Mild Heat Treatment and Biopreservatives for Artisanal Raw Milk Cheeses: Reducing Microbial Spoilage and Extending Shelf-Life through Thermisation, Plant Extracts and Lactic Acid Bacteria
Abstract
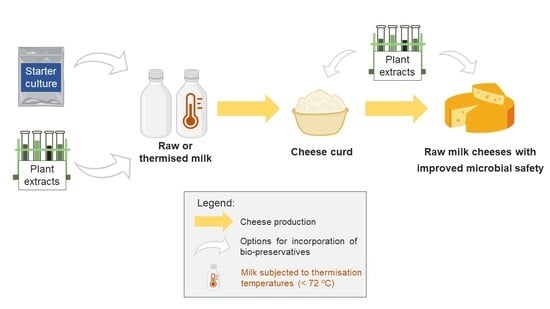
:1. Introduction
2. Spoilage Microorganisms in Raw Milk and Raw Milk Cheeses
3. Biopreservation Strategies
3.1. Plant Extracts
3.2. Lactic Acid Bacteria (LAB)
4. Thermisation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reis, P.J.M.; Malcata, F.X. Current state of Portuguese dairy products from ovine and caprine milks. Small Rumin. Res. 2011, 10, 122–133. [Google Scholar] [CrossRef]
- Arias-Roth, E.; Bachmann, H.-P.; Fröhlich-Wyder, M.-T.; Schmidt, R.S.; Wechsler, D.; Beuvier, E.; Buchin, S.; Delbès, C. Raw Milk Cheeses. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Elsevier Ltd.: London, UK, 2022; pp. 299–308. [Google Scholar] [CrossRef]
- Singh, H.; Waungana, A. Influence of heat treatment of milk on cheesemaking properties. Int. Dairy J. 2001, 11, 543–551. [Google Scholar] [CrossRef]
- Grappin, R.; Beuvier, E. Possible implications of milk pasteurization on the manufacture and sensory quality of ripened cheese. Int. Dairy J. 1997, 7, 751–761. [Google Scholar] [CrossRef]
- Gonzales-Barron, U.; Gonçalves-Tenório, A.; Rodrigues, V.; Cadavez, V. Foodborne pathogens in raw milk and cheese of sheep and goat origin: A meta-analysis approach. Curr. Opin. Food Sci. 2017, 18, 7–13. [Google Scholar] [CrossRef]
- Costanzo, N.; Ceniti, C.; Santoro, A.; Clausi, M.T.; Casalinuovo, F. Foodborne Pathogen Assessment in Raw Milk Cheeses. Int. J. Food Sci. 2020, 2020, 3616713. [Google Scholar] [CrossRef] [PubMed]
- Salazar, J.K.; Gonsalves, L.J.; Natarajan, V.; Shazer, A.; Reineke, K.; Mhetras, T.; Sule, C.; Carstens, C.K.; Schill, K.M.; Tortorello, M.L. Population Dynamics of Listeria monocytogenes, Escherichia coli O157:H7, and Native Microflora during Manufacture and Aging of Gouda Cheese Made with Unpasteurized Milk. J. Food Prot. 2020, 83, 266–276. [Google Scholar] [CrossRef]
- Johler, S.; Giannini, P.; Jermini, M.; Hummerjohann, J.; Baumgartner, A.; Stephan, R. Further Evidence for Staphylococcal Food Poisoning Outbreaks Caused by egc-Encoded Enterotoxins. Toxins 2015, 7, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.; Trmcic, A.; Taylor, M.; Shyng, S.; Hasselback, P.; Man, S.; Tchao, C.; Stone, J.; Janz, L.; Hoang, L.; et al. Escherichia coli O121 outbreak associated with raw milk Gouda-like cheese in British Columbia, Canada, 2018. Can. Commun. Dis. Rep. 2021, 47, 11. [Google Scholar] [CrossRef]
- Anses. AVIS de l’Anses Relatif Aux Modalités de Maîtrise du Risque Lié à la pRESENCE de Dangers Microbiologiques dans les Fromages et Autres Produits Laitiers Fabriqués à Partir de Lait Cru. 2022. Available online: https://www.anses.fr/fr/system/files/BIORISK2019SA0033.pdf (accessed on 28 December 2022).
- Food Safety News. Raw Milk Cheese Recalled Because of Risk of Listeria monocytogenes. Available online: https://www.foodsafetynews.com/2019/07/raw-milk-cheese-recalled-because-of-risk-of-listeria-monocytogenes/ (accessed on 28 December 2022).
- Food Safety News. More Ill in Listeria, E. coli Outbreaks Linked to Raw Milk Cheese. Available online: https://www.foodsafetynews.com/2019/05/more-ill-in-listeria-e-coli-outbreaks-linked-to-raw-milk-cheese/ (accessed on 28 December 2022).
