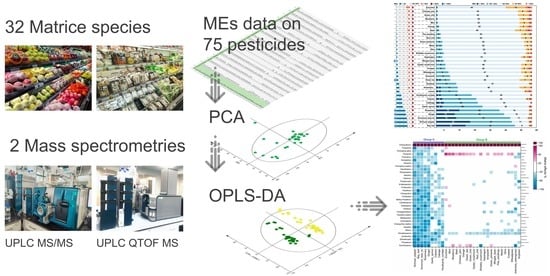
Impact of Matrix Species and Mass Spectrometry on Matrix Effects in Multi-Residue Pesticide Analysis Based on QuEChERS-LC-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Food Materials
2.2. Sample Preparation
2.3. Analysis
2.4. MEs
2.5. Multivariate Analysis
3. Results and Discussion
3.1. Overview of Matrix Effects (MEs) under Multiple Reaction Monitoring (MRM) Scans
3.2. Matrix Typing by MEs under the MRM Scan
3.3. Overview of Matrix Effects (MEs) under Information-Dependent Acquisition (IDA) Mode
3.4. Matrix Typing in the TOF-MS Scan under IDA Mode
3.5. Impact of Different Mass Spectrometry on MEs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raposo, F.; Barcelo, D. Challenges and strategies of matrix effects using chromatography-mass spectrometry: An overview from research versus regulatory viewpoints. TrAC-Trend. Anal. Chem. 2021, 134, 116068. [Google Scholar] [CrossRef]
- EU Reference Laboratories for Residues of Pesticides. No SANTE/11312/2021 Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; EU Reference Laboratories for Residues of Pesticides: Almería, Spain, 2021. [Google Scholar]
- Michlig, N.; Lehotay, S.J.; Lightfield, A.R.; Beldoménico, H.; Repetti, M.R. Validation of a high-throughput method for analysis of pesticide residues in hemp and hemp products. J. Chromatogr. 2021, 1645, 462097. [Google Scholar] [CrossRef] [PubMed]
- GB 23200.121-2021; National Food Safety Standard—Determination of 331 Pesticides and Metabolites in Foods of Plant Origin—Liquid Chromatography—Mass Spectrometry Method. National Standardization Committee: Beijing, China, 2021.
- Stahnke, H.; Kittlaus, S.; Kempe, G.; Alder, L. Reduction of matrix effects in liquid chromatography-electrospray ionization-mass spectrometry by dilution of the sample extracts: How much dilution is needed? Anal. Chem. 2012, 84, 1474–1482. [Google Scholar] [CrossRef] [PubMed]
- Kasperkiewicz, A.; Lendor, S.; Pawliszyn, J. Impact of pesticide formulation excipients and employed analytical approach on relative matrix effects of pesticide determination in strawberries. Talanta 2022, 236, 122825. [Google Scholar] [CrossRef]
- Uclés Moreno, A.; Herrera López, S.; Reichert, B.; Lozano Fernández, A.; Hernando Guil, M.D.; Fernández-Alba, A.R. Microflow liquid chromatography coupled to mass spectrometry-an approach to significantly increase sensitivity, decrease matrix effects, and reduce organic solvent usage in pesticide residue analysis. Anal. Chem. 2015, 87, 1018–1025. [Google Scholar] [CrossRef]
- Saito-Shida, S.; Hamasaka, T.; Nemoto, S.; Akiyama, H. Multiresidue determination of pesticides in tea by liquid chromatography-high-resolution mass spectrometry: Comparison between orbitrap and time-of-flight mass analyzers. Food Chem. 2018, 256, 140–148. [Google Scholar] [CrossRef]
- Ng, K.T.; Rapp-Wright, H.; Egli, M.; Hartmann, A.; Steele, J.C.; Eduardo Sosa-Hernandez, J.; Melchor-Martinez, E.M.; Jacobs, M.; White, B.; Regan, F.; et al. High-Throughput multi-residue quantification of contaminants of emerging concern in wastewaters enabled using direct injection liquid chromatography-tandem mass spectrometry. J. Hazard. Mater. 2020, 398, 122933. [Google Scholar] [CrossRef]
- Qi, P.; Wang, J.; Liu, Z.; Wang, Z.; Xu, H.; Di, S.; Zhao, H.; Wang, X. Integrated QuEChERS strategy for high-throughput multi-pesticide residues analysis of vegetables. J. Chromatogr. A 2021, 1659, 462589. [Google Scholar] [CrossRef]
- Mert, A.; Qi, A.; Bygrave, A.; Stotz, H.U. Trends of pesticide residues in foods imported to the United Kingdom from 2000 to 2020. Food Control 2022, 133, 108616A. [Google Scholar] [CrossRef]
