Dry Fractionation and Gluten-Free Sourdough Bread Baking from Quinoa and Sorghum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Chemical Analyses
2.3. Pasting Properties
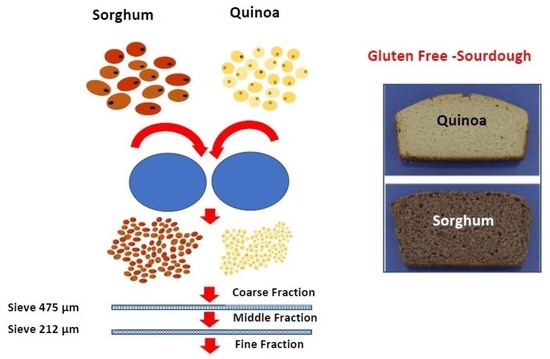
2.4. Dry Fractionation by Roller Milling
2.5. Gluten-Free Bread Baking
2.6. Storage Tests of Gluten-Free Bread
2.7. Evaluation of Physical Bread Properties
2.8. Scanning Electron Micrographs
2.9. Statistical Analysis
3. Results and Discussion
3.1. Milling Performance—Yield of Obtained Fractions
3.2. Chemical Composition of the Milling Fractions
3.3. Pasting Properties of the Milling Fractions (RVA)
3.4. Bread-Baking Quality
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, J.R.N. Sorghum and Millets: Taxonomy, History, Distribution, and Production. In Sorghum and Millets Chemistry, Technology, and Nutritional Attributes, 2nd ed.; Taylor, J.R.N., Duodu, K.G., Eds.; Woodhead Publishing: Duxford, UK, 2019; pp. 10–52. [Google Scholar]
- FAO. Quinoa. Available online: https://www.fao.org/quinoa (accessed on 2 May 2023).
- Akin, P.A.; Demirkesen, I.; Bean, S.R.; Aramouni, F.; Boyaci, I.H. Sorghum Flour Application in Bread: Technological Challenges and Opportunities. Foods 2022, 11, 2466. [Google Scholar] [CrossRef] [PubMed]
- Coţovanu, I.; Ungureanu-Iuga, M.; Mironeasa, S. Investigation of quinoa seeds fractions and their application in wheat bread production. Plants 2021, 10, 2150. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.A.; Sokolova, N. Optimization of bread quality with quinoa flour of different particle size and degree of wheat flour replacement. Food Sci. Technol. 2020, 40, 307–314. [Google Scholar] [CrossRef]
- Serna-Saldivar, S.O.; Espinosa-Ramírez, J. Grain structure and grain chemical composition. In Sorghum and Millets: Chemistry, Technology, and Nutritional Attributes, 2nd ed.; Taylor, J.R.N., Duodu, K.G., Eds.; Woodhead Publishing: Duxford, UK, 2019; pp. 85–129. [Google Scholar]
- Pereira, E.; Encina-Zelada, C.; Barros, L.; Gonzales-Barron, U.; Cadavez, V.; Isabel, C.F.R.; Ferreira, I. Chemical and nutritional characterization of Chenopodium quinoa Willd (quinoa) grains: A good alternative to nutritious food. Food Chem. 2019, 280, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Bender, D.; Schoenlechner, R. Recent developments and knowledge in pseudocereals including technological aspects. Acta Aliment. 2021, 50, 583–609. [Google Scholar] [CrossRef]
- Rumler, R.; Bender, D.; Schoenlechner, R. Sorghum and its potential for the Western diet. J. Cereal Sci. 2022, 1014, 103425. [Google Scholar] [CrossRef]
- Rumler, R.; Bender, D.; Speranza, S.; Frauenlob, J.; Gamper, L.; Hoek, J.; Jager, H.; Schoenlechner, R. Chemical and physical characterization of sorghum milling fractions and sorghum whole meal flours obtained via stone or roller milling. Foods 2021, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- ICC Standard 110/1; Determination of the Moisture Content of Cereals and Cereal Products (Practical Method). ICC: Dubai, United Arab Emirates, 1976.
- ICC Standard 104/1; Determination of Ash in Cereals and Cereal Products. ICC: Dubai, United Arab Emirates, 1990.
- ICC Standard 168; Measurement of Total Starch Content of Cereal Grains, Food and Feed Products Using α-Amylase/Amyloglucosidase Method. ICC: Dubai, United Arab Emirates, 2017.
- ICC Standard 105/2; Determination of Crude Protein in Cereals and Cereal Products for Food and for Feed. ICC: Dubai, United Arab Emirates, 1994.
- ICC Standard 156; Determination of Total Dietary Fibre. ICC: Dubai, United Arab Emirates, 1994.
