Degradation and Transformation Mechanisms of Zanthoxylum Alkylamides Exposed to UVB Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Experimental Material
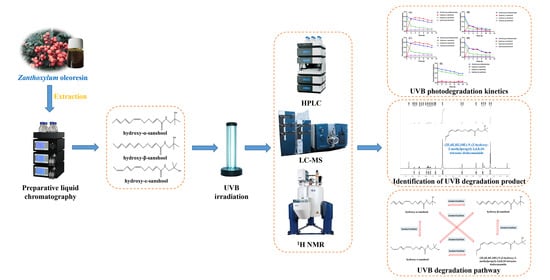
2.2. Extraction of Zanthoxylum Alkylamides
2.3. Isolation and Purification of Zanthoxylum Alkylamides
2.4. Detection of Zanthoxylum Alkylamides
2.4.1. Detection of Zanthoxylum Alkylamides Using High-Performance Liquid Chromatography–Mass Spectrometry (HPLC-MS)
2.4.2. Determining Zanthoxylum Alkylamides Using HPLC Method
2.4.3. HPLC Methodology in Detecting Zanthoxylum Alkylamides
Linearity and Correlation Coefficient
Repeatability and Precision
Recovery
2.5. UVB Photodegradation Kinetics of Zanthoxylum Alkylamides
2.6. Analysis of UVB Light Degradation products of Zanthoxylum Alkylamides and Degradation Pathway
2.6.1. HPLC Analysis of the UVB Degradation Products of Zanthoxylum Alkylamides
2.6.2. Preparative Liquid Chromatographic Separation and Purification of the UVB Degradation Products of Zanthoxylum Alkylamides
2.6.3. 1H Nuclear Magnetic Resonance Spectroscopy (1H NMR) of the UVB-Light Degradation Products of Zanthoxylum Alkylamides
2.6.4. UVB Degradation Pathways of Zanthoxylum Alkylamides
2.7. Data Processing
3. Results and Discussion
3.1. Liquid Chromatogram for Preparing Zanthoxylum Alkylamides
3.2. Component Analysis of Zanthoxylum Alkylamides
3.3. High-Performance Liquid Chromatograms of Zanthoxylum Alkylamides
3.4. Methodological Testing of the HPLC Method for Detecting Zanthoxylum Alkylamides
3.4.1. Linear
3.4.2. Repeatability and Precision
3.4.3. Recovery
3.5. Degradation Kinetics of Zanthoxylum Alkylamides under UVB Irradiation
3.6. Analysis of UV Light Degradation Products and Degradation Pathways of Zanthoxylum Alkylamides
3.6.1. HPLC Detection of UVB Light Degradation Products of Zanthoxylum Alkylamides
3.6.2. Preparative Liquid Chromatographic Separation and Purification of the UVB-Irradiated Degradation Products of Zanthoxylum Alkylamides
3.6.3. HPLC-MS Analysis of UVB Light Degradation Products of Zanthoxylum Alkylamides
3.6.4. Nuclear Magnetic Resonance H-Spectrum Analysis of UVB Degradation Products of Zanthoxylum Alkylamides
3.6.5. Testing of UVB Degradation Products
3.7. UVB Light Degradation Pathways of Zanthoxylum Alkylamides
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.Y.; Shen, X.; Zhang, Y.; Cao, W.; Wang, K.; Xu, S.Z.; Wang, S.W. Analysis of chemical constituents of volatiles and petroleum ether extract from the branch of Zanthoxylum bungeanum by GC-MS. Asian J. Chem. 2014, 26, 3863–3867. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, W. Study on the numb-taste components of Zanthoxylum L. Food Drug 2006, 8, 26–29. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, F.; Kan, J. Characterization of chlorophyll breakdown in green prickleyashes (Zanthoxylum schinifolium Zucc.) during slow drying. Eur. Food Res. Technol. 2012, 234, 1023–1031. [Google Scholar] [CrossRef]