- Shin, J.M.; Choi, S.; Park, Y.H.; Kwak, B.; Ha Moon, S.; Yoon, Y.T.; Jo, S.A.; Yi, H.; Kim, S.J.; Park, S.K.; et al. Comparison of QuEChERS and liquid-liquid extraction methods for the simultaneous analysis of pesticide residues using LC-MS/MS. Food Control 2022, 141, 109202. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Zhu, Q.; Du, H.; Guan, S.; Wang, F.; Zhang, W.; Fan, W.; Chen, Z.; Yang, G.; et al. Reduction of matrix effects through a simplified QuEChERS method and using small injection volumes in a LC-MS/MS system for the determination of 28 pesticides in fruits and vegetables. Anal. Methods 2016, 8, 561–569. [Google Scholar] [CrossRef]
- Li, Y.; Pang, G.F.; Fan, C.L.; Chen, X. Hierarchical cluster analysis of matrix effects on 110 pesticide residues in 28 tea matrixes. J. AOAC Int. 2013, 96, 1453–1465. [Google Scholar] [CrossRef]
- Besil, N.; Cesio, V.; Heinzen, H.; Fernandez-Alba, A.R. Matrix effects and interferences of different citrus fruit coextractives in pesticide residue analysis using ultrahigh-performance liquid chromatography–high-resolution mass spectrometry. J. Agr. Food Chem. 2017, 65, 4819–4829. [Google Scholar] [CrossRef]
- Pang, G.; Chang, Q.; Bai, R.; Fan, C.; Zhang, Z.; Yan, H.; Wu, X. Simultaneous screening of 733 pesticide residues in fruits and vegetables by a GC/LC-Q-TOFMS combination technique. Engineering 2020, 6, 432–441. [Google Scholar] [CrossRef]
- Kanrar, B.; Ghosh, P.; Khan, P.; Sengupta, S. Alternative strategies for the calibration and elimination of matrix effects in LC-MS/MS multiresidue analysis of tea matrix. J. Anal. Chem. 2022, 77, 224–234. [Google Scholar] [CrossRef]
- GB 2763-2021; National Food Safety Standard—Maximum Residue Limits for Pesticides in Food. National Standardization Committee: Beijing, China, 2021.
- Zhao, M.; Feng, Y.; Zhu, Y.; Kim, J. Multi-residue Method for Determination of 238 Pesticides in Chinese cabbage and cucumber by liquid chromatography-tandem mass spectrometry: Comparison of different purification procedures. J. Agr. Food Chem. 2014, 62, 11449–11456. [Google Scholar] [CrossRef]
- Greer, B.; Chevallier, O.; Quinn, B.; Botana, L.M.; Elliott, C.T. Redefining dilute and shoot: The evolution of the technique and its application in the analysis of foods and biological matrices by liquid chromatography mass spectrometry. TrAC-Trend. Anal. Chem. 2021, 141, 116284. [Google Scholar] [CrossRef]
- Lara-Ortega, F.J.; Robles-Molina, J.; Brandt, S.; Schütz, A.; Gilbert-López, B.; Molina-Díaz, A.; García-Reyes, J.F.; Franzke, J. Use of dielectric barrier discharge ionization to minimize matrix effects and expand coverage in pesticide residue analysis by liquid chromatography-mass spectrometry. Anal. Chim. Acta 2018, 1020, 76–85. [Google Scholar] [CrossRef]
- Tripathy, V.; Sharma, K.K.; Yadav, R.; Devi, S.; Tayade, A.; Sharma, K.; Pandey, P.; Singh, G.; Patel, A.N.; Gautam, R.; et al. Development, validation of QuEChERS-based method for simultaneous determination of multiclass pesticide residue in milk, and evaluation of the matrix effect. J. Environ. Sci. Health. B 2019, 54, 394–406. [Google Scholar] [CrossRef]
- Goon, A.; Khan, Z.; Oulkar, D.; Shinde, R.; Gaikwad, S.; Banerjee, K. A simultaneous screening and quantitative method for the multiresidue analysis of pesticides in spices using ultra-high performance liquid chromatography-high resolution (Orbitrap) mass spectrometry. J. Chromatogr. A 2018, 1532, 105–111. [Google Scholar]
