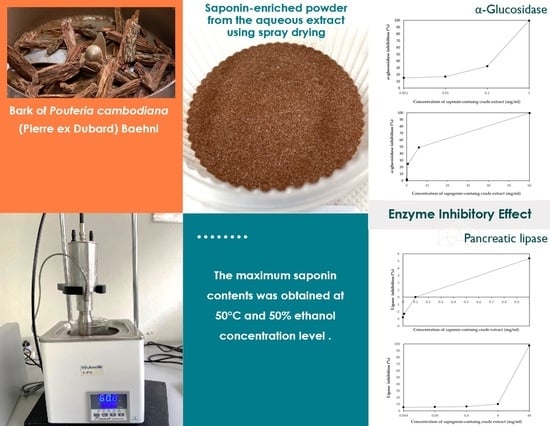
Inhibitory Effects of Saponin-Rich Extracts from Pouteria cambodiana against Digestive Enzymes α-Glucosidase and Pancreatic Lipase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Ultrasound-Assisted Extraction (UAE)
2.3. Determination of Total Phenolic Contents (TPC)
2.4. Determination of Total Flavonoid Content (TFC)
2.5. Determination of Total Saponin Contents (TSC)
2.6. Preparation of the Saponin-Rich Extracts
2.7. Screening Process of Triterpenes and Steroids
2.8. Preparation of the Sapogenin-Rich Extracts through Acid Hydrolysis of Saponins
2.9. α-Glucosidase Inhibition Assay Using Saponin and Sapogenin-Rich Extracts
2.10. Assay for Pancreatic Lipase Enzyme Inhibition Using Saponin and Sapogenin-Rich Extracts
2.11. Storage Stability Test on the Saponin-Rich Extract Powder
2.12. Antioxidant Analysis
2.12.1. DPPH Radical Scavenging Capacity Assay
2.12.2. Ferric-Reducing Antioxidant Power (FRAP) Assay
2.13. Moisture Content and Water Activity
2.14. Colour Determination
2.15. Statistical Analysis
3. Results and Discussion
3.1. Influence of UAE Parameters on the Extraction Efficiency
3.2. Comparative Effect of Non-Hydrolysed and Hydrolysed Extracts on α-Glucosidase Inhibition
3.3. Effects of Non-Hydrolysed and Hydrolysed P. cambodiana Bark Extract on Pancreatic Lipase Activity
3.4. Effect of Storage Temperature on the Stability of Saponin-Rich Extract Powder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manosroi, A.; Saraphanchotiwitthaya, A.; Manosroi, J. In Vitro Immunomodulatory Effect of Pouteria cambodiana (Pierre ex Dubard) Baehni Extract. J. Ethnopharmacol. 2005, 101, 90–94. [Google Scholar] [CrossRef]
- Fitriansyah, S.N.; Fidrianny, I.; Hartati, R. Pharmacological Activities and Phytochemical Compounds: Overview of Pouteria Genus. Pharmacogn. J. 2021, 13, 577–584. [Google Scholar] [CrossRef]
- da Silva Magedans, Y.V.; Phillips, M.A.; Fett-Neto, A.G. Production of Plant Bioactive Triterpenoid Saponins: From Metabolites to Genes and Back. Phytochem. Rev. 2020, 20, 461–482. [Google Scholar] [CrossRef]
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-based, biological-active surfactants from plants. Appl. Charact. Surfactants 2017, 6, 184–205. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Araniti, F.; Statti, G.A. Effects of Saponins on Lipid Metabolism: A Review of Potential Health Benefits in the Treatment of Obesity. Molecules 2016, 21, 1404. [Google Scholar] [CrossRef]
- Dias, S.; Paredes, S.; Ribeiro, L. Drugs Involved in Dyslipidemia and Obesity Treatment: Focus on Adipose Tissue. Int. J. Endocrinol. 2018, 2018, 2637418. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Ramanand, S.; Ramanand, J.; Akat, P.; Patwardhan, M.; Joshi, S. Evaluation of Efficacy and Safety of Orlistat in Obese Patients. Indian J. Endocrinol. Metab. 2011, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C.; Biofisika Bizkaia, F.; Sarriena, B. Molecular Sciences Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Amoo, S.O.; Mudau, T.E.; Olowoyo, J.O. In Vitro α-glucosidase Inhibitory Activity of Medicinal Plants Used Traditionally for Treating Diabetes in Vhembe District, South Africa. J. Herbmed Pharmacol. 2022, 11, 513–521. [Google Scholar] [CrossRef]