- You, Y.; Zhou, M.; Wang, Q.; Ren, T.; Liu, X. Antioxidant Activity in vitro of Sanshool from Sichuan Peppers (Zanthoxylum bungeanum). Food Sci. 2015, 36, 27–31. [Google Scholar] [CrossRef]
- Wansi, J.D.; Tcho, A.T.; Toze, F.A.A.; Nahar, L.; Martin, C.; Sarker, S.D. Cytotoxic acridone and indoloquinazoline alkaloids from Zanthoxylum poggei. Phytochem. Lett. 2016, 17, 293–298. [Google Scholar] [CrossRef]
- Guetchueng, S.T.; Nahar, L.; Ritchie, K.J.; Ismail, F.M.D.; Wansi, J.D.; Evans, A.; Sarker, S.D. Kaurane diterpenes from the fruits of Zanthoxylum leprieurii (Rutaceae). Rec. Nat. Prod. 2017, 11, 304–309. [Google Scholar]
- Shu, J.; Zhang, X.; Song, S.; Fan, J. Research progress on extraction and application of Zanthoxylum bungeanum essential oil. China Condiment 2023, 48, 203–207. [Google Scholar] [CrossRef]
- Wang, S.; Xie, J.; Yang, W.; Sun, B. Preparative separation and purification of alkylamides from Zanthoxylum bungeanum maxim by high-speed counter-current chromatography. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2640–2652. [Google Scholar] [CrossRef]
- Artaria, C.; Maramaldi, G.; Bonfigli, A.; Rigano, L.; Appendino, G. Lifting properties of the alkamide fraction from the fruit husks of Zanthoxylum bungeanum. Int. J. Cosmet. Sci. 2011, 33, 328–333. [Google Scholar] [CrossRef]
- Bader, M.; Stark, T.D.; Dawid, C.; Loesch, S.; Hofmann, T. All-trans-configuration in Zanthoxylum alkylamides swaps the tingling with a numbing sensation and diminishes salivation. J. Agric. Food Chem. 2014, 62, 2479–2488. [Google Scholar] [CrossRef]
- Albin, K.C.; Simons, C.T. Psychophysical evaluation of a sanshool derivative (alkylamide) and the elucidation of mechanisms subserving tingle. PLoS ONE 2010, 5, e9520. [Google Scholar] [CrossRef] [PubMed]
- Tsunozaki, M.; Lennertz, R.C.; Vilceanu, D.; Katta, S.; Stucky, C.L.; Bautista, D.M. A ‘toothache tree’ alkylamide inhibits a delta mechanonociceptors to alleviate mechanical pain. J. Physiol. Lond. 2013, 591, 3325–3340. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Chien, C.T.; Teng, K.Y.; Chou, S.T. Antioxidative and mutagenic properties of Zanthoxylum ailanthoides sieb & zucc. Food Chem. 2006, 97, 418–425. [Google Scholar] [CrossRef]
- Dupont, E.; Gomez, J.; Bilodeau, D. Beyond UV radiation: A skin under challenge. Int. J. Cosmetic. Sci. 2013, 35, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, S.; Huang, Y.; Wang, Y. Effects of stratospheric ozone recovery on photochemistry and ozone air quality in the troposphere. Atmos. Chem. Phys. 2014, 14, 4079–4086. [Google Scholar] [CrossRef]
- Simon, R.R.; Phillips, K.M.; Horst, R.L.; Munro, I.C. Vitamin D Mushrooms: Comparison of the omposition of button mushrooms (agaricus bisporus) treated postharvest with UVB light or sunlight. J. Agric. Food Chem. 2011, 59, 8724–8732. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, Z.; Zeng, P.; Gong, X.; Tang, Y.; Zhang, Y.; Wen, K.; WANG, H. Quality of Zanthoxylum armatumwith Different Maturity. Guizhou Agric. Sci. 2021, 49, 82–87. [Google Scholar] [CrossRef]
