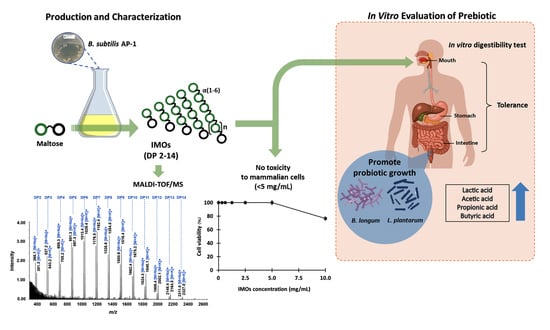
Production of a Series of Long-Chain Isomaltooligosaccharides from Maltose by Bacillus subtilis AP-1 and Associated Prebiotic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microorganisms, Media and Culture Conditions
2.3. Production of IMOs from Maltose by B. subtilis Strain AP-1
2.4. α-Glucosidase Assay and Protein Determination
2.5. Purification of IMOs Produced from B. subtilis Strain AP-1
2.6. TLC
2.7. Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry (MALDI-TOF/MS)
2.8. Linkage Analysis by Enzymatic Hydrolysis
2.9. In Vitro Digestibility of IMOs
2.9.1. Simulated Oral Digestion
2.9.2. Simulated Gastric Digestion
2.9.3. Simulated Intestinal Digestion
2.10. In Vitro Fermentation of IMOs by Probiotic and Pathogenic Bacteria
2.11. Short-Chain Fatty Acid (SCFA) Analysis
2.12. In Vitro Cytotoxicity Assay of IMOs on Mammalian Cells
2.13. Statistical Analysis
3. Results and Discussion
3.1. Production of IMOs by B. subtilis Strain AP-1
3.2. Purification and Linkage Analysis of IMOs
3.3. In Vitro Determination of Prebiotic Properties of IMOs
3.3.1. In Vitro Digestibility of IMOs
3.3.2. Effect of IMOs on the Growth of Probiotic and Pathogenic Bacteria
3.3.3. Production of SCFAs by Probiotic Bacteria
3.4. In Vitro Cytotoxicity of IMOs with Mammalian Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Mutturi, S.; Prapulla, S.G. Modeling of enzymatic production of isomaltooligosaccharides: A mechanistic approach. Catal. Sci. Technol. 2015, 5, 2945–2958. [Google Scholar] [CrossRef]
- Sorndech, W. Isomaltooligosaccharides as Prebiotics and their Health Benefits. In Probiotics, Prebiotics and Synbiotics: Technological Advancements Towards Safety and Industrial Applications; Panesar, P.S., Anal, A.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2022; pp. 361–377. [Google Scholar]
- Hu, Y.; Winter, V.; Gänzle, M. In vitro digestibility of commercial and experimental isomalto-oligosaccharides. Int. Food Res. J. 2020, 134, 109250. [Google Scholar] [CrossRef]
- Seo, E.S.; Nam, S.H.; Kang, H.K.; Cho, J.Y.; Lee, H.S.; Ryu, H.W.; Kim, D. Synthesis of thermo- and acid-stable novel oligosaccharides by using dextransucrase with high concentration of sucrose. Enzym. Microb. Technol. 2007, 40, 1117–1123. [Google Scholar] [CrossRef]
- Plongbunjong, V.; Graidist, P.; Knudsen, K.E.B.; Wichienchot, S. Starch-based carbohydrates display the bifidogenic and butyrogenic properties in pH-controlled faecal fermentation. Int. J. Food Sci. 2017, 52, 2647–2653. [Google Scholar] [CrossRef]
- Wu, Q.; Pi, X.; Liu, W.; Chen, H.; Yin, Y.; Hongwei, D.Y.; Wang, X.; Zhu, L. Fermentation properties of isomaltooligosaccharides are affected by human fecal enterotypes. Anaerobe 2017, 48, 206–214. [Google Scholar] [CrossRef]
- Lan, J.; Wang, K.; Chen, G.; Cao, G.; Yang, C. Effects of inulin and isomalto-oligosaccharide on diphenoxylate-induced constipation, gastrointestinal motility-related hormones, short chain fatty acids, and the intestinal flora in rats. Food Funct. 2020, 11, 9216–9225. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Lee, D.G.; Apu, M.A.I.; Jung, J.H.; Kim, M.K.; Lim, S.; Chung, B.; Pal, K.; Kim, D. The bifidogenic effects and dental plaque deformation of non-digestible isomaltooligosaccharides synthesized by dextransucrase and alternansucrase. Enzym. Microb. Technol. 2022, 153, 109955. [Google Scholar] [CrossRef]