- Leksawasdi, N.; Taesuwan, S.; Prommajak, T.; Techapun, C.; Khonchaisri, R.; Sittilop, N.; Halee, A.; Jantanasakulwong, K.; Phongthai, S.; Nunta, R.; et al. Ultrasonic Extraction of Bioactive Compounds from Green Soybean Pods and Application in Green Soybean Milk Antioxidants Fortification. Foods 2022, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Khonchaisri, R.; Sumonsiri, N.; Prommajak, T.; Rachtanapun, P.; Leksawasdi, N.; Techapun, C.; Taesuwan, S.; Halee, A.; Nunta, R.; Khemacheewakul, J. Optimization of Ultrasonic-Assisted Bioactive Compound Extraction from Green Soybean (Glycine max L.) and the Effect of Drying Methods and Storage Conditions on Procyanidin Extract. Foods 2022, 11, 1775. [Google Scholar] [CrossRef]
- Yusnawan, E. Effects of different extraction methods on total phenolic content and antioxidant activity in soybean cultivars. IOP Conf. Ser. Earth Environ. Sci. 2018, 102, 12039. [Google Scholar] [CrossRef]
- Le, A.V.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Improving the Vanillin-Sulphuric Acid Method for Quantifying Total Saponins. Technologies 2018, 6, 84. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Effect of Vacuum-Drying, Hot Air-Drying and Freeze-Drying on Polyphenols and Antioxidant Capacity of Lemon (Citrus limon) Pomace Aqueous Extracts. Int. J. Food Sci. Technol. 2017, 52, 880–887. [Google Scholar] [CrossRef]
- Chan, K.W.; Iqbal, S.; Khong, N.M.H.; Ooi, D.J.; Ismail, M. Antioxidant Activity of Phenolics–Saponins Rich Fraction Prepared from Defatted Kenaf Seed Meal. LWT-Food Sci. Technol. 2014, 56, 181–186. [Google Scholar] [CrossRef]
- Taesotikul, T.; Kitcharoen, N.; Chinpaisal, C.; Phuagphong, P.; Nawanopparatsakul, S. Extraction and Analyses of Phytochemical Compounds from Citrus Hystrix Peels for Molluscicidal Activities. Thai Bull. Pharm. Sci. 2023, 18, 17–28. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Reglero, G.; Martin, D. Chemical Characterization and Bioaccessibility of Bioactive Compounds from Saponin-Rich Extracts and Their Acid-Hydrolysates Obtained from Fenugreek and Quinoa. Foods 2020, 9, 1159. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Zaidul, I.S.M.; Ghafoor, K.; Sahena, F.; Hakim, M.A.; Rafii, M.Y.; Abir, H.M.; Bostanudin, M.F.; Perumal, V.; Khatib, A. In Vitro Antioxidant and, α-Glucosidase Inhibitory Activities and Comprehensive Metabolite Profiling of Methanol Extract and Its Fractions from Clinacanthus nutans. BMC Complement. Altern. Med. 2017, 17, 181. [Google Scholar] [CrossRef] [PubMed]