- Yang, X. Aroma constituents and alkylamides of red and green huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 2008, 56, 1689–1696. [Google Scholar] [CrossRef]
- Wang, Q.; Chu, L.; Kou, L. UV-C Treatment maintains quality and delays senescence of oyster mushroom (Pleurotus ostreatus). Sci. Hortic-Amst. 2017, 225, 380–385. [Google Scholar] [CrossRef]
- Luo, J.J.; Hou, X.Y.; Li, S.S.; Luo, Q.Y.; Wu, H.J.; Shen, G.H.; Gu, X.Q.; Mo, X.Y.; Zhang, Z.Q. Degradation and transformation mechanisms of numbing substances: Hydroxyl-alpha-sanshool & hydroxyl-beta-sanshool from Zanthoxylum bungeanum exposed to acid environment. Food Chem. X 2022, 14, 9. [Google Scholar] [CrossRef]
- You, Y.M.; Ren, T.; Zhang, S.Q.; Shirima, G.G.; Cheng, Y.J.; Liu, X. Hypoglycemic effects of Zanthoxylum alkylamides by enhancing glucose metabolism and ameliorating pancreatic dysfunction in streptozotocin-induced diabetic rats. Food Funct. 2015, 6, 3144–3154. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Jacobo-Velazquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC light enhances the biosynthesis of phenolic antioxidants in fresh-cut carrot through a synergistic effect with wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef]
- Lin, C.C.; Lee, L.T.; Hsu, L.J. Degradation of polyvinyl alcohol in aqueous solutions using UV-365 nm/S2O82− process. Int. J. Environ. Sci. Technol. 2014, 11, 831–838. [Google Scholar] [CrossRef]
- Couteau, C.; Stojanovic, S.; Peigne, F.; Coiffard, L.J.M. Photodegradation kinetics under UV light of aspartame. Sci. Aliment. 2000, 20, 523–526. [Google Scholar] [CrossRef]
- Popova, S.A.; Matafonova, G.G.; Batoev, V.B. Dual-wavelength UV degradation of bisphenol A and bezafibrate in aqueous solution using excilamps (222, 282 nm) and led (365 nm): Yes or no synergy? J. Environ. Sci. Health A 2023, 58, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Shad, A.; Chen, J.; Qu, R.J.; Dar, A.A.; Bin-Jumah, M.; Allam, A.A.; Wang, Z.Y. Degradation of sulfadimethoxine in phosphate buffer solution by UV alone, UV/PMS and UV/H2O2: Kinetics, degradation products, and reaction pathways. Chem. Eng. J. 2020, 398, 14. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, W.; Sun, L.; Wei, A.; Wang, D. Accumulation and biosynthesis of hydroxyl-α-sanshool in varieties of Zanthoxylum bungeanum Maxim. by HPLC-fingerprint and transcriptome analyses. Ind. Crop. Prod. 2020, 145, 111998. [Google Scholar] [CrossRef]
- Zhuo, Z.; Xu, D.; Li, Y.; Pu, B.; Ye, M. Fingerprint analysis of Zanthoxylum armatum DC. By HPLC. J. Food Compos. Anal. 2021, 96, 103736. [Google Scholar] [CrossRef]
