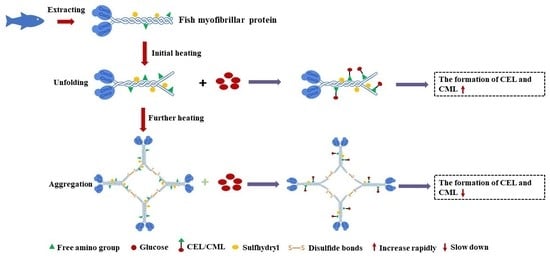
Formation of Nε-Carboxymethyl-Lysine and Nε-Carboxyethyl-Lysine in Heated Fish Myofibrillar Proteins with Glucose: Relationship with Its Protein Structural Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Analysis of CML and CEL
2.4. Analysis of Furosine
2.5. Total Sulfhydryl (T-SH)
2.6. Protein Surface Hydrophobicity (H0)
2.7. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.8. Fourier Transform Infrared Spectroscopy (FTIR)
2.9. Particle Size and ζ-Potential
2.10. Free Amino Content
2.11. Statistical Analysis
3. Results and Discussion
3.1. CML and CEL Levels
3.2. Furosine
3.3. Total Sulfhydryl Content
3.4. Surface Hydrophobicity Content
3.5. SDS–PAGE
3.6. FTIR Analysis
3.7. Particle Size and ζ-Potential
3.8. Free Amino Content
3.9. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, Z.; Huang, M.; Cheng, Y.; Khan, I.A.; Huang, J. A comprehensive review of Nε-Carboxymethyllysine and Nε-Carboxyethyllysine in thermal processed meat products. Trends Food Sci. Technol. 2020, 98, 30–40. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef]
- Han, J.R.; Yan, J.N.; Sun, S.G.; Tang, Y.; Shang, W.H.; Li, A.T.; Guo, X.K.; Du, Y.N.; Wu, H.T.; Zhu, B.W.; et al. Characteristic antioxidant activity and comprehensive flavor compound profile of scallop (Chlamys Farreri) mantle hydrolysates-Ribose Maillard reaction products. Food Chem. 2018, 261, 337–347. [Google Scholar] [CrossRef]
- Lin, J.A.; Wu, C.H.; Yen, G.C. Perspective of advanced glycation end products on human health. J. Agric. Food Chem. 2018, 66, 2065–2070. [Google Scholar] [CrossRef]
- Brownlee, M. Glycation and diabetic complications. Diabetes 1993, 43, 836–842. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, X.; Li, L.; Li, B.; Yang, Z. The fate of dietary advanced glycation end products in the body: From oral intake to excretion. Crit. Rev. Food Sci. Nutr. 2019, 60, 3475–3491. [Google Scholar] [CrossRef]
- Goldberg, T.; Cai, W.; Peppa, M.; Dardaine, V.; Baliga, B.S.; Uribarri, J.; Vlassara, H. Advanced glycoxidation end products in commonly consumed foods. J. Am. Diet. Assoc. 2004, 104, 1287–1291. [Google Scholar] [CrossRef]
- Han, L.; Li, L.; Li, B.; Zhao, D.I.; Li, Y.; Xu, Z.; Liu, G. Review of the characteristics of Food-Derived and endogenous Nɛ-Carboxymethyllysine. J. Food Prot. 2013, 76, 912–918. [Google Scholar] [CrossRef]
- Sun, X.; Tang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Huang, Y. Formation of free and protein-bound carboxymethyllysine and carboxyethyllysine in meats during commercial sterilization. Meat Sci. 2016, 116, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Semedo Tavares, W.P.; Dong, S.; Jin, W.; Yang, Y.; Han, K.; Zha, F.; Zhao, Y.; Zeng, M. Effect of different cooking conditions on the profiles of Maillard reaction products and nutrient composition of hairtail (Thichiurus lepturus) fillets. Food Res. Int. 2018, 103, 390–397. [Google Scholar] [CrossRef]
- Niu, L.; Sun, X.; Tang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Huang, Y. Free and protein-bound Nε-carboxymethyllysine and Nε-carboxyethyllysine in fish muscle: Biological variation and effects of heat treatment. J. Food Compos. Anal. 2017, 57, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Wang, S.; Wang, X.; Dong, S.; Zhao, Y.; Zeng, M. The relationship between the formation of advanced glycation end products and quality attributes of fried sturgeon fillets. LWT 2022, 159, 113161. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, D.; Sheng, B.; Zhu, Z.; Li, H.; Nian, Y.; Wang, C.; Li, C.; Xu, X.; Zhou, G. Application of preheating treatment in up-and down-regulating the glycation process of dietary proteins. Food Hydrocoll. 2020, 98, 105264. [Google Scholar] [CrossRef]