- Bustanji, Y.; Al-Masri, I.M.; Mohammad, M.; Hudaib, M.; Tawaha, K.; Tarazi, H.; Alkhatib, H.S. Pancreatic Lipase Inhibition Activity of Trilactone Terpenes of Ginkgo biloba. J. Enzym. Inhib. Med. Chem. 2011, 26, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.G.; Zhan, L.B.; Feng, B.A.; Qu, M.Y.; Yu, L.H.; Xie, J.H. Inhibition of Growth and Metastasis of Human Gastric Cancer Implanted in Nude Mice by D-Limonene. World J. Gastroenterol. 2004, 10, 2140–2144. [Google Scholar] [CrossRef]
- Sharma, S.; Singh, A.; Singh, B. Characterization of In Vitro Antioxidant Activity, Bioactive Components, and Nutrient Digestibility in Pigeon Pea (Cajanus cajan) as Influenced by Germination Time and Temperature. J. Food Biochem. 2019, 43, e12706. [Google Scholar] [CrossRef] [PubMed]
- Sancheti, S.V.; Gogate, P.R. A Review of Engineering Aspects of Intensification of Chemical Synthesis Using Ultrasound. Ultrason. Sonochem. 2017, 36, 527–543. [Google Scholar] [CrossRef] [PubMed]
- Vartolomei, A.; Calinescu, I.; Vinatoru, M.; Gavrila, A.I. A Parameter Study of Ultrasound Assisted Enzymatic Esterification. Sci. Rep. 2022, 12, 1421. [Google Scholar] [CrossRef] [PubMed]
- Khoang, L.T.; Huyen, H.T.T.; Chung, H.V.; Duy, L.X.; Toan, T.Q.; Bich, H.T.; Minh, P.T.H.; Pham, D.T.N.; Hien, T.T. Optimization of Total Saponin Extraction from Polyscias fruticosa Roots Using the Ultrasonic-Assisted Method and Response Surface Methodology. Processes 2022, 10, 2034. [Google Scholar] [CrossRef]
- Anh, T.T.; Thanh Huyen, N.T.; Lam, T.D. Effect of Extracting Process on Saponin Content and Antioxidant Activity of Gleditschia fera (Lour.) Merr Dried Fruit Extract. IOP Conf. Ser. Mater. Sci. Eng. 2019, 544, 012026. [Google Scholar] [CrossRef]
- Gavrila, A.I.; Tatia, R.; Seciu-Grama, A.M.; Tarcomnicu, I.; Negrea, C.; Calinescu, I.; Zalaru, C.; Moldovan, L.; Raiciu, A.D.; Popa, I. Ultrasound Assisted Extraction of Saponins from Hedera helix L. and an In Vitro Biocompatibility Evaluation of the Extracts. Pharmaceuticals 2022, 15, 1197. [Google Scholar] [CrossRef]
- Mala, P.; Khan, G.A.; Gopalan, R.; Gedefaw, D.; Soapi, K. Fijian Medicinal Plants and Their Role in the Prevention of Type 2 Diabetes Mellitus. Biosci. Rep. 2022, 42, BSR20220461. [Google Scholar] [CrossRef]
- Barber, E.; Houghton, M.J.; Williamson, G.; Nilsson, A.; Hernandez-Hernandez, O. Flavonoids as Human Intestinal α-Glucosidase Inhibitors. Foods 2021, 10, 1939. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Park, Y.H.; Na, M.K.; Kang, S.C. α-Glucosidase and Tyrosinase Inhibitory Effects of an Abietane Type Diterpenoid Taxoquinone from Metasequoia glyptostroboides. BMC Complement. Altern. Med. 2015, 15, 84. [Google Scholar] [CrossRef]
- Silva, C.A.M.; Simeoni, L.A.; Silveira, D. Genus Pouteria: Chemistry and Biological Activity. Rev. Bras. Farmacogn. 2009, 19, 501–509. [Google Scholar] [CrossRef]