- Ke, J.; Qu, Y.; Li, S.; Shen, G.; Chen, A.; Luo, Q.; Liu, X.; Wu, H.; Li, M.; Pu, B. Application of HPLC fingerprint based on acid amide components in Chinese prickly ash Zanthoxylum. Ind. Crops Prod. 2018, 119, 267–276. [Google Scholar] [CrossRef]
- Chang, Y.; Ge, A.; Donnapee, S.; Li, J.; Bai, Y.; Liu, J.; He, J.; Yang, X.; Song, L.; Zhang, B. The multi-targets integrated fingerprinting for screening anti-diabetic compounds from a Chinese medicine jinqi jiangtang Tablet. J. Ethnopharmacol. 2015, 164, 210–222. [Google Scholar] [CrossRef]
- Li, D.; Xiao, J.; Liu, W.; Zhang, C.; Akihisa, T.; Abe, M.; Masters, E.; Zhai, W.; Feng, F.; Zhang, J. Vitellaria paradoxa nutshells from seven sub-Saharan countries as potential herbal medicines for treating diabetes based on chemical compositions, HPLC fingerprints and bioactivity evaluation. Chin. J. Nat. Med. 2019, 17, 446–460. [Google Scholar] [CrossRef]
- Blainski, A.; Antonelli-Ushirobira, T.M.; Godoy, G.; Leite-Mello, E.V.S.; Mello, J.C.P. Pharmacognostic evaluation, and development and validation of a HPLC-DAD technique for gallocatechin and epigallocatechin in rhizomes from Limonium brasiliense. Rev. Bras. Farm. 2017, 27, 162–169. [Google Scholar] [CrossRef]
- Kalmár, J.; Lente, G.; Fábián, I. Kinetics and mechanism of the adsorption of methylene blue from aqueous solution on the surface of a quartz cuvette by on-line UV-Vis spectrophotometry. Dye. Pigment. 2016, 127, 170–178. [Google Scholar] [CrossRef]
- Jeong, J.; Sekiguchi, K.; Sakamoto, K. Photochemical and photocatalytic degradation of gaseous toluene using short-wavelength UV irradiation with TiO2 catalyst: Comparison of three UV sources. Chemosphere 2004, 57, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Kruczala, K.; Aris, W.; Schlick, S. Stabilization and early degradation of UV-irradiated heterophasic propylene-ethylene copolymers based on ESR, ESR imaging, UV-Vis, and DSC: Effect of ethylene content and UV wavelength. Macromolecules 2005, 38, 6979–6987. [Google Scholar] [CrossRef]
- Nagai, Y.; Nakamura, D.; Ueno, H.; Matsumoto, N.; Ohishi, F. Photodegradation mechanisms in poly(2,6-butylenenaphthalate-co-tetramethyleneglycol) (PBN-PTMG). II: Wavelength sensitivity of the photodegradation. Polym. Degrad. Stabil. 2005, 88, 256–260. [Google Scholar] [CrossRef]
- Tasaki, T.; Wada, T.; Fujimoto, K.; Kai, S.; Ohe, K.; Oshima, T.; Baba, Y.; Kukizaki, M. Degradation of methyl orange using short-wavelength UV irradiation with oxygen microbubbles. J. Hazard. Mater. 2009, 162, 1103–1110. [Google Scholar] [CrossRef]
- Vulliet, E.; Emmelin, C.; Chovelon, J.M. Influence of pH and irradiation wavelength on the photochemical degradation of sulfonylureas. J. Photochem. Photobiol. A 2004, 163, 69–75. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, J.G.; Kamiyama, H.; Moriyoshi, Y.; Ishigaki, T. Wavelength-sensitive photocatalytic degradation of methyl orange in aqueous suspension over iron(III)-doped TIO2 nanopowders under UV and Visible light irradiation. J. Phys. Chem. B 2006, 110, 6804–6809. [Google Scholar] [CrossRef]