- ICC Standard 162; Rapid Pasting Method using the Newport Rapid Visco Analyser. ICC: Dubai, United Arab Emirates, 1996.
- Ramos, L.; Alonso-Hernando, A.; Martínez-Castro, M.; Morán-Pérez, J.A.; Cabrero-Lobato, P.; Pascual-Maté, A.; Téllez-Jiménez, E.; Mujico, J.R. Sourdough biotechnology applied to gluten-free baked goods: Rescuing the tradition. Foods 2021, 10, 1498. [Google Scholar] [CrossRef] [PubMed]
- Kulp, K.; Ponte, J.G. Handbook of Cereal Science and Technology, 2nd ed.; CRC Press: New York, NY, USA, 2000; pp. 5–29. ISBN 0-8247-8294-1. [Google Scholar]
- Prego, I.; Maldonado, S.; Otegui, M. Seed structure and localization of reserves in Chenopodium quinoa. Ann. Bot. 1998, 82, 481–488. [Google Scholar] [CrossRef]
- Kebakile, M.M.; Rooney, L.W.; Taylor, J.R.N. Effects of hand pounding, abrasive decortication, roller milling and sorghum type on sorghum meal extraction and quality. Cereal Foods World 2007, 52, 129–137. [Google Scholar]
- Mezgebe, A.G.; Taylor, J.R.N.; de Kock, H.L. Influence of waxy (high amylopectin) and high protein digestibility traits in sorghum on injera sourdough-type flatbread sensory characteristics. Foods 2020, 9, 1749. [Google Scholar] [CrossRef] [PubMed]
- Bourne, M.C. Viscosity Measurement. In Food Texture and Viscosity: Concept and Measurement, 2nd ed.; Bourne, M.C., Ed.; Academic Press: New York, NY, USA, 2002; pp. 249–250. [Google Scholar]
- Balet, S.; Guelpa, A.; Fox, G.; Manley, M. Rapid Visco Analyser (RVA) as a tool for measuring starch-related physiochemical properties in cereals: A review. Food Anal. Methods 2019, 12, 2344–2360. [Google Scholar] [CrossRef]
- De La Hera, E.; Rosell, C.M.; Gomez, M. Effect of water content and flour particle size on gluten-free bread quality and digestibility. Food Chem. 2014, 151, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Armero, E.; Collar, C. Crumb firming kinetics of wheat breads with anti-staling additives. J. Cereal Sci. 1998, 28, 165–174. [Google Scholar] [CrossRef]








| Gap | Coarse Fraction (>475 µm) | Medium Fraction (212–475 µm) | Fine Fraction (<212 µm) |
|---|---|---|---|
| Quinoa | |||
| 0 | 3.1 ± 0.07 c | 1.6 ± 0.01 b | 0.8 ± 0.04 a |
| 5 | 4.6 ± 0.55 c | 2.2 ± 0.15 b | 0.8 ± 0.04 a |
| 8 | 4.5 ± 0.27 c | 2.9 ± 0.00 b | 0.9 ± 0.06 a |
| 10 | 3.6 ± 0.26 c | 3.1 ± 0.03 bc | 1.4 ± 0.0 a |
| Sorghum (Albita) | |||
| 3 | 2.46 ± 0.206 b | 1.07 ± 0.085 a | 1.38 ± 0.048 a |
| 5 | 2.45 ± 0.189 c | 1.23 ± 0.107 a | 1.58 ± 0.065 b |
| 7 | 1.99 ± 0.038 a | 1.27 ± 0.014 b | 1.89 ± 0.12 a |
| Water Addition | Quinoa | Sorghum | ||
|---|---|---|---|---|
| Baking loss [%, n = 4] | 70 | 12.57± 0.81 a | 13.76 ± 0.97 a | |
| 80 | 13.19 ± 0.37 ab | 14.86 ±0.29 ab | ||
| 90 | 14.20 ± 0.39 b | 15.40 ± 0.29 b | ||
| Specific volume [cm³/g, n = 4] | 70 | 1.78 ± 0.03 a | 2.20 ± 0.05 a | |
| 80 | 1.78 ± 0.06 a | 2.23 ± 0.02 a | ||
| 90 | 1.86 ± 0.03 b | 2.46 ± 0.02 b | ||
| Texture | Crumb firmness Fmax [N, n = 6] | 70 | 17.16 ± 0.77 c | 17.04 ± 1.32 b |