- Xu, D.; Li, L.; Wu, Y.; Zhang, X.; Wu, M.; Li, Y.; Gai, Z.; Li, B.; Zhao, D.; Li, C. Influence of ultrasound pretreatment on the subsequent glycation of dietary proteins. Ultrason. Sonochem. 2020, 63, 104910. [Google Scholar] [CrossRef]
- Huang, X.; Tu, Z.; Wang, H.; Zhang, Q.; Shi, Y.; Xiao, H. Increase of ovalbumin glycation by the Maillard reaction after disruption of the disulfide bridge evaluated by liquid chromatography and high resolution mass spectrometry. J. Agric. Food Chem. 2013, 61, 2253–2262. [Google Scholar] [CrossRef]
- Yu, L.; Chai, M.; Zeng, M.; He, Z.; Chen, J. Effect of lipid oxidation on the formation of Nε-carboxymethyl-lysine and Nε-carboxyethyl-lysine in Chinese-style sausage during storage. Food Chem. 2018, 269, 466–472. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, F.; Gao, P.; Yu, D.; Yu, P.; Jiang, Q.; Xu, Y.; Xia, W. Comparative Study on Quality Characteristics of Pickled and Fermented Sturgeon (Acipenser Sinensis) Meat in Retort Cooking. Int. J. Food Sci. Technol. 2019, 54, 2553–2562. [Google Scholar] [CrossRef]
- Han, P.; Zhang, Q.; Wang, X.; Zhou, P.; Dong, S.; Zha, F.; Zeng, M. Formation of Advanced Glycation End Products in Sturgeon Patties Affected by Pan-Fried and Deep-Fried Conditions. Food Res. Int. 2022, 162, 112105. [Google Scholar] [CrossRef]
- Li, X.; Xie, W.; Bai, F.; Wang, J.; Zhou, X.; Gao, R.; Xu, X.; Zhao, Y. Influence of Thermal Processing on Flavor and Sensory Profile of Sturgeon Meat. Food Chem. 2022, 374, 131689. [Google Scholar] [CrossRef]
- Liu, F.; Dong, X.; Shen, S.; Shi, Y.; Ou, Y.; Cai, W.; Chen, Y.; Zhu, B. Changes in the digestion properties and protein conformation of sturgeon myofibrillar protein treated by low temperature vacuum heating during in vitro digestion. Food Funct. 2021, 12, 6981–6991. [Google Scholar] [CrossRef]
- Han, K.; Yao, Y.; Dong, S.; Jin, S.; Xiao, H.; Wu, H.; Zeng, M. Chemical characterization of the glycated myofibrillar proteins from grass carp (Ctenopharyngodon idella) and their impacts on the human gut microbiota in vitro fermentation. Food Funct. 2017, 8, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.C.; Yu, C.C.; Hsu, K.C. Changes in conformation and sulfhydryl groups of tilapia actomyosin by thermal treatment. LWT 2007, 40, 1316–1320. [Google Scholar] [CrossRef]
- Sun, X.; Tang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Huang, Y. Formation of advanced glycation endproducts in ground beef under pasteurisation conditions. Food Chem. 2015, 172, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue Sulfhydryl Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Bertsch, M.; Mayburd, A.L.; Kassner, R.J. The identification of hydrophobic sites on the surface of proteins using absorption difference spectroscopy of bromophenol blue. Anal. Biochem. 2003, 313, 187–195. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, S.; Feng, X.; Wang, R.; Zeng, M.; Zhao, Y. Physicochemical state and in vitro digestibility of heat treated water-soluble protein from Pacific oyster (Crassostrea gigas). Food Biosci. 2020, 34, 100528. [Google Scholar] [CrossRef]