- Townsend, E.M.; Schrock, R.R.; Hoveyda, A.H. Z-selective metathesis homocoupling of 1,3-dienes by molybdenum and tungsten monoaryloxide pyrrolide (MAP) complexes. J. Am. Chem. Soc. 2012, 134, 11334–11337. [Google Scholar] [CrossRef]
- Asano, T.; Okada, T.; Shinkai, S.; Shigematsu, K.; Kusano, Y.; Manabe, O. Temperature and pressure dependences of thermal cis-to-trans isomerization of azobenzenes which evidence an inversion mechanism. J. Am. Chem. Soc. 1981, 103, 5161–5165. [Google Scholar] [CrossRef]
- Cnossen, A.; Kistemaker, J.C.M.; Kojima, T.; Feringa, B.L. Structural dynamics of overcrowded alkene-based molecular motors during thermal isomerization. J. Org. Chem. 2014, 79, 927–935. [Google Scholar] [CrossRef]
- Kazaryan, A.; Kistemaker, J.C.M.; Schäfer, L.V.; Browne, W.R.; Feringa, B.L.; Filatov, M. Understanding the dynamics behind the photoisomerization of a light-driven fluorene molecular rotary motor. J. Phys. Chem. A 2010, 114, 5058–5067. [Google Scholar] [CrossRef] [PubMed]
- Cnossen, A.; Hou, L.L.; Pollard, M.M.; Wesenhagen, P.V.; Browne, W.R.; Feringa, B.L. Driving unidirectional molecular rotary motors with visible light by intra- and intermolecular energy transfer from palladium porphyrin. J. Am. Chem. Soc. 2012, 134, 17613–17619. [Google Scholar] [CrossRef] [PubMed]
- Xing, Q.; Pei, W.; Xing, Q.; Xing, Q. Basic Organic Chemistry (I), 4th ed.; Peking University Press: Beijing, China, 2016; pp. 337–338. [Google Scholar]
- Wang, X.; Hu, X.; Zhang, H.; Chang, F.; Luo, Y. Photolysis Kinetics, Mechanisms, and Pathways of Tetrabromobisphenol A in Water under Simulated Solar Light Irradiation. Environ. Sci. Technol. 2015, 49, 6683–6690. [Google Scholar] [CrossRef] [PubMed]






















| Samples | Standard Curve | Correlation Coefficient (R2) |
|---|---|---|
| Zanthoxylum alkylamides | Y = 120.99X − 4.3988 | 0.987 |
| Hydroxy-ε-sanshool | Y = 49.776X + 0.1358 | 0.991 |
| Hydroxy-α-sanshool | Y = 124.7X − 3.9475 | 0.987 |
| Hydroxy-β-sanshool | Y = 129.64X − 0.2426 | 0.990 |
| Samples | Concentration (mg/mL) | RSD (%) |
|---|---|---|
| Zanthoxylum alkylamides | 0.36 | 0.04 |
| Hydroxy-ε-sanshool | 0.02 | 1.3 |
| Hydroxy-α-sanshool | 0.32 | 0.06 |
| Hydroxy-β-sanshool | 0.02 | 0.3 |
| Zanthoxylum alkylamides | 0.7 | 0.09 |
| Hydroxy-ε-sanshool | 0.04 | 0.9 |
| Hydroxy-α-sanshool | 0.62 | 0.1 |
| Hydroxy-β-sanshool | 0.04 | 0.1 |
| Zanthoxylum alkylamides | 1.0 | 0.08 |
| Hydroxy-ε-sanshool | 0.06 | 1.2 |
| Hydroxy-α-sanshool | 0.88 | 0.09 |
| Hydroxy-β-sanshool | 0.06 | 0.3 |
| Samples | Concentration (mg/mL) | RSD (%) | |
|---|---|---|---|
| Intraday | Interday | ||
| Zanthoxylum alkylamides | 0.36 | 0.75 | 0.72 |
| Hydroxy-ε-sanshool | 0.02 | 5.25 | 1.32 |
| Hydroxy-α-sanshool | 0.32 | 0.36 | 0.64 |
| Hydroxy-β-sanshool | 0.02 | 4.47 | 1.86 |