| 80 | 13.08 ± 0.22 b | 15.39 ± 1.07 b | ||
| 90 | 10.20 ± 0.03 a | 10.23 ± 0.02 a | ||
| Relative elasticity [%, n = 6] | 70 | 59.88 ± 0.54 a | 51.27 ± 1.53 a | |
| 80 | 62.29 ± 0.38 b | 47.58 ± 2.00 a | ||
| 90 | 64.99 ± 0.48 c | 53.99 ± 0.22 b | ||
| Colour crust | L* [n = 4] | 70 | 47.82 ± 1.52 a | 37.59 ± 1.10 a |
| 80 | 42.84 ± 3.81 ab | 35.85 ± 0.96 a | ||
| 90 | 39.28 ± 1.61 b | 39.47 ± 1.44 b | ||
| a* [n = 4] | 70 | 18.39 ± 0.49 a | 13.53 ± 3.41 ab | |
| 80 | 19.44 ± 0.59 a | 13.11 ± 0.16 ab | ||
| 90 | 19.38 ± 0.77 a | 14.11 ± 1.70 b | ||
| b* [n = 4] | 70 | 35.98 ± 0.32 b | 16.12 ± 0.85 a | |
| 80 | 31.39 ± 2.50 ab | 15.89 ± 0.15 a | ||
| 90 | 27.64 ± 1.02 a | 18.04 ± 1.96 b | ||
| Pore properties | Average pore size [mm², n = 8] | 70 | 3.48 ± 0.03 a | 11.55 ± 0.63 a |
| 80 | 2.76 ± 0.06 a | 9.81 ± 1.06 a | ||
| 90 | 3.37 ± 0.39 a | 10.94 ± 1.17 a | ||
| Pore area [%, n = 8] | 70 | 38.92 ± 2.67 a | 48.05 ± 0.78 b | |
| 80 | 37.50 ± 2.63 a | 48.18 ± 0.94 b | ||
| 90 | 37.57 ± 1.61 a | 46.41 ± 1.15 a | ||
| Number of pores [n = 4] | 70 | 45.17 ± 5.72 a | 15.5 ± 1.32 a | |
| 80 | 51.13 ± 7.68 b | 18.38 ± 1.89 a | ||
| 90 | 47.88 ± 3.97 ab | 17.38 ± 1.65 a | ||
| Pore uniformity [n = 4] | 70 | 3.02 ± 0.58 ab | 42.31 ± 3.87 ab | |
| 80 | 2.32 ± 0.22 a | 18.74 ± 3.52 a | ||
| 90 | 3.37 ± 0.18 b | 27.33 ± 2.34 b | ||
| Colour crumb | L* [n = 4] | 70 | 62.14 ± 1.07 a | 35.96 ± 0.62 b |
| 80 | 64.35 ± 0.68 b | 34.47 ± 1.28 a | ||
| 90 | 64.25 ± 1.62 b | 37.57 ± 0.76 c | ||
| a* [n = 4] | 70 | 6.76 ± 0.14 a | 11.06 ± 0.33 b | |
| 80 | 6.78 ± 0.21 a | 10.17 ± 0.30 a | ||
| 90 | 6.71 ± 0.03 a | 11.99 ± 0.14 c | ||
| b* [n = 4] | 70 | 24.86 ± 0.48 a | 12.29 ± 0.17 a | |
| 80 | 25.23 ± 0.44 a | 12.20 ± 0.12 a | ||
| 90 | 25.18 ± 0.57 b | 13.05 ± 0.21 b | ||
| Paramter | Quinoa | Sorghum |
|---|---|---|
| k | 0.049 | 0.026 |
| n | 1.822 | 2.086 |
| Tinf (N) | 20.24 | 32.07 |
| T0 | 8.54 | 14.06 |
| R² | 0.979 | 0.924 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoenlechner, R.; Bender, D.; D’Amico, S.; Kinner, M.; Tömösközi, S.; Yamsaengsung, R. Dry Fractionation and Gluten-Free Sourdough Bread Baking from Quinoa and Sorghum. Foods 2023, 12, 3125. https://doi.org/10.3390/foods12163125
Schoenlechner R, Bender D, D’Amico S, Kinner M, Tömösközi S, Yamsaengsung R. Dry Fractionation and Gluten-Free Sourdough Bread Baking from Quinoa and Sorghum. Foods. 2023; 12(16):3125. https://doi.org/10.3390/foods12163125
Chicago/Turabian StyleSchoenlechner, Regine, Denisse Bender, Stefano D’Amico, Mathias Kinner, Sandor Tömösközi, and Ram Yamsaengsung. 2023. "Dry Fractionation and Gluten-Free Sourdough Bread Baking from Quinoa and Sorghum" Foods 12, no. 16: 3125. https://doi.org/10.3390/foods12163125
APA StyleSchoenlechner, R., Bender, D., D’Amico, S., Kinner, M., Tömösközi, S., & Yamsaengsung, R. (2023). Dry Fractionation and Gluten-Free Sourdough Bread Baking from Quinoa and Sorghum. Foods, 12(16), 3125. https://doi.org/10.3390/foods12163125