- Considine, E.C.; Thomas, G.; Boulesteix, A.L.; Khashan, A.S.; Kenny, L.C. Critical review of reporting of the data analysis step in metabolomics. Metabolomics 2018, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.X.; Hou, L.Z.; Gao, J.; Li, D.F.; Tian, Z.L.; Fan, B.; Wang, F.Z.; Li, S.Y. Metabolomics Approaches for the Comprehensive Evaluation of Fermented Foods: A Review. Foods 2021, 10, 2294. [Google Scholar] [CrossRef] [PubMed]
- Muralidhara, M.; Mithyantha, S.; Rajendran, T.P.; Banerjee, K. Regulatory Landscape of Risk Assessment of Pesticide Residues in Processed Foods in India: A Perspective. J. Food Sci. Tech. Mys. 2022. [Google Scholar] [CrossRef]
- Ngqangashe, Y.; Goldman, S.; Schram, A.; Friel, S. A Narrative Review of Regulatory Governance Factors that Shape Food and Nutrition Policies. Nutr. Rev. 2022, 80, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zeng, J.; Wu, K. Research and application of crop pest monitoring and early warning technology in China. Front. Agric. Sci. Eng. 2022, 9, 19–36. [Google Scholar] [CrossRef]
- He, Z.; Xu, Y.; Wang, L.; Peng, Y.; Luo, M.; Cheng, H.; Liu, X. Wide-scope screening and quantification of 50 pesticides in wine by liquid chromatography/quadrupole time-of-flight mass spectrometry combined with liquid chromatography/quadrupole linear ion trap mass spectrometry. Food Chem. 2016, 196, 1248–1255. [Google Scholar] [CrossRef]
- Saito-Shida, S.; Nemoto, S.; Teshima, R.; Akiyama, H. Quantitative analysis of pesticide residues in vegetables and fruits by liquid chromatography quadrupole time-of-flight mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2016, 33, 119–127. [Google Scholar] [CrossRef]
- Wang, Z.B.; Cao, Y.Z.; Ge, N.; Liu, X.M.; Chang, Q.Y.; Fan, C.L.; Pang, G.F. Wide-scope screening of pesticides in fruits and vegetables using information-dependent acquisition employing UHPLC-QTOF-MS and automated MS/MS library searching. Anal. Bioanal. Chem. 2016, 408, 7795–7810. [Google Scholar] [CrossRef]
- Buhrman, D.L.; Price, P.I.; Rudewicz, P.J. Quantitation of SR 27417 in human plasma using electrospray liquid chromatography tandem mass spectrometry: A study of ion suppression. J. Am. Soc. Mass Spectr. 1996, 7, 1099–1105. [Google Scholar] [CrossRef] [Green Version]
- Oliveri, P.; Malegori, C.; Casale, M. Chemical Analysis of Food, 2nd ed.; Academic Press: Genova, Italy, 2020; pp. 33–76. [Google Scholar]
- He, J.; Sun, R.B. Multivariate statistical analysis for metabolomic data: The key points in principal component analysis. Acta Pharm. Sin. 2018, 53, 929–937. [Google Scholar]
- Antignac, J.; de Wasch, K.; Monteau, F.; De Brabander, H.; Andre, F.; Le Bizec, B. The ion suppression phenomenon in liquid chromatography–mass spectrometry and its consequences in the field of residue analysis. Anal. Chim. Acta 2005, 529, 129–136. [Google Scholar] [CrossRef]
- Zhao, J.; Pu, J.; Wu, X.; Chen, B.; He, Y.; Zhang, Y.; Han, B. Evaluation of the matrix effect of ph value and sugar content on the analysis of pesticides in tropical fruits by UPLC-MS/MS. Microchem. J. 2021, 168, 106375. [Google Scholar] [CrossRef]
- Uclés, S.; Lozano, A.; Sosa, A.; Parrilla Vázquez, P.; Valverde, A.; Fernández-Alba, A.R. Matrix interference evaluation employing GC and LC coupled to triple quadrupole tandem mass spectrometry. Talanta 2017, 17, 72–81. [Google Scholar] [CrossRef]
- Ferrer Amate, C.; Unterluggauer, H.; Fischer, R.J.; Fernández-Alba, A.R.; Masselter, S. Development and validation of a LC-MS/MS method for the simultaneous determination of aflatoxins, dyes and pesticides in spices. Anal. Bioanal. Chem. 2010, 397, 93–107. [Google Scholar] [CrossRef]
- Lacina, O.; Zachariasova, M.; Urbanova, J.; Vaclavikova, M.; Cajka, T.; Hajslova, J. Critical assessment of extraction methods for the simultaneous determination of pesticide residues and mycotoxins in fruits, cereals, spices and oil seeds employing ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2012, 1262, 8–18. [Google Scholar] [CrossRef]
