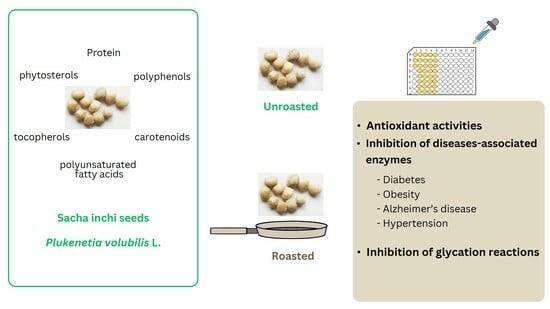
The Effects of Different Roasting Methods on the Phenolic Contents, Antioxidant Potential, and In Vitro Inhibitory Activities of Sacha Inchi Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Extraction
2.2. Determination of Phenolic Profile and Total Phenolic Content
2.3. Determination of Antioxidant Activity
2.4. Determination of Enzyme Inhibitory Activity
2.5. Determination of Glycation Inhibitory Activity
2.6. Statistical Analysis
3. Results
3.1. The Effect of Roasting Processes on Total Phenolic Content
3.2. The Effect of Roasting Processes on Antioxidant Activity
3.3. The Effect of Roasting Processes on Enzyme Inhibitory Activity
3.4. Effect of Roasting Processes on Glycation Inhibitory Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chirinos, R.; Zuloeta, G.; Pedreschi, R.; Mignolet, E.; Larondelle, Y.; Campos, D. Sacha inchi (Plukenetia volubilis): A seed source of polyunsaturated fatty acids, tocopherols, phytosterols, phenolic compounds and antioxidant capacity. Food Chem. 2013, 141, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Kodahl, N.; Sørensen, M. Sacha inchi (Plukenetia volubilis L.) is an underutilized crop with a great potential. Agronomy 2021, 11, 1066. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Valles, C.; Gilman, R.; Hardmeier, R.M.; Clark, D.; Garcia, H.H.; Gonzales, A.E.; Kohlstad, I.; Castro, M.; Valdivia, R.; et al. Amino acid and fatty acid profiles of the Inca peanut (Plukenetia volubilis L.). Cereal Chem. 1992, 69, 461–463. [Google Scholar]
- Follegatti-Romero, L.A.; Piantino, C.R.; Grimaldi, R.; Cabral, A. Supercritical CO2 extraction of omega-3 rich oil from Sacha inchi (Plukenetia volubilis L.) seeds. J. Supercrit. Fluids 2009, 49, 323–329. [Google Scholar] [CrossRef]
- Fanali, C.; Dugo, L.; Cacciola, F.; Beccaria, M.; Grasso, S.; Dachà, M.; Dugo, P.; Mondello, L. Chemical characterization of sacha inchi (Plukenetia volubilis L.) oil. J. Agric. Food Chem. 2011, 59, 13043–13049. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Tanwar, B.; Sihag, M.K.; Sharma, V. Sacha inchi (Plukenetia volubilis L.): An emerging source of nutrients, omega-3 fatty acid and phytochemicals. Food Chem. 2022, 373, 131459. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, D.M.; Gómez Rave, L.J.; Soto, J.A. Biological activity of sacha inchi (Plukenetia volubilis Linneo) and potential uses in human health: A review. Food Technol. Biotechnol. 2021, 59, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, F.; Pando, R.; Ronceros, G. Effect of sacha inchi oil (Plukenetia volúbilis L.) on the lipid profile of patients with hyper-lipoproteinemia. Rev. Peru. Med. Exp. Salud Publica 2011, 28, 628–632. [Google Scholar]
- Saavedra, J.J.H.; Silva, B.E.F.; Pairazamán, P.I.E.; Minaya, K.Y.C. Effects of the ingestión of Plukenetia volubilis Linneo (a.k.a. ‘Sacha inchi’) on the lipid profile of young adults. Acta Méd. Peru. 2012, 29, 155–160. [Google Scholar]
- Schiessel, D.L.; Yamazaki, R.K.; Kryczyk, M.; Coelho, I.; Yamaguchi, A.A.; Pequito, D.C.T.; Brito, G.A.P.; Borghetti, G.; Fernandes, L.C. α-Linolenic fatty acid supplementation decreases tumor growth and cachexia parameters in walker 256 tumor-bearing rats. Nutr. Cancer 2015, 67, 839–846. [Google Scholar] [CrossRef]