- Zhou, P.; Feng, Q.; Yang, X.; Gao, R.; Li, Y.; Bai, F.; Wang, J.; Zhou, X.; Wang, H.; Xiao, F.; et al. Sous vide pretreatment in cooking sturgeon fish burger: Effects on physicochemical properties and sensory characteristics. Int. J. Food Sci. Technol. 2021, 56, 2973–2982. [Google Scholar] [CrossRef]
- Ren, Y.; Mao, Z.; Zhang, Q.; Dong, S.; Zha, F.; Chen, B. Formation of advanced glycation end-products in glycation of silver carp myofibrillar protein with glucose: Relationship with its chemical indicators. Int. J. Food Sci. Technol. 2022, 57, 7840–7851. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Erbersdobler, H.F.; Somoza, V. Forty years of furosine–Forty years of using Maillard reaction products as indicators of the nutritional quality of foods. Mol. Nutr. Food Res. 2007, 51, 423–430. [Google Scholar] [CrossRef]
- Trevisan, A.J.B.; de Almeida Lima, D.; Sampaio, G.R.; Soares, R.A.M.; Markowicz Bastos, D.H. Influence of home cooking conditions on Maillard reaction products in beef. Food Chem. 2016, 196, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Lametsch, R.; Greco, I.; Ruiz-Carrascal, J. Advanced glycation end products, protein crosslinks and post translational modifications in pork subjected to different heat treatments. Meat Sci. 2018, 145, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castaño, L.; López-Fandiño, R.; Olano, A.; Villamiel, M. Study on β-lactoglobulin glycosylation with dextran: Effect on solubility and heat stability. Food Chem. 2005, 93, 689–695. [Google Scholar] [CrossRef]
- Qu, W.; Zhang, X.; Han, X.; Wang, Z.; He, R.; Ma, H. Structure and functional characteristics of rapeseed protein isolate-dextran conjugates. Food Hydrocoll. 2018, 82, 329–337. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, H.; Chen, W.; Gantumur, M.A.; Bilawala, A.; Hou, J.; Wang, H. Comparisons of characteristics, kinetics and biological activities of glycosylated α-lactalbumin produced by microwave and conventional heating. LWT 2021, 151, 112111. [Google Scholar] [CrossRef]
- Bian, G.; Xue, S.; Xu, Y.; Xu, X.; Han, M. Improved gelation functionalities of myofibrillar protein from pale, soft and exudative chicken breast meat by nonenzymatic glycation with glucosamine. Int. J. Food Sci. Technol. 2018, 53, 2006–2014. [Google Scholar] [CrossRef]
- Mu, L.; Zhao, M.; Yang, B.; Zhao, H.; Cui, C.; Zhao, Q. Effect of ultrasonic treatment on the graft reaction between soy protein isolate and gum acacia and on the physicochemical properties of conjugates. J. Agric. Food Chem. 2010, 58, 4494–4499. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Y.; Wei, Z.; Zhang, H.; Dong, M.; Huang, M.; Han, M.; Xu, X.; Zhou, G. Modification of myofibrillar protein via glycation: Physicochemical characterization, rheological behavior and solubility property. Food Hydrocoll. 2020, 105, 105852. [Google Scholar] [CrossRef]
- Zhu, D.; Damodaran, S.; Lucey, J.A. Formation of whey protein isolate (WPI)− dextran conjugates in aqueous solutions. J. Agric. Food Chem. 2008, 56, 7113–7118. [Google Scholar] [CrossRef]