- Mizubuchi, H.; Yajima, T.; Aoi, N.; Tomita, T.; Yoshikai, Y. Isomalto-oligosaccharides polarize th1-like responses in intestinal and systemic immunity in mice. J. Nutr. 2005, 135, 2857–2861. [Google Scholar] [CrossRef] [Green Version]
- Mumpuni, H.; Yasmine, N.; Marsono, Y.; Fibri, D.L.N.; Murdiati, A. FiberCreme as a Functional Food Ingredient Reduces Hyperlipidemia and Risk of Cardiovascular Diseases in Subjects with Hyperlipidemia. Prev. Nutr. Food Sci. 2022, 27, 165–171. [Google Scholar]
- Zhang, L.; Gu, X.; Wang, J.; Liao, S.; Duan, Y.; Li, H.; Song, Z.; He, X.; Fan, Z. Effects of Dietary Isomaltooligosaccharide Levels on the Gut Microbiota, Immune Function of Sows, and the Diarrhea Rate of Their Offspring. Front. Microbiol. 2020, 11, 588986. [Google Scholar] [CrossRef]
- Sorndech, W.; Nakorn, K.N.; Tongta, S.; Blennow, A. Isomalto-oligosaccharides: Recent insights in production technology and their use for food and medical applications. LWT—Food Sci. Technol. 2018, 95, 135–142. [Google Scholar] [CrossRef]
- Kumar, S.; Basu, A.; Anu-Appaiah, K.A.; Gnanesh Kumar, B.S.; Mutturi, S. Identification and characterization of novel transglycosylating α-glucosidase from Aspergillus neoniger. J. Appl. Microbiol. 2020, 129, 1644–1656. [Google Scholar] [CrossRef]
- Goffin, D.; Wathelet, B.; Blecker, C.; Deroanne, C.; Malmendier, Y.; Paquot, M. Comparison of the glucooligosaccharide profiles produced from maltose by two different transglucosidases from Aspergillus niger. Biotechnol. Agron. Soc. Environ. 2010, 14, 607–616. [Google Scholar]
- Nimpiboon, P.; Nakapong, S.; Pichyangkura, R.; Ito, K.; Pongsawasdi, P. Synthesis of a novel prebiotic trisaccharide by a type I α-glucosidase from Bacillus licheniformis strain TH4-2. Process Biochem. 2011, 46, 448–457. [Google Scholar] [CrossRef]
- Chen, P.; Xu, R.; Wang, J.; Wu, Z.; Yan, L.; Zhao, W.; Liu, Y.; Ma, W.; Shi, X.; Li, H. Starch biotransformation into isomaltooligosaccharides using thermostable alpha-glucosidase from Geobacillus stearothermophilus. PeerJ 2018, 6, e5086. [Google Scholar] [CrossRef]
- Fernández-Arrojo, L.; Marín, D.; De Segura, A.G.; Linde, D.; Alcalde, M.; Gutiérrez-Alonso, P.; Ghazi, I.; Plou, F.J.; Fernández-Lobato, M.; Ballesteros, A. Transformation of maltose into prebiotic isomaltooligosaccharides by a novel α-glucosidase from Xantophyllomyces dendrorhous. Process Biochem. 2007, 42, 1530–1536. [Google Scholar] [CrossRef] [Green Version]
- Shin, K.S. Isolation and structural characterization of an oligosaccharide produced by Bacillus subtilis in a maltose-containing medium. Nutr. Food Sci. 2016, 21, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Goffin, D.; Delzenne, N.; Blecker, C.; Hanon, E.; Deroanne, C.; Paquot, M. Will isomalto-oligosaccharides, a well-established functional food in Asia, break through the European and American market? The status of knowledge on these prebiotics. Crit. Rev. Food Sci. Nutr. 2011, 51, 394–409. [Google Scholar] [CrossRef]