- Kim, D.S.; Joo, N. Nutritional composition of sacha inchi (Plukenetia volubilis L.) as affected by different cooking methods. Int. J. Food Prop. 2019, 22, 1235–1241. [Google Scholar] [CrossRef]
- Kittibunchakul, S.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Temviriyanukul, P.; Suttisansanee, U. Evaluation of sacha inchi (Plukenetia volubilis L.) by-products as valuable and sustainable sources of health benefits. Horticulturae 2022, 8, 344. [Google Scholar] [CrossRef]
- Štěrbová, L.; Čepková, P.H.; Viehmannová, I.; Huansi, D.C. Effect of thermal processing on phenolic content, tocopherols and antioxidant activity of sacha inchi kernels. J. Food Process. Preserv. 2017, 41, e12848. [Google Scholar] [CrossRef]
- Temviriyanukul, P.; Sritalahareuthai, V.; Promyos, N.; Thangsiri, S.; Pruesapan, K.; Srinuanchai, W.; Nuchuchua, O.; Siriwan, D.; On-nom, N.; Suttisansanee, U. The effect of sacred lotus (Nelumbo nucifera) and its mixtures on phenolic profiles, antioxidant activities, and inhibitions of the key enzymes relevant to Alzheimer’s disease. Molecules 2020, 25, 3713. [Google Scholar] [CrossRef] [PubMed]
- Hinkaew, J.; Sahasakul, Y.; Tangsuphoom, N.; Suttisansanee, U. The effect of cultivar variation on total phenolic contents and antioxidant activities of date palm fruit (Phoenix dactylifera L.). Curr. Res. Nutr. Food Sci. 2020, 8, 155–163. [Google Scholar] [CrossRef]
- Sripum, C.; Kukreja, R.K.; Charoenkiatkul, S.; Kriengsinyos, W.; Suttisansanee, U. The effect of extraction conditions on antioxidant activities and total phenolic contents of different processed Thai Jasmine rice. Int. Food Res. J. 2017, 24, 1644–1650. [Google Scholar]
- Sirichai, P.; Kittibunchakul, S.; Thangsiri, S.; On-Nom, N.; Chupeerach, C.; Temviriyanukul, P.; Inthachat, W.; Nuchuchua, O.; Aursalung, A.; Sahasakul, Y.; et al. Impact of drying processes on phenolics and in vitro health-related activities of indigenous plants in Thailand. Plants 2022, 11, 294. [Google Scholar] [CrossRef]
- Chupeerach, C.; Aursalung, A.; Watcharachaisoponsiri, T.; Whanmek, K.; Thiyajai, P.; Yosphan, K.; Sritalahareuthai, V.; Sahasakul, Y.; Santivarangkna, C.; Suttisansanee, U. The effect of steaming and fermentation on nutritive values, antioxidant activities, and inhibitory properties of tea leaves. Foods 2021, 10, 117. [Google Scholar] [CrossRef]
- Bueno-Borges, L.B.; Sartim, M.A.; Gil, C.C.; Sampaio, S.V.; Rodrigues, P.H.V.; Regitano-d’Arce, M.A.B. Sacha inchi seeds from sub-tropical cultivation: Effects of roasting on antinutrients, antioxidant capacity and oxidative stability. J. Food Sci. Technol. 2018, 55, 4159–4166. [Google Scholar] [CrossRef]
- Amrein, T.M.; Lukac, H.; Anres, L.; Rainer, P.; Escher, F.; Amado, R. Acrylamide in roasted almonds and hazelnuts. J. Agric. Food Chem. 2005, 53, 7819–7825. [Google Scholar] [CrossRef]
- Yaacoub, R.; Saliba, R.; Nsouli, B.; Khalaf, G.; Birlouez-Aragon, I. Formation of lipid oxidation and isomerization products during processing of nuts and sesame seeds. J. Agric. Food Chem. 2008, 56, 7082–7090. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R.P.F. Food Drying and Dehydration: Technology and Effect on Food Properties; LAP Lambert Academic Publishing: Saarbrücken, Germany, 2015. [Google Scholar]