- Abiega-Franyutti, P.; Freyre-Fonseca, V. Chronic consumption of food-additives lead to changes via microbiota gut-brain axis. Toxicology 2021, 464, 153001. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Zhou, D.D.; Shang, A.; Gan, R.Y.; Li, H.B. Influences of food contaminants and additives on gut microbiota as well as protective effects of dietary bioactive compounds. Trends Food Sci. Technol. 2021, 113, 180–192. [Google Scholar] [CrossRef]
- Peterson, C.T. Dysfunction of the Microbiota-Gut-Brain Axis in Neurodegenerative Disease: The Promise of Therapeutic Modulation with Prebiotics, Medicinal Herbs, Probiotics, and Synbiotics. J. Evid.-Based Integr. Med. 2020, 25. [Google Scholar] [CrossRef]
- Bulajic, S. Biopreservation of traditional raw milk cheeses with an emphasis on Serbian artisanal cheeses and their historical production. Meat Technol. 2017, 58, 52–61. [Google Scholar]
- Ali, A.M.M.; Sant’Ana, A.S.; Bavisetty, S.C.B. Sustainable preservation of cheese: Advanced technologies, physicochemical properties and sensory attributes. Trends Food Sci. Technol. 2022, 129, 306–326. [Google Scholar] [CrossRef]
- Dupas, C.; Métoyer, B.; El Hatmi, H.; Adt, I.; Mahgoub, S.A.; Dumas, E. Plants: A natural solution to enhance raw milk cheese preservation? Food Res. Int. 2020, 130, 108883. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, D.; Robertson, R.; Fraser, R.; Engstrom, S.; Jordan, K. Heat induced inactivation of microorganisms in milk and dairy products. Int. Dairy J. 2021, 121, 105096. [Google Scholar] [CrossRef]
- Giaccone, D.; Revello-Chion, A.; Galassi, L.; Bianchi, P.; Battelli, G.; Coppa, M.; Tabacco, E.; Borreani, G. Effect of milk thermisation and farming system on cheese sensory profile and fatty acid composition. Int. Dairy J. 2016, 59, 10–19. [Google Scholar] [CrossRef]
- Dash, K.K.; Fayaz, U.; Dar, A.H.; Shams, R.; Manzoor, S.; Sundarsingh, A.; Deka, P.; Khan, S.A. A comprehensive review on heat treatments and related impact on the quality and microbial safety of milk and milk-based products. Food Chem. Adv. 2022, 1, 100041. [Google Scholar] [CrossRef]
- Bintsis, T. Yeasts in different types of cheese. AIMS Microbiol. 2021, 7, 447. [Google Scholar] [CrossRef]
- Sørhaug, T. Yeasts and Molds: Spoilage Molds in Dairy Products. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Elsevier Ltd.: London, UK, 2011; pp. 780–784. [Google Scholar] [CrossRef]
- Kure, C.F.; Skaar, I. The fungal problem in cheese industry. Curr. Opin. Food Sci. 2019, 29, 14–19. [Google Scholar] [CrossRef]
- Lavoie, K.; Touchette, M.; St-Gelais, D.; Labrie, S. Characterization of the fungal microflora in raw milk and specialty cheeses of the province of Quebec. Dairy Sci. Technol. 2012, 92, 455. [Google Scholar] [CrossRef]
- Yuan, L.; Sadiq, F.A.; Burmølle, M.; Wang, N.; He, G. Insights into psychrotrophic bacteria in raw milk: A review. J. Food Prot. 2019, 82, 1148–1159. [Google Scholar] [CrossRef]
- Podrzaj, L.; Burtscher, J.; Küller, F.; Domig, K.J. Strain-Dependent Cheese Spoilage Potential of Clostridium tyrobutyricum. Microorganisms 2020, 8, 1836. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.K.; Tamime, A.Y.; Wszolek, M. Microbiology of fermented milks. In Dairy Microbiology Handbook, the Microbiology of Milk and Milk Products; Robinson, R.K., Ed.; John Wiley and Sons, Inc.: New York, NY, USA, 2002; pp. 367–430. [Google Scholar]
- Tabla, R.; Gómez, A.; Simancas, A.; Rebollo, J.E.; Molina, F.; Roa, I. Enterobacteriaceae species during manufacturing and ripening of semi–hard and soft raw ewe’s milk cheese: Gas production capacity. Small Rumin. Res. 2016, 145, 123–129. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand. Microbiological Risk Assessment of Raw Milk Cheeses—Risk Assessment Microbiology Section. 2009. Available online: https://www.foodstandards.gov.au/code/proposals/documents/P1007%20PPPS%20for%20raw%20milk%201AR%20SD3%20Cheese%20Risk%20Assessment.pdf (accessed on 2 March 2023).