- Mohamed Abed El Aziz, M.; Said Ashour, A.; Sadek Gomha Melad, A. A Review on Saponins From Medicinal Plants: Chemistry, Isolation, and Determination. J. Nanomed. Res. 2019, 8, 6–12. [Google Scholar] [CrossRef]
- Li, H.; Zhai, B.; Sun, J.; Fan, Y.; Zou, J.; Cheng, J.; Zhang, X.; Shi, Y.; Guo, D. Ultrasound-Assisted Extraction of Total Saponins from Aralia taibaiensis: Process Optimization, Phytochemical Characterization, and Mechanism of α-Glucosidase Inhibition. Drug Des. Dev. Ther. 2022, 6, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Dou, F.; Xi, M.; Wang, J.; Tian, X.; Hong, L.; Tang, H.; Wen, A. α-Glucosidase and α-Amylase Inhibitory Activities of Saponins from Traditional Chinese medicines in the Treatment of Diabetes Mellitus. Pharmazie 2013, 68, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Shen, W.; Gao, W.; Namujia, L.; Yang, X.; Cao, J.; Sun, L. Essential Moieties of Myricetins, Quercetins and Catechins for Binding and Inhibitory Activity Against α-Glucosidase. Bioorg. Chem. 2021, 115, 105235. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Li, J.; Hu, N.; Wang, H.; Cao, J.; Chi, X.; Dong, Q. Screening for α-Glucosidase-Inhibiting Saponins from Pressurized Hot Water Extracts of Quinoa Husks. Foods 2022, 11, 3026. [Google Scholar] [CrossRef]
- Ding, H.; Hu, X.; Xu, X.; Zhang, G.; Gong, D. Inhibitory Mechanism of Two Allosteric Inhibitors, Oleanolic Acid and Ursolic Acid on α-Glucosidase. Int. J. Biol. Macromol. 2018, 107, 1844–1855. [Google Scholar] [CrossRef]
- Murugesu, S.; Ibrahim, Z.; Ahmed, Q.U.; Yusoff, N.I.N.; Uzir, B.F.; Perumal, V.; Abas, F.; Saari, K.; El-Seedi, H.; Khatib, A. Characterization of α-Glucosidase Inhibitors from Clinacanthus nutans Lindau Leaves by Gas Chromatography-Mass Spectrometry-Based Metabolomics and Molecular Docking Simulation. Molecules 2018, 23, 2402. [Google Scholar] [CrossRef]
- Loveid, J.; Simonsid, C.R. Acid Hydrolysis of Saponins Extracted in Tincture. PLoS ONE 2020, 15, e0244654. [Google Scholar] [CrossRef]
- Padilla-Camberos, E.; Flores-Fernandez, J.M.; Fernandez-Flores, O.; Gutierrez-Mercado, Y.; Carmona-De La Luz, J.; Sandoval-Salas, F.; Mendez-Carreto, C.; Allen, K. Hypocholesterolemic Effect and In Vitro Pancreatic Lipase Inhibitory Activity of an Opuntia Ficus-Indica Extract. BioMed Res. Int. 2015, 2015, 837452. [Google Scholar] [CrossRef]
- Navarro del Hierro, J.; Herrera, T.; Fornari, T.; Reglero, G.; Martin, D. The Gastrointestinal Behavior of Saponins and Its Significance for Their Bioavailability and Bioactivities. J. Funct. Foods 2018, 40, 484–497. [Google Scholar] [CrossRef]