- Chua, L.Y.W.; Chua, B.L.; Figiel, A.; Chong, C.H.; Wojdyło, A.; Szumny, A.; Lech, K. Characterisation of the convective hot-air drying and vacuum microwave drying of Cassia alata: Antioxidant activity, essential oil volatile composition and quality studies. Molecules 2019, 24, 1625. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.K.; Kirkman, T.; Nguyen, M.; Vuong, Q.V. Effects of drying on physical properties, phenolic compounds and antioxidant capacity of Robusta wet coffee pulp (Coffea canephora). Heliyon 2020, 6, e04498. [Google Scholar] [CrossRef] [PubMed]
- Kittibunchakul, S.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Suttisansanee, U.; Sahasakul, Y. Effects of maturity and thermal treatment on phenolic profiles and in vitro health-related properties of sacha inchi leaves. Plants 2022, 11, 1515. [Google Scholar] [CrossRef]
- Maghsoudlou, Y.; Ghajari, M.A.; Sedighe, T. Effects of heat treatment on the phenolic compounds and antioxidant capacity of quince fruit and its tisane’s sensory properties. J. Food Sci. Technol. 2019, 56, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.K.; Betti, M. Non-enzymatic browning reaction of glucosamine at mild conditions: Relationship between colour formation, radical scavenging activity and α-dicarbonyl compounds production. Food Chem. 2016, 212, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Açar, Ö.Ç.; Gökmen, V.; Pellegrini, N.; Fogliano, V. Direct evaluation of the total antioxidant capacity of raw and roasted pulses, nuts and seeds. Eur. Food Res. Technol. 2009, 229, 961–969. [Google Scholar] [CrossRef]
- Ghazzawi, H.A.; Al-Ismail, K. A comprehensive study on the effect of roasting and frying on fatty acids profiles and antioxidant capacity of almonds, pine, cashew, and pistachio. J. Food Qual. 2017, 2017, 9038257. [Google Scholar] [CrossRef]
- Meng, Y.; Su, A.; Yuan, S.; Zhao, H.; Tan, S.; Hu, C.; Deng, H.; Guo, Y. Evaluation of total flavonoids, myricetin, and quercetin from Hovenia dulcis Thunb. as inhibitors of α-amylase and α-glucosidase. Plant Foods Hum. Nutr. 2016, 71, 444–449. [Google Scholar] [CrossRef]
- Szwajgier, D. Anticholinesterase activity of phenolic acids and their derivatives. Z. Naturforschung C 2013, 68, 125–132. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative properties and effect of quercetin and its glycosylated form (rutin) on acetylcholinesterase and butyrylcholinesterase activities. J. Evid. Based Complement. Altern. Med. 2016, 21, NP11–NP17. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Medica 2015, 81, 771–783. [Google Scholar] [CrossRef]
- Zhou, J.F.; Wang, W.J.; Yin, Z.P.; Zheng, G.D.; Chen, J.G.; Li, J.E.; Chen, L.L.; Zhang, Q.F. Quercetin is a promising pancreatic lipase inhibitor in reducing fat absorption in vivo. Food Biosci. 2021, 43, 101248. [Google Scholar] [CrossRef]
- Kumar, A.; Chauhan, S. Pancreatic lipase inhibitors: The road voyaged and successes. Life Sci. 2021, 271, 119115. [Google Scholar] [CrossRef]