- Martin, N.H.; Torres-Frenzel, P.; Wiedmann, M. Invited review: Controlling dairy product spoilage to reduce food loss and waste. J. Dairy Sci. 2021, 104, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Hayaloglu, A.A.; Farkye, N.Y. Cheese | Cheese with Added Herbs, Spices and Condiments. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Elsevier Ltd.: London, UK, 2011; pp. 783–789. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- European Union. Directive 2009/32/EC of the European Parliament and of the Council of 23 April 2009 on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. Eur. Union 2009, 5, 3–9. [Google Scholar]
- Azwanida, N.N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 196. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Genisheva, Z.; Botelho, C.; Santos, J.; Ramos, C.; Teixeira, J.A.; Rocha, C.M.R. Unravelling the biological potential of pinus pinaster bark extracts. Antioxidants 2020, 9, 334. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, Isolation and Characterization of Bioactive Compounds From Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2010, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado-Adámez, J. Application of Phenolic Compounds for Food Preservation: Food Additive and Active Packaging. In Phenolic Compounds—Biological Activity; Soto-Hernández, M., Palma-Tenango, M., García-Mateos, R., Eds.; InTechOpen: Vienna, Austria, 2017; pp. 39–58. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019, 8, 1–28. [Google Scholar] [CrossRef]
- Ritota, M.; Manzi, P. Natural Preservatives from Plant in Cheese Making. Animals 2020, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Hayek, S.A.; Gyawali, R.; Ibrahim, S.A. Antimicrobial Natural Products. In Microbial Pathogens and Strategies for Combating Them: Science, Technology And Education; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2013; pp. 910–921. [Google Scholar]
- Gouvea, F.S.; Rosenthal, A.; Ferreira, E.H.R. Plant extract and essential oils added as antimicrobials to cheeses: A review. Cienc. Rural 2017, 47, 1–9. [Google Scholar] [CrossRef]
- Cetin-Karaca, H. Evaluation of Natural Antimicrobial Phenolic Compounds against Foodborne Pathogens. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2011. Available online: https://uknowledge.uky.edu/gradschool_theses/652 (accessed on 2 March 2023).
- Giner, M.J.; Vegara, S.; Funes, L.; Marti, N.; Saura, D.; Micol, V.; Valero, M. Antimicrobial activity of food-compatible plant extracts and chitosan against naturally occurring micro-organisms in tomato juice. J. Sci. Food Agric. 2012, 92, 1917–1923. [Google Scholar] [CrossRef]
- Hołderna-Kędzia, E.; Kędzia, B. Działanie preparatów pochodzenia roślinnego na drobnoustroje probiotyczne. Post. Fitoter. 2012, 2, 72–77. [Google Scholar]
- Ziarno, M.; Kozłowska, M.; Ratusz, K.; Hasalliu, R. Effect of the Addition of Selected Herbal Extracts on the Quality Characteristics of Flavored Cream and Butter. Foods 2023, 12, 471. [Google Scholar] [CrossRef]
- Zaika, L.L.; Kissinger, J.C.; Wasserman, A.E. Inhibition of Lactic Acid Bacteria by Herbs. J. Food Sci. 1983, 48, 1455–1459. [Google Scholar] [CrossRef]
- Behrad, S.; Yusof, M.Y.; Goh, K.L.; Baba, A.S. Manipulation of Probiotics Fermentation of Yogurt by Cinnamon and Licorice: Effects on Yogurt Formation and Inhibition of Helicobacter pylori Growth in vitro. Int. J. Nutr. Food Eng. 2009, 3, 563–567. [Google Scholar] [CrossRef]
- Shori, A.B.; Yong, Y.S.; Baba, A.S. Effects of medicinal plants extract enriched cheese with fish collagen on proteolysis and in vitro angiotensin-I converting enzyme inhibitory activity. LWT—Food Sci. Technol. 2022, 159, 113218. [Google Scholar] [CrossRef]
- Ziarno, M.; Kozłowska, M.; Scibisz, I.; Kowalczyk, M.; Pawelec, S.; Stochmal, A.; Szleszyński, B. The Effect of Selected Herbal Extracts on Lactic Acid Bacteria Activity. Appl. Sci. 2021, 11, 3898. [Google Scholar] [CrossRef]
- Mohamed, F.A.E.F.; Salama, H.H.; El-Sayed, S.M.; El-Sayed, H.S.; Zahran, H.A.H. Utilization of Natural Antimicrobial and Antioxidant of Moringa oleifera Leaves Extract in Manufacture of Cream Cheese. J. Biol. Sci. 2018, 18, 92–106. [Google Scholar] [CrossRef]
- Chouchouli, V.; Kalogeropoulos, N.; Konteles, S.J.; Karvela, E.; Makris, D.P.; Karathanos, V.T. Fortification of yoghurts with grape (Vitis vinifera) seed extracts. LWT—Food Sci. Technol. 2013, 53, 522–529. [Google Scholar] [CrossRef]
- Aktypis, A.; Christodoulou, E.D.; Manolopoulou, E.; Georgala, A.; Daferera, D.; Polysiou, M. Fresh ovine cheese supplemented with saffron (Crocus sativus L.): Impact on microbiological, physicochemical, antioxidant, color and sensory characteristics during storage. Small Rumin. Res. 2018, 167, 32–38. [Google Scholar] [CrossRef]