- Zhao, H.L.; Sim, J.S.; Shim, S.H.; Ha, Y.W.; Kang, S.S.; Kim, Y.S. Antiobese and Hypolipidemic Effects of Platycodin Saponins in Diet-Induced Obese Rats: Evidences for Lipase Inhibition and Calorie Intake Restriction. Int. J. Obes. 2005, 29, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ji, G.E. Effects of Various Ginsenosides and Ginseng Root and Ginseng Berry on the Activity of Pancreatic Lipase. Food Sci. Biotechnol. 2017, 26, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Navarro del Hierro, J.; Casado-Hidalgo, G.; Reglero, G.; Martin, D. The Hydrolysis of Saponin-Rich Extracts from Fenugreek and Quinoa Improves Their Pancreatic Lipase Inhibitory Activity and Hypocholesterolemic Effect. Food Chem. 2021, 338, 128113. [Google Scholar] [CrossRef]
- Uemura, T.; Goto, T.; Kang, M.S.; Mizoguchi, N.; Hirai, S.; Lee, J.Y.; Nakano, Y.; Shono, J.; Hoshino, S.; Taketani, K.; et al. Diosgenin, the Main Aglycon of Fenugreek, Inhibits LXRα Activity in HepG2 Cells and Decreases Plasma and Hepatic Triglycerides in Obese Diabetic Mice. J. Nutr. 2011, 141, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Forsido, S.F.; Welelaw, E.; Belachew, T.; Hensel, O. Effects of Storage Temperature and Packaging Material on Physico-Chemical, Microbial and Sensory Properties and Shelf Life of Extruded Composite Baby Food Flour. Heliyon 2021, 7, e06821. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kang, J.Y.; Park, S.K.; Han, H.J.; Lee, K.Y.; Kim, A.N.; Kim, J.C.; Choi, S.G.; Heo, H.J. Effect of Storage Temperature on the Antioxidant Activity and Catechins Stability of Matcha (Camellia sinensis). Food Sci. Biotechnol. 2020, 29, 1261–1271. [Google Scholar] [CrossRef] [PubMed]



| Temperature (°C) | Ethanol Concentration (%) | Total Phenolic Content (mg GAE/g Sample) | Total Flavonoid Content (mg CAE/g Sample) | Saponin Content (mg/g) |
|---|---|---|---|---|
| 40 | 50 | 234.9 ± 0.89 g | 59.76 ± 0.95 e | 20.23 ± 0.48 f |
| 60 | 283.5 ± 0.55 d | 70.88 ± 0.14 c | 31.70 ± 0.70 c | |
| 70 | 308.9 ± 1.89 b | 76.63 ± 0.91 b | 31.94 ± 0.18 c | |
| 50 | 50 | 314.7 ± 0.55 a | 79.85 ± 0.64 a | 36.04 ± 0.08 a |
| 60 | 313.8 ± 0.36 a | 79.12 ± 0.61 a | 35.64 ± 0.09 a | |
| 70 | 313.6 ± 1.15 a | 80.43 ± 0.85 a | 35.69 ± 0.09 a | |
| 60 | 50 | 246.5 ± 0.95 f | 63.76 ± 0.38 d | 26.57 ± 0.09 e |
| 60 | 272.4 ± 1.09 e | 68.11 ± 0.27 c | 29.27 ± 0.14 d | |
| 70 | 294.4 ± 0.62 c | 73.00 ± 0.69 bc | 33.08 ± 0.61 b |
| Bioactive Compounds | Saponin-Rich Extract Powder |
|---|---|
| Total phenolic content (mg GAE/g sample) | 60.71 ± 0.20 |
| Total flavonoid content (mg CAE/g sample) | 136.6 ± 0.45 |
| Saponin content (mg/g) | 283.3 ± 0.93 |
| Samples | IC50 (mg/mL) |
|---|---|
| Acarbose | 0.15 ± 0.01 |
| Saponin-rich extracts | 0.10 ± 0.01 |
| Sapogenin-rich extracts | 2.98 ± 0.33 |
| Samples | IC50 (mg/mL) |
|---|---|
| Orlistat | 2.36 ± 0.02 |
| Saponin-rich extracts | ˃1000 |
| Sapogenin-rich extracts | 7.60 ± 0.01 |
| Temperature (°C) | Time (Months) | Moisture Content (% db.) | Water Activity | L* | a* | b* | TPC (mg GAE/g) | TSC (mg/g) | DPPH (mg Trolox/g) | FRAP (mg Trolox/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 0 | 5.26 ± 0.21 ef | 0.43 ± 0.00 a | 79.15 ± 0.34 ab | 8.06 ± 0.10 ab | 14.65 ± 0.18 ns | 241.42 ± 1.51 a | 10.19 ± 0.15 a | 262.34 ± 5.70 a | 131.73 ± 3.28 a |