| Zanthoxylum alkylamides | 0.7 | 0.03 | 0.48 |
| Hydroxy-ε-sanshool | 0.04 | 1.72 | 3.01 |
| Hydroxy-α-sanshool | 0.62 | 0.03 | 0.27 |
| Hydroxy-β-sanshool | 0.04 | 0.14 | 2.34 |
| Zanthoxylum alkylamides | 1.0 | 0.11 | 0.31 |
| Hydroxy-ε-sanshool | 0.06 | 1.17 | 3.48 |
| Hydroxy-α-sanshool | 0.88 | 0.10 | 0.35 |
| Hydroxy-β-sanshool | 0.06 | 0.26 | 0.43 |
| Samples | Concentration (mg/mL) | RSD (%) |
|---|---|---|
| Zanthoxylum alkylamides | 0.15 | 94.0 |
| Hydroxy-ε-sanshool | 0.01 | 89.8 |
| Hydroxy-α-sanshool | 0.13 | 93.0 |
| Hydroxy-β-sanshool | 0.01 | 110.0 |
| Zanthoxylum alkylamides | 0.30 | 102.8 |
| Hydroxy-ε-sanshool | 0.02 | 115.7 |
| Hydroxy-α-sanshool | 0.26 | 101.2 |
| Hydroxy-β-sanshool | 0.02 | 110.0 |
| Zanthoxylum alkylamides | 0.50 | 100.1 |
| Hydroxy-ε-sanshool | 0.03 | 104.3 |
| Hydroxy-α-sanshool | 0.44 | 99.1 |
| Hydroxy-β-sanshool | 0.03 | 116.4 |
| Solvent | Kinetic Equation | Degradation Rate Constant k (mg·mL−1·h−1) | Correlation Coefficient (R2) |
|---|---|---|---|
| methanol | Ct = 0.268 − 0.023t | 0.023 | 0.852 |
| ethanol | Ct = 0.272 − 0.023t | 0.023 | 0.860 |
| soybean oil | Ct = 0.254 − 0.009t | 0.009 | 0.984 |
| 50% methanol | Ct = 0.259 − 0.025t | 0.025 | 0.809 |
| 50% ethanol | Ct = 0.259 − 0.024t | 0.024 | 0.831 |
| Solvent | Kinetic Equation | Degradation Rate Constant k (h−1) | Correlation Coefficient (R2) |
|---|---|---|---|
| methanol | ln(Ct/C0) = −0.162t | 0.162 | 0.909 |
| ethanol | ln(Ct/C0) = −0.154t | 0.154 | 0.918 |
| soybean oil | ln(Ct/C0) = −0.042t | 0.042 | 0.977 |
| 50% methanol | ln(Ct/C0) = −0.222t | 0.222 | 0.861 |
| 50% ethanol | ln(Ct/C0) = −0.189t | 0.189 | 0.887 |
| Solvent | Kinetic Equation | Degradation Rate Constant k (mg−1·mL·h−1) | Correlation Coefficient (R2) |
|---|---|---|---|
| methanol | 1/Ct = 1.351t + 1/0.268 | 1.351 | 0.965 |
| ethanol | 1/Ct = 1.214t + 1/0.272 | 1.214 | 0.972 |
| soybean oil | 1/Ct = 0.207t + 1/0.254 | 0.207 | 0.962 |
| 50% methanol | 1/Ct = 2.655t + 1/0.259 | 2.655 | 0.930 |
| 50% ethanol | 1/Ct = 1.885t + 1/0.259 | 1.885 | 0.950 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Rao, C.; Liu, Q.; Liu, X. Degradation and Transformation Mechanisms of Zanthoxylum Alkylamides Exposed to UVB Light. Foods 2023, 12, 4392. https://doi.org/10.3390/foods12244392
Wang R, Rao C, Liu Q, Liu X. Degradation and Transformation Mechanisms of Zanthoxylum Alkylamides Exposed to UVB Light. Foods. 2023; 12(24):4392. https://doi.org/10.3390/foods12244392
Chicago/Turabian StyleWang, Rui, Chaolong Rao, Qiuyan Liu, and Xiong Liu. 2023. "Degradation and Transformation Mechanisms of Zanthoxylum Alkylamides Exposed to UVB Light" Foods 12, no. 24: 4392. https://doi.org/10.3390/foods12244392
APA StyleWang, R., Rao, C., Liu, Q., & Liu, X. (2023). Degradation and Transformation Mechanisms of Zanthoxylum Alkylamides Exposed to UVB Light. Foods, 12(24), 4392. https://doi.org/10.3390/foods12244392