- Hassanien, M.F.R.; Mahgoub, S.A.; El-Zahar, K.M. Soft cheese supplemented with black cumin oil: Impact on food borne pathogens and quality during storage. Saudi J. Biol. Sci. 2014, 21, 280–288. [Google Scholar] [CrossRef]
- Gavriil, A.; Zilelidou, E.; Papadopoulos, A.-E.; Siderakou, D.; Kasiotis, K.M.; Haroutounian, S.A.; Gardeli, C.; Giannenas, I.; Skandamis, P.N. Evaluation of antimicrobial activities of plant aqueous extracts against Salmonella Typhimurium and their application to improve safety of pork meat. Sci. Rep. 2021, 11, 21971. [Google Scholar] [CrossRef]
- Tassou, C.C.; Nychas, G.J.E. Inhibition of Staphylococcus aureus by olive phenolics in broth and in a model food system. J. Food Prot. 1994, 57, 120–124. [Google Scholar] [CrossRef]
- Boziaris, I.S.; Proestos, C.; Kapsokefalou, M.; Komaitis, M. Antimicrobial Effect of Filipendula ulmaria Plant Extract Against Selected Foodborne Pathogenic and Spoilage Bacteria in Laboratory Media, Fish Flesh and Fish Roe Product. Food Technol. Biotechnol. 2011, 49, 263–270. [Google Scholar]
- Del Campo, J.; Amiot, M.-J.; Nguyen-The, C. Antimicrobial effect of rosemary extracts. J. Food Prot. 2000, 63, 1359–1368. [Google Scholar] [CrossRef]
- Oulahal, N.; Degraeve, P. Phenolic-Rich Plant Extracts With Antimicrobial Activity: An Alternative to Food Preservatives and Biocides? Front. Microbiol. 2022, 1, 753518. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Shelef, L.A.; Jyothi, E.K.; Bulgarellii, M.A. Growth of Enteropathogenic and Spoilage Bacteria in Sage-Containing Broth and Foods. J. Food Sci. 1984, 49, 737–740. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Bains, A.; Krishna, T.C.; Chawla, P.; Dyduch-Siemińska, M.; Klepacka, J.; Kaushik, R. A Comprehensive Review on the Interaction of Milk Protein Concentrates with Plant-Based Polyphenolics. Int. J. Mol. Sci. 2021, 22, 13548. [Google Scholar] [CrossRef] [PubMed]
- Moro, A.; Librán, C.M.; Berruga, M.I.; Carmona, M.; Zalacain, A. Dairy matrix effect on the transference of rosemary (Rosmarinus officinalis) essential oil compounds during cheese making. J. Sci. Food Agric. 2015, 95, 1507–1513. [Google Scholar] [CrossRef]
- Brocklehurst, T.F.; Wilson, P.D.G. The role of lipids in controlling microbial growth. Grasas Aceites 2000, 51, 66–73. [Google Scholar] [CrossRef]
- Verheyen, D.; Xu, X.M.; Govaert, M.; Baka, M.; Skåra, T.; Van Impe, J.F. Food microstructure and fat content affect growth morphology, growth kinetics, and preferred phase for cell growth of Listeria monocytogenes in fish-based model systems. Appl. Environ. Microbiol. 2019, 85, e00707-19. [Google Scholar] [CrossRef]
- Peng, K.; Liu, W.; Xiong, Y.; Lu, L.; Liu, J.; Huang, X. Emulsion microstructural evolution with the action of environmentally friendly demulsifying bacteria. Colloids Surf. 2018, 553, 528–538. [Google Scholar] [CrossRef]
- Lopez, C.; Maillard, M.B.; Briard-Bion, V.; Camier, B.; Hannon, J.A. Lipolysis during ripening of emmental cheese considering organization of fat and preferential localization of bacteria. J. Agric. Food Chem. 2006, 54, 5855–5867. [Google Scholar] [CrossRef]
- Tayel, A.A.; Hussein, H.; Sorour, N.M.; El-Tras, W.F. Foodborne Pathogens Prevention and Sensory Attributes Enhancement in Processed Cheese via Flavoring with Plant Extracts. J. Food Sci. 2015, 80, M2886–M2891. [Google Scholar] [CrossRef]
- Lee, N.K.; Jeewanthi, R.K.C.; Park, E.H.; Paik, H.D. Short communication: Physicochemical and antioxidant properties of Cheddar-type cheese fortified with Inula britannica extract. J. Dairy Sci. 2016, 99, 83–88. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.; Mohamed, S.H.S.; Seleet, F.L. Production and Evaluation of Soft Cheese Fortified with Ginger Extract as a Functional Dairy Food. Polish J. Food Nutr. Sci. 2012, 62, 77–83. [Google Scholar] [CrossRef]
- Mahajan, D.; Bhat, Z.F.; Kumar, S. Pine needles (Cedrus deodara (Roxb.) Loud.) extract as a novel preservative in cheese. Food Pack. Shelf Life 2016, 7, 20–25. [Google Scholar] [CrossRef]
- Evstigneeva, T.; Skvortsova, N.; Yakovleva, R. The application of green tea Extract as a source of antioxidants in the processing of dairy products. Agron.Res. 2016, 14, 1284–1298. [Google Scholar]
- European Union. Regulation No 1334/2008 of the European Parliament and of the Council of 16 December 2008 on flavourings and certain food ingredients with flavouring properties for use in and on foods and amending Council Regulation (EEC) No 1601/91, Regulations (EC) No 2232/96 and (EC) No 110/2008 and Directive 2000/13/EC. Off. J. Eur. Union 2008, 354, 34–49. [Google Scholar]
- Code of Federal Regulations. eCFR: 21 CFR 172.510—Natural Flavoring Substances and Natural Substances Used in Conjunction with Flavors. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-172/subpart-F/section-172.510 (accessed on 2 March 2023).