- Moctezuma-Dávila, K.M.; Aguilar-García, R.D.; Cuellar-Rincón, I.R.; Wong-Paz, J.E.; Aguilar-Zárate, P.; Muñiz-Márquez, D.B. Enzymatic synthesis of prebiotics from conventional food and beverages rich in sugars. In Value-Addition in Food Products and Processing Through Enzyme Technology; Kuddus, M., Aguilar, C.N., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 111–122. [Google Scholar]
- Madsen, L.R.; Stanley, S.; Swann, P.; Oswald, J. A survey of commercially available isomaltooligosaccharide—Based food ingredients. J. Food Sci. 2017, 82, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kaulpiboon, J.; Rudeekulthamrong, P.; Watanasatitarpa, S.; Ito, K.; Pongsawasdi, P. Synthesis of long-chain isomaltooligosaccharides from tapioca starch and an in vitro investigation of their prebiotic properties. J. Mol. Catal. B Enzym. 2015, 120, 127–135. [Google Scholar] [CrossRef]
- Lee, B.H.; Rose, D.R.; Lin, A.H.M.; Quezada-Calvillo, R.; Nichols, B.L.; Hamaker, B.R. Contribution of the individual small Intestinal α-glucosidases to digestion of unusual α-linked glycemic disaccharides. J. Agric. Food Chem. 2016, 64, 6487–6494. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Lin, A.H.M.; Nichols, B.L.; Jones, K.; Rose, D.R.; Quezada-Calvillo, R.; Hamaker, B.R. Mucosal C-terminal maltase-glucoamylase hydrolyzes large size starch digestion products that may contribute to rapid postprandial glucose generation. Mol. Nutr. Food Res. 2014, 58, 1111–1121. [Google Scholar] [CrossRef]
- Hara, H.; Kume, S.; Iizuka, T.; Fujimoto, Y.; Kimura, A. Enzymatically synthesized megalo-type isomaltosaccharides enhance the barrier function of the tight junction in the intestinal epithelium. Biosci. Biotechnol. Biochem. 2018, 82, 629–635. [Google Scholar] [CrossRef] [Green Version]
- Joe, G.H.; Andoh, M.; Shinoki, A.; Lang, W.; Kumagai, Y.; Sadahiro, J.; Okuyama, M.; Kimura, A.; Shimizu, H.; Hara, H.; et al. Megalo-type α-1,6-glucosaccharides induce production of tumor necrosis factor α in primary macrophages via toll-like receptor 4 signaling. Biomed. Res. J. 2016, 37, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Lin, Q.; Liu, M.; Ding, W.; Weng, N.; Ni, H.; Lu, J.; Lyu, M.; Wang, S. Molecular Docking and Site-Directed Mutagenesis of GH49 Family Dextranase for the Preparation of High-Degree Polymerization Isomaltooligosaccharide. Biomolecules 2023, 13, 300. [Google Scholar] [CrossRef]
- Park, B.R.; Park, J.Y.; Lee, S.H.; Hong, S.J.; Jeong, J.H.; Choi, J.H.; Park, S.Y.; Park, C.S.; Lee, H.N.; Kim, Y.M. Synthesis of improved long-chain isomaltooligosaccharide, using a novel glucosyltransferase derived from Thermoanaerobacter thermocopriae, with maltodextrin. Enzym. Microb. Technol. 2021, 147, 109788. [Google Scholar] [CrossRef]
- Ojha, S.; Mishra, S.; Chand, S. Production of isomalto-oligosaccharides by cell bound α-glucosidase of Microbacterium sp. LWT—Food Sci. Technol. 2015, 60, 486–494. [Google Scholar] [CrossRef]
- Rengarajan, S.; Palanivel, R. High purity prebiotic isomalto-oligosaccharides production by cell associated transglucosidase of isolated strain Debaryomyces hansenii SCY204 and selective fermentation by Saccharomyces cerevisiae SYI065. Process Biochem. 2020, 98, 93–105. [Google Scholar] [CrossRef]