- Suttisansanee, U.; Thiyajai, P.; Chalermchaiwat, P.; Wongwathanarat, K.; Pruesapan, K.; Charoenkiatkul, S.; Temviriyanukul, P. Phytochemicals and in vitro bioactivities of aqueous ethanolic extracts from common vegetables in Thai food. Plants 2021, 10, 1563. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Huang, S.M.; Lin, J.A.; Yen, G.C. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2011, 2, 224. [Google Scholar] [CrossRef] [PubMed]
- Julius, A.; Hopper, W. Inhibition of advanced glycation end-product formation by quercetin and catechin: An alternative therapy for treating diabetic complications. Asian J. Pharm. Clin. Res. 2017, 10, 173. [Google Scholar] [CrossRef]
- Wu, Q.; Li, S.; Li, X.; Fu, X.; Sui, Y.; Guo, T.; Xie, B.; Sun, Z. A significant inhibitory effect on advanced glycation end product formation by catechin as the major metabolite of lotus seedpod oligomeric procyanidins. Nutrients 2014, 6, 3230–3244. [Google Scholar] [CrossRef]
- Selvakumar, G.V.D.; Kuttalam, I.; Lonchin, S. Inhibition of advanced glycation end product formation in rat tail tendons by polydatin and p-coumaric acid: An in vitro study. Appl. Biochem. Biotechnol. 2022, 194, 339–353. [Google Scholar] [CrossRef]
- Ávila, F.; Ravello, N.; Manriquez, C.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; Theoduloz, C. Antiglycating effect of phenolics from the Chilean currant Ribes cucullatum under thermal treatment. Antioxidants 2021, 10, 665. [Google Scholar] [CrossRef]
- Liao, G.Z.; Wang, G.Y.; Zhang, Y.J.; Xu, X.L.; Zhou, G.H. Formation of heterocyclic amines during cooking of duck meat. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1668–1678. [Google Scholar] [CrossRef]
- Xie, Q.; Wang, C.; Peng, L.; Dong, Y.; Gao, Y.; Xu, J.; Ping, H.; Liu, S. Effect of vacuum roasting on total selenium content of selenium-enriched rapeseed, Maillard reaction products, oxidative stability and physicochemical properties of selenium-enriched rapeseed oil. Foods 2023, 12, 3204. [Google Scholar] [CrossRef]
- Gang, K.Q.; Wu, Z.X.; Zhou, D.Y.; Zhao, Q.; Zhou, X.; Lv, D.D.; Rakariyatham, K.; Liu, X.Y.; Shahidi, F. Effects of hot air drying process on lipid quality of whelks Neptunea arthritica cumingi Crosse and Neverita didyma. J. Food Sci. Technol. 2019, 56, 4166–4176. [Google Scholar] [CrossRef]

| Assay | Enzyme | Substrate | Indicator | Detected Wavelength |
|---|---|---|---|---|
| α-Amylase | ≥10 units/mg (type VII), porcine pancreatin | p-nitrophenyl-α-D-maltopentaoside | 405 nm | |
| α-Glucosidase | ≥10 units/mg (type I), Saccharomyces cerevisiae | p-nitrophenyl-α-D-glucopyranoside | ||
| Lipase | ≥700 units/mg (type VII), Candida rugose | DMPTB | DTNB | 412 nm |
| AChE | 1000 units/mg, Electrophorus electricus | acetylthiocholine | ||
| BChE | ≥10 units/mg, equine serum | butyrylthiocholine | ||
| BACE-1 | BACE-1 FRET assay kit (Sigma-Aldrich, St. Louis, MO, USA) | λex = 320 nm, λem = 405 nm | ||
| ACE | ≥2 units/mg, rabbit lung | hippuryl-histidyl-leucine | o-phthaldialdehyde | λex = 360 nm, λem = 485 nm |
| Phenolic Compounds | Retention Times (min) | Concentrations (µg/100 g DW) |
|---|---|---|
| Flavonoids | ||
| Myricetin | 37.82–37.91 | ND |