| 25 | 1 | 5.93 ± 0.33 d | 0.43 ± 0.00 a | 79.69 ± 0.13 ab | 7.91 ± 0.05 ab | 14.27 ± 0.03 ns | 227.03 ± 0.10 b | 10.00 ± 0.07 a | 174.71 ± 3.16 b | 124.03 ± 1.12 b |
| 2 | 7.68 ± 0.12 a | 0.43 ± 0.00 a | 78.71 ± 0.22 bc | 7.88 ± 0.25 ab | 14.27 ± 0.53 ns | 227.20 ± 1.54 b | 9.53 ± 0.30 b | 161.23 ± 3.29 c | 112.84 ± 1.33 e | |
| 3 | 7.19 ± 0.03 b | 0.44 ± 0.00 a | 79.29 ± 0.90 ab | 8.13 ± 0.28 a | 14.66 ± 0.31 ns | 214.29 ± 1.08 e | 8.65 ± 0.49 c | 133.25 ± 3.53 f | 95.76 ± 0.24 h | |
| 35 | 1 | 5.88 ± 0.08 d | 0.43 ± 0.00 a | 78.59 ± 0.32 bc | 7.67 ± 0.27 b | 14.72 ± 0.25 ns | 222.35 ± 1.59 c | 8.56 ± 0.20 cd | 165.32 ± 2.15 c | 121.12 ± 0.76 c |
| 2 | 6.93 ± 0.05 bc | 0.44 ± 0.00 b | 78.12 ± 0.57 cd | 7.92 ± 0.25 ab | 14.45 ± 0.35 ns | 219.75 ± 0.82 d | 8.45 ± 0.21 cd | 149.80 ± 3.29 d | 109.13 ± 1.23 f | |
| 3 | 6.67 ± 0.01 c | 0.42 ± 0.00 c | 79.75 ± 0.25 a | 7.85 ± 0.20 ab | 14.57 ± 0.17 ns | 207.00 ± 1.51 f | 8.26 ± 0.03 cd | 125.49 ± 2.36 g | 84.13 ± 0.63 i | |
| 45 | 1 | 4.92 ± 0.07 f | 0.37 ± 0.01 d | 78.49 ± 0.23 cd | 7.81 ± 0.25 ab | 14.23 ± 0.36 ns | 218.62 ± 0.63 d | 8.55 ± 0.14 cd | 143.67 ± 2.25 e | 115.89 ± 1.29 d |
| 2 | 5.53 ± 0.18 e | 0.39 ± 0.00 d | 77.71 ± 0.49 d | 7.92 ± 0.60 ab | 14.65 ± 0.23 ns | 215.85 ± 0.75 e | 7.29 ± 0.14 e | 141.01 ± 1.94 e | 105.57 ± 239 g | |
| 3 | 5.27 ± 0.04 ef | 0.36 ± 0.06 d | 79.03 ± 0.88 abc | 7.99 ± 0.18 ab | 14.68 ± 0.03 ns | 202.06 ± 0.72 g | 7.29 ± 0.06 e | 120.38 ± 2.53 h | 79.48 ± 1.56 j |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanneur, K.; Leksawasdi, N.; Sumonsiri, N.; Techapun, C.; Taesuwan, S.; Nunta, R.; Khemacheewakul, J. Inhibitory Effects of Saponin-Rich Extracts from Pouteria cambodiana against Digestive Enzymes α-Glucosidase and Pancreatic Lipase. Foods 2023, 12, 3738. https://doi.org/10.3390/foods12203738
Sanneur K, Leksawasdi N, Sumonsiri N, Techapun C, Taesuwan S, Nunta R, Khemacheewakul J. Inhibitory Effects of Saponin-Rich Extracts from Pouteria cambodiana against Digestive Enzymes α-Glucosidase and Pancreatic Lipase. Foods. 2023; 12(20):3738. https://doi.org/10.3390/foods12203738
Chicago/Turabian StyleSanneur, Kawisara, Noppol Leksawasdi, Nutsuda Sumonsiri, Charin Techapun, Siraphat Taesuwan, Rojarej Nunta, and Julaluk Khemacheewakul. 2023. "Inhibitory Effects of Saponin-Rich Extracts from Pouteria cambodiana against Digestive Enzymes α-Glucosidase and Pancreatic Lipase" Foods 12, no. 20: 3738. https://doi.org/10.3390/foods12203738
APA StyleSanneur, K., Leksawasdi, N., Sumonsiri, N., Techapun, C., Taesuwan, S., Nunta, R., & Khemacheewakul, J. (2023). Inhibitory Effects of Saponin-Rich Extracts from Pouteria cambodiana against Digestive Enzymes α-Glucosidase and Pancreatic Lipase. Foods, 12(20), 3738. https://doi.org/10.3390/foods12203738