- Casa-Villegas, M.; Marín-Navarro, J.; Polaina, J. Synthesis of isomaltooligosaccharides by Saccharomyces cerevisiae cells expressing Aspergillus niger α-glucosidase. ACS Omega 2017, 2, 8062–8068. [Google Scholar] [CrossRef] [Green Version]
- Schallmey, M.; Singh, A.; Ward, O.P. Developments in the use of Bacillus species for industrial production. Can. J. Microbiol. 2004, 50, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Brutscher, L.M.; Borgmeier, C.; Garvey, S.M.; Spears, J.L. Preclinical Safety Assessment of Bacillus subtilis BS50 for Probiotic and Food Applications. Microorganisms 2022, 10, 1038. [Google Scholar] [CrossRef]
- Nhim, S.; Laothong, S.; Tachaapaikoon, C.; Pason, P.; Ratanakhanokchai, K.; Waeonukul, R. Selection of oligosaccharides producing bacteria from Thai fermented foods. Agric. Sci. J. Thail. J. 2018, 49, 161–164. [Google Scholar]
- Teeravivattanakit, T.; Baramee, S.; Ketbot, P.; Waeonukul, R.; Pason, P.; Tachaapaikoon, C.; Ratanakhanokchai, K.; Phitsuwan, P. Digestibility of Bacillus firmus K-1 pretreated rice straw by different commercial cellulase cocktails. Prep. Biochem. Biotechnol. 2022, 52, 508–513. [Google Scholar] [CrossRef]
- Basu, A.; Mutturi, S.; Prapulla, S.G. Production of isomaltooligosaccharides (IMO) using simultaneous saccharification and transglucosylation from starch and sustainable sources. Process Biochem. 2016, 51, 1464–1471. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Baramee, S.; Phitsuwan, P.; Waeonukul, R.; Pason, P.; Tachaapaikoon, C.; Kosugi, A.; Ratanakhanokchai, K. Alkaline xylanolytic–cellulolytic multienzyme complex from the novel anaerobic alkalithermophilic bacterium Cellulosibacter alkalithermophilus and its hydrolysis of insoluble polysaccharides under neutral and alkaline conditions. Process Biochem. 2015, 50, 643–650. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.O.R.S.T.E.N.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Biochem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Yan, Q.; You, X.; Yang, S.; Jiang, Z. In vitro digestibility and prebiotic potential of curdlan (1 → 3)-β-D-glucan oligosaccharides in Lactobacillus species. Carbohydr. Polym. 2018, 188, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.A.N.; Rodrigues, S. Optimization of panose production by enzymatic synthesis using neural networks. Process Biochem. 2006, 41, 1090–1096. [Google Scholar] [CrossRef]
- Pan, Y.C.; Lee, W.C. Production of high-purity isomalto-oligosaccharides syrup by the enzymatic conversion of transglucosidase and fermentation of yeast cells. Biotechnol. Bioeng. 2005, 89, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Yatake, T. Anomalously linked oligosaccharides in oligosaccharides. In Production, Properties and Application; Nakakuki, T., Ed.; Gordon and Breech Science: Tokyo, Japan, 1993; pp. 79–89. [Google Scholar]
- Goulas, A.K.; Grandison, A.S.; Rastall, R.A. Fractionation of oligosaccharides by Nanofiltration. J. Sci. Food Agric. 2003, 83, 675–680. [Google Scholar] [CrossRef]
- Crittenden, R.G.; Playne, M.J. Purification of food-grade oligosaccharides using immobilised cells of Zymomonas mobilis. Appl. Microbiol. Biotechnol. 2002, 58, 297–302. [Google Scholar]