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci.Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Coelho, M.C.; Malcata, F.X.; Silva, C.C.G. Lactic Acid Bacteria in Raw-Milk Cheeses: From Starter Cultures to Probiotic Functions. Foods 2022, 11, 2276. [Google Scholar] [CrossRef]
- Bettera, L.; Levante, A.; Bancalari, E.; Bottari, B.; Gatti, M. Lactic acid bacteria in cow raw milk for cheese production: Which and how many? Front. Microbiol. 2023, 13, 5413. [Google Scholar] [CrossRef]
- Silva, C.C.G.; Silva, S.P.M.; Ribeiro, S.C. Application of bacteriocins and protective cultures in dairy food preservation. Front. Microbiol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Beresford, T.P.; Fitzsimons, N.A.; Brennan, N.L.; Cogan, T.M. Recent advances in cheese microbiology. Int. Dairy J. 2001, 11, 259–274. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Cheng, P.C.; Pan, T.M. The immunomodulatory effects of lactic acid bacteria for improving immune functions and benefits. Appl. Microbiol. Biotechnol. 2012, 96, 853–862. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Ladero, V.; Fernández, M.; Fiocco, D.; Alvarez, M.A.; Grieco, F.; Spano, G. Biogenic amines degradation by Lactobacillus plantarum: Toward a potential application in wine. Front. Microbiol. 2012, 3, 122. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, S.; Lu, J.; Zhang, C.; Pang, X.; Lv, J. Screening for Cholesterol-Lowering Probiotics from Lactic Acid Bacteria Isolated from Corn Silage Based on Three Hypothesized Pathways. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Damodharan, K.; Palaniyandi, S.A.; Yang, S.H.; Suh, J.W. Functional probiotic characterization and in vivo cholesterol-lowering activity of Lactobacillus helveticus isolated from fermented cow milk. J. Microbiol. Biotechnol. 2016, 26, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Hati, S.; Patel, M.; Mishra, B.K.; Das, S. Short-chain fatty acid and vitamin production potentials of Lactobacillus isolated from fermented foods of Khasi Tribes, Meghalaya, India. Ann. Microbiol. 2019, 69, 1191–1199. [Google Scholar] [CrossRef]
- Renes, E.; Linares, D.M.; González, L.; Fresno, J.M.; Tornadijo, M.E.; Stanton, C. Production of conjugated linoleic acid and gamma-aminobutyric acid by autochthonous lactic acid bacteria and detection of the genes involved. J. Funct. Foods 2017, 34, 340–346. [Google Scholar] [CrossRef]
- Łepecka, A.; Szymański, P.; Rutkowska, S.; Iwanowska, K.; Kołożyn-Krajewska, D. The Influence of Environmental Conditions on the Antagonistic Activity of Lactic Acid Bacteria Isolated from Fermented Meat Products. Foods 2021, 10, 2267. [Google Scholar] [CrossRef]
- Mellefont, L.A.; McMeekin, T.A.; Ross, T. Effect of relative inoculum concentration on Listeria monocytogenes growth in co-culture. Int. J. Food Microbiol. 2008, 121, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Gonzales-Barron, U.; Campagnollo, F.B.; Schaffner, D.W.; Sant’Ana, A.S.; Cadavez, V.A.P. Behavior of Listeria monocytogenes in the presence or not of intentionally-added lactic acid bacteria during ripening of artisanal Minas semi-hard cheese. Food Microbiol. 2020, 91, 103545. [Google Scholar] [CrossRef]
- Campagnollo, F.B.; Margalho, L.P.; Kamimura, B.A.; Feliciano, M.D.; Freire, L.; Lopes, L.S.; Alvarenga, V.O.; Cadavez, V.A.P.; Gonzales-Barron, U.; Schaffner, D.W.; et al. Selection of indigenous lactic acid bacteria presenting anti-listerial activity, and their role in reducing the maturation period and assuring the safety of traditional Brazilian cheeses. Food Microbiol. 2018, 73, 288–297. [Google Scholar] [CrossRef]
- Callon, C.; Picque, D.; Corrieu, G.; Montel, M.C. Ripening conditions: A tool for the control of Listeria monocytogenes in uncooked pressed type cheese. Food Control 2011, 22, 1911–1919. [Google Scholar] [CrossRef]
- Ammor, S.; Tauveron, G.; Dufour, E.; Chevallier, I. Antibacterial activity of lactic acid bacteria against spoilage and pathogenic bacteria isolated from the same meat small-scale facility. 1-Screening and characterization of the antibacterial compounds. Food Control 2006, 17, 454–461. [Google Scholar] [CrossRef]
- Tan, P.L.; Peh, K.K.; Gan, C.Y.; Liong, M.T. Bioactive dairy ingredients for food and non-food applications. Acta Aliment. 2014, 43, 113–123. [Google Scholar] [CrossRef]
- Lindgren, S.E.; Dobrogosz, W.J. Antagonistic activities of lactic acid bacteria in food and feed fermentations. FEMS Microbiol. Rev. 1990, 7, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Elliot, R.M.; McLay, J.C.; Kennedy, M.J.; Simmonds, R.S. Inhibition of foodborne bacteria by the lactoperoxidase system in a beef cube system. Int. J. Food Microbiol. 2004, 91, 73–81. [Google Scholar] [CrossRef] [PubMed]