- Chawla, S.; Patel, H.K.; Gor, H.N.; Vaghela, K.M.; Solanki, P.P.; Shah, P.G. Evaluation of Matrix Effects in Multiresidue Analysis of Pesticide Residues in Vegetables and Spices by LC-MS/MS. J. AOAC Int. 2017, 100, 616–623. [Google Scholar] [CrossRef]
- Jadhav, M.; Thekkumpurath, A.S.; Nakade, M.; Gadgil, M.; Oulkar, D.; Arimboor, R.; Ramkrishna, M.; Banerjee, K. Multiresidue method for targeted screening of pesticide residues in spice cardamom (Elettaria cardamomum) by Liquid Chromatography with tandem mass spectrometry. J. AOAC Int. 2017, 100, 603–609. [Google Scholar] [CrossRef]
- Nantia, E.A.; Moreno-González, D.; Manfo, F.P.; Gámiz-Gracia, L.; García-Campaña, A.M. QuEChERS-based method for the determination of carbamate residues in aromatic herbs by UHPLC-MS/MS. Food Chem. 2017, 216, 334–341. [Google Scholar] [CrossRef]
- Gómez-Ramos, M.D.M.; Rajski, Ł.; Lozano, A.; Fernández-Alba, A.R. The evaluation of matrix effects in pesticide multi-residue methods via matrix fingerprinting using liquid chromatography electrospray high-resolution mass spectrometry. Anal. Methods 2016, 8, 4664–4673. [Google Scholar] [CrossRef]
- Meng, X.; Song, W.; Xiao, Y.; Zheng, P.; Cui, C.; Gao, W.; Hou, R. Rapid determination of 134 pesticides in tea through multi-functional filter cleanup followed by UPLC-QTOF-MS. Food Chem. 2022, 370, 130846. [Google Scholar] [CrossRef]
- Makni, Y.; Diallo, T.; Guérin, T.; Parinet, J. Improving the monitoring of multi-class pesticides in baby foods using QuEChERS-UHPLC-Q-TOF with automated identification based on MS/MS similarity algorithms. Food Chem. 2022, 395, 133573. [Google Scholar] [CrossRef] [PubMed]
- Pizzutti, I.R.; de Kok, A.; Hiemstra, M.; Wickert, C.; Prestes, O.D. Method validation and comparison of acetonitrile and acetone extraction for the analysis of 169 pesticides in soya grain by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 4539–4552. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Krska, R.; Malachova, A.; Taschl, I.; Sulyok, M. Evaluation of matrix effects and extraction efficiencies of lc-ms/ms methods as the essential part for proper validation of multiclass contaminants in complex feed. J. Agr. Food Chem. 2020, 68, 3868–3880. [Google Scholar] [CrossRef] [PubMed]
- Constantinou, M.; Louca-Christodoulou, D.; Agapiou, A. Method validation for the determination of 314 pesticide residues using tandem ms systems (GC–MS/MS and LC-MS/MS) in Raisins: Focus on risk exposure assessment and respective processing factors in real samples (a pilot survey). Food Chem. 2021, 360, 129964. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; He, Z.; Zeng, M.; Chen, J. Impact of Matrix Species and Mass Spectrometry on Matrix Effects in Multi-Residue Pesticide Analysis Based on QuEChERS-LC-MS. Foods 2023, 12, 1226. https://doi.org/10.3390/foods12061226
Zhang S, He Z, Zeng M, Chen J. Impact of Matrix Species and Mass Spectrometry on Matrix Effects in Multi-Residue Pesticide Analysis Based on QuEChERS-LC-MS. Foods. 2023; 12(6):1226. https://doi.org/10.3390/foods12061226
Chicago/Turabian StyleZhang, Shuang, Zhiyong He, Maomao Zeng, and Jie Chen. 2023. "Impact of Matrix Species and Mass Spectrometry on Matrix Effects in Multi-Residue Pesticide Analysis Based on QuEChERS-LC-MS" Foods 12, no. 6: 1226. https://doi.org/10.3390/foods12061226
APA StyleZhang, S., He, Z., Zeng, M., & Chen, J. (2023). Impact of Matrix Species and Mass Spectrometry on Matrix Effects in Multi-Residue Pesticide Analysis Based on QuEChERS-LC-MS. Foods, 12(6), 1226. https://doi.org/10.3390/foods12061226