| Quercetin | 42.91–43.16 | 71.65 ± 0.12 c |
| Luteolin | 43.81–43.88 | ND |
| Naringenin | 43.86–43.90 | ND |
| Hesperitin | 45.27–45.33 | ND |
| Kaempferol | 46.20–46.25 | ND |
| Apigenin | 46.57–46.64 | ND |
| Isorhamnitin | 46.92–46.96 | ND |
| Phenolic acids | ||
| Gallic acid | 4.82–4.87 | ND |
| 4-Hydroxybenzoic acid | 11.95–12.06 | ND |
| Chlorogenic acid | 13.17–13.30 | ND |
| Vanillic acid | 14.43–14.54 | ND |
| Caffeic acid | 15.43–15.56 | ND |
| Syringic acid | 16.15–16.26 | ND |
| p-Coumaric acid | 22.32–22.48 | 84.38 ± 0.43 b |
| Ferulic acid | 25.45–25.60 | 90.26 ± 0.85 a |
| Sinapic acid | 26.17–26.34 | ND |
| Sacha Inchi Seeds | TPCs (mg GAE/100 g DW) | Antioxidant Activities (µmol TE/100 g DW) | ||
|---|---|---|---|---|
| ORAC Assay | FRAP Assay | DPPH Radical Scavenging Assay | ||
| Raw | 45.15 ± 3.26 b | 106.14 ± 9.35 d | 2.47 ± 0.41 c | 0.012 ± 0.001 b |
| Roasted-CP | 58.13 ± 4.99 a | 284.48 ± 14.76 a | 4.27 ± 0.30 a | 0.015 ± 0.001 a |
| Roasted-VO | 56.03 ± 3.64 a | 161.42 ± 14.20 c | 3.68 ± 0.49 b | 0.014 ± 0.000 b |
| Roasted-TD | 40.83 ± 0.84 c | 251.92 ± 17.74 b | 4.07 ± 0.88 ab | 0.018 ± 0.001 a |
| Sacha Inchi Seeds | Inhibitory Activities (% Inhibition) | |||
|---|---|---|---|---|
| α-Amylase | Lipase | AChE | BchE | |
| Raw | ND | 14.63 ± 0.57 d | ND | ND |
| Roasted-CP | ND | 16.82 ± 0.38 c | 12.25 ± 1.83 | 22.48 ± 2.95 a |
| Roasted-VO | 16.33 ± 1.03 | 24.19 ± 1.60 a | ND | 4.46 ± 1.13 b |
| Roasted-TD | ND | 19.50 ± 1.23 b | ND | ND |
| Sacha Inchi Seeds | Inhibitory Activities (% Inhibition) | |
|---|---|---|
| MG-Induced Glycation | D-Glucose-Induced Glycation | |
| Raw | 15.18 ± 1.24 c* | 19.40 ± 0.93 c |
| Roasted-CP | 72.66 ± 1.10 a* | 75.99 ± 2.05 a |
| Roasted-VO | 67.27 ± 1.98 b | 68.55 ± 4.91 b |
| Roasted-TD | 66.80 ± 2.99 b | 68.74 ± 1.26 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittibunchakul, S.; Kemsawasd, V.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Suttisansanee, U. The Effects of Different Roasting Methods on the Phenolic Contents, Antioxidant Potential, and In Vitro Inhibitory Activities of Sacha Inchi Seeds. Foods 2023, 12, 4178. https://doi.org/10.3390/foods12224178
Kittibunchakul S, Kemsawasd V, Hudthagosol C, Sanporkha P, Sapwarobol S, Suttisansanee U. The Effects of Different Roasting Methods on the Phenolic Contents, Antioxidant Potential, and In Vitro Inhibitory Activities of Sacha Inchi Seeds. Foods. 2023; 12(22):4178. https://doi.org/10.3390/foods12224178
Chicago/Turabian StyleKittibunchakul, Suwapat, Varongsiri Kemsawasd, Chatrapa Hudthagosol, Promluck Sanporkha, Suwimol Sapwarobol, and Uthaiwan Suttisansanee. 2023. "The Effects of Different Roasting Methods on the Phenolic Contents, Antioxidant Potential, and In Vitro Inhibitory Activities of Sacha Inchi Seeds" Foods 12, no. 22: 4178. https://doi.org/10.3390/foods12224178
APA StyleKittibunchakul, S., Kemsawasd, V., Hudthagosol, C., Sanporkha, P., Sapwarobol, S., & Suttisansanee, U. (2023). The Effects of Different Roasting Methods on the Phenolic Contents, Antioxidant Potential, and In Vitro Inhibitory Activities of Sacha Inchi Seeds. Foods, 12(22), 4178. https://doi.org/10.3390/foods12224178