- Boostani, S.; Aminlari, M.; Moosavi-nasab, M.; Niakosari, M.; Mesbahi, G. Fabrication and characterisation of soy protein isolate-grafted dextran biopolymer: A novel ingredient in spray-dried soy beverage formulation. Int. J. Biol. Macromol. 2017, 102, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.L.; Kim, J.M.; Abbas, S.; Zhang, X.M.; Xia, S.Q.; Chen, Z.X. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein–glucose. Food Chem. 2010, 120, 505–511. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, R.; Zhang, W.; Hu, Z.; Zhao, W. Structural characterization and physicochemical properties of protein extracted from soybean meal assisted by steam flash-explosion with dilute acid soaking. Food Chem. 2017, 219, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xue, H.; Chen, Z.; Ding, Q.; Wang, X. Comparative studies on the physicochemical properties of peanut protein isolate–polysaccharide conjugates prepared by ultrasonic treatment or classical heating. Food Res. Int. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Zhu, Z.; Yang, J.; Zhou, X.; Khan, I.A.; Bassey, A.P.; Huang, M. Comparison of two kinds of peroxyl radical pretreatment at chicken myofibrillar proteins glycation on the formation of Nε-carboxymethyllysine and Nε-carboxyethyllysine. Food Chem. 2021, 353, 129487. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Bassey, A.P.; Khan, I.A.; Huang, M.; Zhang, X. Inhibitory mechanism of catechins against advanced glycation end products of glycated myofibrillar protein through anti-aggregation and anti-oxidation. LWT 2021, 147, 111550. [Google Scholar] [CrossRef]
- Spickett, C.M.; Pitt, A.R. Modification of proteins by reactive lipid oxidation products and biochemical effects of lipoxidation. Essays Biochem. 2020, 64, 19–31. [Google Scholar] [CrossRef]
- Sun, N.; Cui, P.; Jin, Z.; Wu, H.; Wang, Y.; Lin, S. Contributions of molecular size, charge distribution, and specific amino acids to the iron-binding capacity of sea cucumber (Stichopus japonicus) ovum hydrolysates. Food Chem. 2017, 230, 627–636. [Google Scholar] [CrossRef]
- Vate, N.K.; Benjakul, S. Combined effect of squid ink tyrosinase and tannic acid on heat induced aggregation of natural actomyosin from sardine. Food Hydrocoll. 2016, 56, 62–70. [Google Scholar] [CrossRef]
- Namli, S.; Sumnu, S.G.; Oztop, M.H. Microwave glycation of soy protein isolate with rare sugar (D-allulose), fructose and glucose. Food Biosci. 2021, 40, 100897. [Google Scholar] [CrossRef]
- De Oliveira, F.C.; Coimbra, J.S.d.R.; de Oliveira, E.B.; Zuñiga, A.D.G.; Rojas, E.E.G. Food protein-polysaccharide conjugates obtained via the Maillard reaction: A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Zhou, P.; Han, P.; Zhang, H.; Dong, S.; Zeng, M. Formation of Nε-Carboxymethyl-Lysine and Nε-Carboxyethyl-Lysine in Heated Fish Myofibrillar Proteins with Glucose: Relationship with Its Protein Structural Characterization. Foods 2023, 12, 1039. https://doi.org/10.3390/foods12051039
Zhang S, Zhou P, Han P, Zhang H, Dong S, Zeng M. Formation of Nε-Carboxymethyl-Lysine and Nε-Carboxyethyl-Lysine in Heated Fish Myofibrillar Proteins with Glucose: Relationship with Its Protein Structural Characterization. Foods. 2023; 12(5):1039. https://doi.org/10.3390/foods12051039
Chicago/Turabian StyleZhang, Siqi, Pengcheng Zhou, Peng Han, Hao Zhang, Shiyuan Dong, and Mingyong Zeng. 2023. "Formation of Nε-Carboxymethyl-Lysine and Nε-Carboxyethyl-Lysine in Heated Fish Myofibrillar Proteins with Glucose: Relationship with Its Protein Structural Characterization" Foods 12, no. 5: 1039. https://doi.org/10.3390/foods12051039
APA StyleZhang, S., Zhou, P., Han, P., Zhang, H., Dong, S., & Zeng, M. (2023). Formation of Nε-Carboxymethyl-Lysine and Nε-Carboxyethyl-Lysine in Heated Fish Myofibrillar Proteins with Glucose: Relationship with Its Protein Structural Characterization. Foods, 12(5), 1039. https://doi.org/10.3390/foods12051039