- García-Quintans, N.; Blancato, V.; Repizo, G.; Magni, C.; López, P. Citrate metabolism and aroma compound production in lactic acid bacteria. In Molecular Aspects of Lactic Acid Bacteria for Traditional and New Applications; Mayo, B., López, P., Pérez-Martínez, G., Eds.; Research Signpost: Kerala, India, 2008. [Google Scholar]
- Domingos-Lopes, M.F.P.; Stanton, C.; Ross, P.R.; Dapkevicius, M.L.E.; Silva, C.C.G. Genetic diversity, safety and technological characterization of lactic acid bacteria isolated from artisanal Pico cheese. Food Microbiol. 2017, 63, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Jay, J.M. Antimicrobial properties of diacetyl. Appl. Environ. Microbiol. 1982, 44, 525. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; García, R.; Valle, D.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef] [PubMed]
- Desbois, A.P.; Smith, V.J. Antibacterial free fatty acids: Activities, mechanisms of action and biotechnological potential. Appl. Microbiol. Biotechnol. 2010, 85, 1629–1642. [Google Scholar] [CrossRef]
- Engels, C.; Schwab, C.; Zhang, J.; Stevens, M.J.A.; Bieri, C.; Ebert, M.-O.; Mcneill, K.; Sturla, S.J.; Lacroix, C. Acrolein contributes strongly to antimicrobial and heterocyclic amine transformation activities of reuterin. Sci. Rep. 2016, 6, 36246. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V.; Mohsin Waheed, S.; Pradhan, D. Efficacy of Reuterin and Bacteriocins Nisin and Pediocin in the Preservation of Raw Milk from Dairy Farms. Food Technol. Biotechnol. 2020, 58, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Dalié, D.K.D.; Deschamps, A.M.; Richard-Forget, F. Lactic acid bacteria—Potential for control of mould growth and mycotoxins: A review. Food Control 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Schaefer, L.; Auchtung, T.A.; Hermans, K.E.; Whitehead, D.; Borhan, B.; Britton, R.A. The antimicrobial compound reuterin (3-hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology 2010, 156, 1589. [Google Scholar] [CrossRef]
- Soltani, S.; Couture, F.; Boutin, Y.; Said, L.B.; Cashman-Kadri, S.; Subirade, M.; Biron, E.; Fliss, I. In vitro investigation of gastrointestinal stability and toxicity of 3-hyrdox-ypropionaldehyde (reuterin) produced by Lactobacillus reuteri. Toxicol. Rep. 2021, 8, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Kaškonienė, V.; Stankevičius, M.; Bimbiraitė-Survilienė, K.; Naujokaitytė, G.; Šernienė, L.; Mulkytė, K.; Malakauskas, M.; Maruška, A. Current state of purification, isolation and analysis of bacteriocins produced by lactic acid bacteria. Appl. Microbiol. Biotechnol. 2017, 101, 1323–1335. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Line, J.E.; Svetoch, E.A.; Eruslanov, B.V.; Perelygin, V.V.; Mitsevich, E.V.; Mitsevich, I.P.; Levchuk, V.P.; Svetoch, O.E.; Seal, B.S.; Siragusa, G.R.; et al. Isolation and purification of enterocin E-760 with broad antimicrobial activity against gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 2008, 52, 1094–1100. [Google Scholar] [CrossRef]
- Hernández-González, J.C.; Martínez-Tapia, A.; Lazcano-Hernández, G.; García-Pérez, B.E.; Castrejón-Jiménez, N.S. Bacteriocins from Lactic Acid Bacteria. A Powerful Alternative as Antimicrobials, Probiotics, and Immunomodulators in Veterinary Medicine. Animals 2021, 11, 979. [Google Scholar] [CrossRef]
- Crowley, S.; Mahony, J.; Van Sinderen, D. Current perspectives on antifungal lactic acid bacteria as natural bio-preservatives. Trends Food Sci. Technol. 2013, 33, 93–109. [Google Scholar] [CrossRef]
- Abouloifa, H.; Hasnaoui, I.; Rokni, Y.; Bellaouchi, R.; Ghabbour, N.; Karboune, S.; Brasca, M.; Abousalham, A.; Jaouadi, B.; Saalaoui, E.; et al. Antifungal activity of lactic acid bacteria and their application in food biopreservation. Adv. Appl. Microbiol. 2022, 120, 33–77. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic Acid Bacteria as Antimicrobial Agents: Food Safety and Microbial Food Spoilage Prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Rukke, E.O.; Sørhaug, T.; Stepaniak, L. Heat Treatment of Milk: Thermization of Milk. In Encyclopedia of Dairy Sciences; Fuquay, J.W., Ed.; Elsevier Ltd.: London, UK, 2011; pp. 693–698. [Google Scholar] [CrossRef]
- Panthi, R.R.; Jordan, K.N.; Kelly, A.L.; Sheehan, J.J.D. Selection and Treatment of Milk for Cheesemaking. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Elsevier Ltd.: London, UK, 2017; pp. 23–50. [Google Scholar] [CrossRef]
- Eugster, E.; Jakob, E. Pre-treatments of Milk and their Effect on the Food Safety of Cheese. Milk Sci. Int. 2019, 72, 45–52. [Google Scholar]
- Codex Committee on Food Hygiene. Code of Hygienic Practice for Milk and Milk Products (CAC/RCP 57-2004). 2009. Available online: https://www.fao.org/fileadmin/user_upload/livestockgov/documents/CXP_057e.pdf (accessed on 1 June 2023).
