2.1 ± 0.1 B | 2.68 ± 0.03 A | 2.7 ± 0.1 A | 1.85–2.31 | 3.38–6.08 |
| Oleic (O) | (C18:1) | 79.5 ± 0.1 A | 80.1 ± 0.5 A | 79 ± 1 A | 79.84–80.12 | 76.31–80.08 |
| Linoleic (Li) | (C18:2) | 6.1 ± 0.1 A | 4.9 ± 0.4 A | 5 ± 1 A | 2.57–3.74 | 1.47–3.56 |
| Linolenic (Le) | (C18:3) | nd | nd | 0.1 ± 0.2 | nd | 0.42–0.69 |
| Arachidic (A) | (C20:0) | 1.01 ± 0.02 B | 1.08 ± 0.04 AB | 1.17 ± 0.01 A | 1.12–1.37 | 1.51–2.23 |
| Gadoleic (Ga) | (C20:1) | 1.81 ± 0.01 A | 1.69 ± 0.02 B | 1.72 ± 0.01 B | 1.93–2.56 | 0.99–1.44 |
| Behenic (Be) | (C22:0) | 2.26 ± 0.01 B | 2.08 ± 0.03 C | 2.37 ± 0.01 A | 2.99–3.34 | 2.55–3.37 |
| Erucic (E) | (C22:1) | 0.1 ± 0.1 | nd | nd | nd | nd |
| Lignoceric (Lg) | (C24:0) | 1.53 ± 0.01 A | 1.24 ± 0.05 B | 1.52 ± 0.02 A | 1.93–2.30 | 1.46–1.65 |
| Average molar mass (g·mol −1) | 283.7 ± 0.1 | 283.2 ± 0.1 | 283.63 ± 0.03 | - | - | |
| Iodine value (IV) (g iodine/100 g oil) | 82.1 | 80.2 | 80.8 | - | - | |
| Saturated fatty acids (mass%) | 12.4 | 13.3 | 13.6 | - | 15.62–19.62 | |
| Unsaturated fatty acids (mass%) | 87.6 | 86.7 | 86.4 | - | 80.38–84.38 | |
| Unsaturated/Saturated mass ratio | 7.0 | 6.5 | 6.4 | - | 4.1–5.4 | |
| Group a | Triacyl Glycerol b | CPPO | POEt | POHx | Dong et al. [54] | |
|---|---|---|---|---|---|---|
| TAG composition | 50:1 | POP | 0.72 C | 0.94 A | 0.84 B | 0.95–1.38 |
| 52:1 | POS | 0.53 C | 0.77A | 0.74 B | 0.37–0.76 | |
| 52:2 | OOP | 12.18 C | 14.01 A | 13.07 B | 9.26–10.30 | |
| 52:3 | POLi | 1.87 A | 1.72 C | 1.79 B | 0.50–1.04 | |
| 54:2 | OOS | 4.86 C | 6.17 A | 6.11 B | 5.97–10.52 | |
| 54:3 | OOO | 51.63 B | 52.45 A | 50.99 C | 59.60–67.81 | |
| 54:4 | OLiO | 11.80 A | 9.56 C | 10.37 B | 0.40–1.00 | |
| 54:5 | LiLiO | 0.91 A | 0.59 C | 0.90 B | 0.03–0.29 | |
| 56:1 | POBe | 0.58 C | 0.64 B | 0.70 A | 0.37–0.69 | |
| 56:2 | OOA | 2.21 C | 2.38 B | 2.53 A | 3.43–4.80 | |
| 56:3 | OOGa | 3.59 A | 3.35 C | 3.40 B | 0.95–1.40 | |
| 56:4 | OLiGa | 0.61 | nd | nd | 0.03–0.07 | |
| 58:1 | POLg | 0.52 C | 0.54 B | 0.62 A | 0.23–0.26 | |
| 58:2 | OOBe | 4.26 B | 3.97 C | 4.46 A | 4.18–7.17 | |
| 58:3 | OLiBe | 0.89 A | 0.54 C | 0.66 B | 0.24–0.39 | |
| 60:2 | OOLg | 2.85 A | 2.35 C | 2.83 B | nd | |
| Free acidity (mass%) | 0.43 ± 0.02 C | 1.47 ± 0.03 A | 0.82 ± 0.04 B | |||
| Refractive index (25 °C) | 1.47 ± 0.01 B | 1.4671 ± 0.0002 A | 1.4672 ± 0.0001 A | |||
| Moisture (mass%) | 0.32 ± 0.02 A | 0.02 ± 0.01 C | 0.17 ± 0.01 B | |||
| Color characteristics | L* | 91.54 ± 0.04 A | 84 ± 3 B | 89.4 ± 0.1 A | ||
| a* | −1.00 ± 0.03 A | −3.3 ± 0.2 B | −3.00 ± 0.01 B | |||
| b* | 3.4 ± 0.2 C | 41 ± 1 A | 18.6 ± 0.1 B | |||
| ΔE | nd | 39 ± 1 A | 15.6 ± 0.1 B | |||
| °h | −81.49 ± 0.01 A | −85.5 ± 0.5 B | −80.76 ± 0.03 A | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, P.J.C.; Gonçalves, D.; Aracava, K.K.; Rodrigues, C.E.d.C. Experimental Comparison between Ethanol and Hexane as Solvents for Oil Extraction from Peanut Press Cake. Foods 2023, 12, 2886. https://doi.org/10.3390/foods12152886
Magalhães PJC, Gonçalves D, Aracava KK, Rodrigues CEdC. Experimental Comparison between Ethanol and Hexane as Solvents for Oil Extraction from Peanut Press Cake. Foods. 2023; 12(15):2886. https://doi.org/10.3390/foods12152886
Chicago/Turabian StyleMagalhães, Paloma Jamily Cristina, Daniel Gonçalves, Keila Kazue Aracava, and Christianne Elisabete da Costa Rodrigues. 2023. "Experimental Comparison between Ethanol and Hexane as Solvents for Oil Extraction from Peanut Press Cake" Foods 12, no. 15: 2886. https://doi.org/10.3390/foods12152886
APA StyleMagalhães, P. J. C., Gonçalves, D., Aracava, K. K., & Rodrigues, C. E. d. C. (2023). Experimental Comparison between Ethanol and Hexane as Solvents for Oil Extraction from Peanut Press Cake. Foods, 12(15), 2886. https://doi.org/10.3390/foods12152886