- Samelis, J.; Lianou, A.; Kakouri, A.; Delbès, C.; Rogelj, I.; Bogovic-Matijasić, B.; Montel, M.-C. Changes in the Microbial Composition of Raw Milk Induced by Thermization Treatments Applied Prior to Traditional Greek Hard Cheese Processing. J. Food Prot. 2009, 72, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, G.; Condón, S.; Mañas, P. Physiology of the Inactivation of Vegetative Bacteria by Thermal Treatments: Mode of Action, Influence of Environmental Factors and Inactivation Kinetics. Foods 2017, 6, 107. [Google Scholar] [CrossRef]
- Russell, A.D. Lethal effects of heat on bacterial physiology and structure. Sci. Prog. 2003, 86, 115–137. [Google Scholar] [CrossRef]
- Tsuchido, T.; Katsui, N.; Takeuchi, A.; Takano, M.; Shibasaki, I. Destruction of the outer membrane permeability barrier of Escherichia coli by heat treatment. Appl. Environ. Microbiol. 1985, 50, 298. [Google Scholar] [CrossRef]
- Baumgarten, T.; Sperling, S.; Seifert, J.; von Bergen, M.; Steiniger, F.; Wick, L.Y.; Heipieper, H.J. Membrane vesicle formation as a multiple-stress response mechanism enhances Pseudomonas putida DOT-T1E cell surface hydrophobicity and biofilm formation. Appl. Environ. Microbiol. 2012, 78, 6217–6224. [Google Scholar] [CrossRef]
- Katsui, N.; Tsuchido, T.; Hiramatsu, R.; Fujikawa, S.; Takano, M.; Shibasaki, I. Heat-induced blebbing and vesiculation of the outer membrane of Escherichia coli. J. Bacteriol. 1982, 151, 1523. [Google Scholar] [CrossRef]
- Mackey, B.M. Changes in antibiotic sensitivity and cell surface hydrophobicity in Escherichia coli injured by heating, freezing, drying or gamma radiation. FEMS Microbiol. Lett. 1983, 20, 395–399. [Google Scholar] [CrossRef]
- Beuchat, L.R. Injury and Repair of Gram-Negative Bacteria, with Special Consideration of the Involvement of the Cytoplasmic Membrane. Adv. Appl. Microbiol. 1978, 23, 219–243. [Google Scholar] [CrossRef]
- Witter, L.D. Thermal Injury and Recovery of Selected Microorganisms. J. Dairy Sci. 1979, 64, 174–177. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Csonka, L.N.; Alam, M.A. Analyzing Thermal Stability of Cell Membrane of Salmonella Using Time-Multiplexed Impedance Sensing. Biophys. J. 2018, 114, 609. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Suo, Y.; Liao, X.; Ahn, J.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Analysis of Staphylococcus aureus cell viability, sublethal injury and death induced by synergistic combination of ultrasound and mild heat. Ultrason. Sonochem. 2017, 39, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Mitsuzawa, S.; Deguchi, S.; Horikoshi, K. Cell structure degradation in Escherichia coli and Thermococcus sp. strain Tc-1-95 associated with thermal death resulting from brief heat treatment. FEMS Microbiol. Lett. 2006, 260, 100–105. [Google Scholar] [CrossRef]
- Karni, M.; Zidon, D.; Polak, P.; Zalevsky, Z.; Shefi, O. Thermal Degradation of DNA. DNA Cell Biol. 2013, 32, 298–301. [Google Scholar] [CrossRef] [PubMed]
- Hanlin, J.H.; Lombardi, S.J.; Slepecky, R.A. Heat and UV light resistance of vegetative cells and spores of Bacillus subtilis Rec-mutants. J. Bacteriol. 1985, 163, 774. [Google Scholar] [CrossRef]
- Zamenhof, S. Gene unstabilization induced by heat and by nitrous acid. J. Bacteriol. 1961, 81, 111. [Google Scholar] [CrossRef]
- Varela-Ramirez, A.; Abendroth, J.; Mejia, A.A.; Phan, I.Q.; Lorimer, D.D.; Edwards, T.E.; Aguilera, R.J. Structure of acid deoxyribonuclease. Nucleic Acids Res. 2017, 45, 6217. [Google Scholar] [CrossRef]
- Earnshaw, R.G.; Appleyard, J.; Hurst, R.M. Understanding physical inactivation processes: Combined preservation opportunities using heat, ultrasound and pressure. Int. J. Food Microbiol. 1995, 28, 197–219. [Google Scholar] [CrossRef]
- Tolker-Nielsen, T.; Molin, S. Role of ribosome degradation in the death of heat-stressed Salmonella typhimurium. FEMS Microbiol. Lett. 1996, 142, 155–160. [Google Scholar] [CrossRef]
- Allwood, M.C.; Russell, A.D. Thermally Induced Ribonucleic Acid Degradation and Leakage of Substances from the Metabolic Pool in Staphylococcus aureus. J. Bacteriol. 1968, 95, 345. [Google Scholar] [CrossRef] [PubMed]
- Allwood, M.C.; Russell, A.D. Mechanism of Thermal Injury in Staphylococcus aureus: I. Relationship Between Viability and Leakage. Appl. Microbiol. 1967, 15, 1266–1269. [Google Scholar]
- Weids, A.J.; Ibstedt, S.; Tamás, M.J.; Grant, C.M. Distinct stress conditions result in aggregation of proteins with similar properties. Sci. Rep. 2016, 6, 24554. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Kemeny, G.; Switzer, R.C.; Hamilton, T.C. Quantitative evidence for protein denaturation as the cause of thermal death. Nature 1971, 232, 471–473. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Corry, J.E.L.; Miles, C.A. Heat Resistance and Mechanism of Heat Inactivation in Thermophilic Campylobacters. Appl. Environ. Microbiol. 2006, 72, 908–913. [Google Scholar] [CrossRef]
- Mackey, B.M.; Derrick, C.M. Elevation of the heat resistance of Salmonella typhimurium by sublethal heat shock. J. Appl. Bacteriol. 1986, 61, 389–393. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, D.J.; Donnelly, C.W. Growth and Survival of Microbial Pathogens in Cheese. In Cheese: Chemistry, Physics and Microbiology, 4th ed.; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Elsevier Ltd.: London, UK, 2017; pp. 573–594. [Google Scholar] [CrossRef]
- Engstrom, S.K.; Mays, M.F.; Glass, K.A. Determination and validation of D-values for Listeria monocytogenes and Shiga toxin–producing Escherichia coli in cheese milk. J. Dairy Sci. 2012, 104, 12332–12341. [Google Scholar] [CrossRef]
- Peng, S.; Hummerjohann, J.; Stephan, R.; Hammer, P. Short communication: Heat resistance of Escherichia coli strains in raw milk at different subpasteurization conditions. J. Dairy Sci. 2013, 96, 3543–3546. [Google Scholar] [CrossRef]
- Van Den Tempel, T.; Jakobsen, M. Yeasts associated with Danablu. Int. Dairy J. 1998, 8, 25–31. [Google Scholar] [CrossRef]


 Enzymatic reaction; ⇄ Equilibrium reactions.
Enzymatic reaction; ⇄ Equilibrium reactions.
 Enzymatic reaction; ⇄ Equilibrium reactions.
Enzymatic reaction; ⇄ Equilibrium reactions.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, B.N.; Teixeira, J.A.; Cadavez, V.; Gonzales-Barron, U. Mild Heat Treatment and Biopreservatives for Artisanal Raw Milk Cheeses: Reducing Microbial Spoilage and Extending Shelf-Life through Thermisation, Plant Extracts and Lactic Acid Bacteria. Foods 2023, 12, 3206. https://doi.org/10.3390/foods12173206
Silva BN, Teixeira JA, Cadavez V, Gonzales-Barron U. Mild Heat Treatment and Biopreservatives for Artisanal Raw Milk Cheeses: Reducing Microbial Spoilage and Extending Shelf-Life through Thermisation, Plant Extracts and Lactic Acid Bacteria. Foods. 2023; 12(17):3206. https://doi.org/10.3390/foods12173206
Chicago/Turabian StyleSilva, Beatriz Nunes, José António Teixeira, Vasco Cadavez, and Ursula Gonzales-Barron. 2023. "Mild Heat Treatment and Biopreservatives for Artisanal Raw Milk Cheeses: Reducing Microbial Spoilage and Extending Shelf-Life through Thermisation, Plant Extracts and Lactic Acid Bacteria" Foods 12, no. 17: 3206. https://doi.org/10.3390/foods12173206
APA StyleSilva, B. N., Teixeira, J. A., Cadavez, V., & Gonzales-Barron, U. (2023). Mild Heat Treatment and Biopreservatives for Artisanal Raw Milk Cheeses: Reducing Microbial Spoilage and Extending Shelf-Life through Thermisation, Plant Extracts and Lactic Acid Bacteria. Foods, 12(17), 3206. https://doi.org/10.3390/foods12173206