- Wang, Y.; Chen, G.; Peng, Y.; Rui, Y.; Zeng, X.; Ye, H. Simulated digestion and fermentation in vitro with human gut microbiota of polysaccharides from Coralline pilulifera. LWT—Food Sci. Technol. 2019, 100, 167–174. [Google Scholar] [CrossRef]
- Jeong, W.S.; Lee, Y.R.; Hong, S.J.; Choi, S.J.; Choi, J.H.; Park, S.Y.; Woo, E.J.; Kim, Y.M.; Park, B.R. Carboxy-terminal region of a thermostable CITase from Thermoanaerobacter thermocopriae has the ability to produce long isomaltooligosaccharides. J. Microbiol. Biotechnol. 2019, 29, 1938–1946. [Google Scholar] [CrossRef]
- Shinoki, A.; Lang, W.; Thawornkuno, C.; Kang, H.K.; Kumagai, Y.; Okuyama, M.; Mori, H.; Kimura, A.; Ishizuka, S.; Hara, H. A novel mechanism for the promotion of quercetin glycoside absorption by megalo α-1,6-glucosaccharide in the rat small intestine. Food Chem. 2013, 136, 293–296. [Google Scholar] [CrossRef] [Green Version]
- You, S.; Ma, Y.; Yan, B.; Pei, W.; Wu, Q.; Ding, C.; Huang, C. The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 2022, 9, 2223. [Google Scholar] [CrossRef]
- Hu, Y.; Ketabi, A.; Buchko, A.; Gänzle, M.G. Metabolism of isomalto-oligosaccharides by Lactobacillus reuteri and Bifidobacteria. Lett. Appl. Microbiol. 2013, 57, 108–114. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Mennia, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [Green Version]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two routes of metabolic cross-feeding between Bifdobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol. 2006, 72, 3593–3599. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Fan, X.; Lu, Y.; Chen, D.; Zhao, Y.; Qi, K. Dietary acetic acid suppress high-fat diet-induced obesity in mice by altering taurine conjugated bile acids metabolism. Curr. Res. Food Sci. 2022, 5, 1976–1984. [Google Scholar] [CrossRef]
- Kumar, J.; Rani, K.; Datt, C. Molecular link between dietary fbre, gut microbiota and health. Mol. Biol. Rep. 2020, 47, 6229–6237. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef]
- Perrin, P.; Pierre, F.; Patry, Y.; Champ, M.; Berreur, M.; Pradal, G.; Bornet, F.; Meflah, K.; Menanteau, J. Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats. Gut 2001, 48, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, H.; Dirven, H.A.A.M.; Grant, D.; Johnsen, H.; Midtvedt, T. Etiology of cecal and hepatic lesions in mice after administration of gas-carrier contrast agents used in ultrasound imaging. Toxicol. Appl. Pharmacol. 2003, 188, 176–184. [Google Scholar] [CrossRef]






| Treatments | Time (min) | Reducing Sugar Content (mg/mL) | Hydrolysis Degree (HD, %) |
|---|---|---|---|
| Oral phase | 0 | 1.40 ± 0.02 a | NA |
| 5 | 1.42 ± 0.06 a | 0.22 ± 0.02 a | |
| 10 | 1.42 ± 0.08 a | 0.21 ± 0.05 a | |
| Gastric phase | 0 | 0.71 ± 0.01 a | NA |
| 30 | 0.71 ± 0.02 a | 0.26 ± 0.07 a | |
| Intestinal phase | 0 | 0.34 ± 0.01 a | NA |
| 30 | 0.36 ± 0.05 ab | 1.06 ± 0.23 a | |
| 60 | 0.37 ± 0.03 ab | 1.91 ± 0.10 ab | |
| 90 | 0.38 ± 0.06 b | 2.21 ± 0.17 b | |
| 120 | 0.38 ± 0.02 b | 2.38 ± 0.21 b | |
| 180 | 0.38 ± 0.04 b | 2.45 ± 0.28 b | |
| 240 | 0.38 ± 0.11 b | 2.45 ± 0.35 b |
| Bacterium | 0 h | 24 h | |||
|---|---|---|---|---|---|
| No Sugar | 2% (w/v) Glucose | 2% (w/v) FOSs | 2% (w/v) IMOs | ||
| B. longum | 7.79 ± 0.02 a | 7.81 ± 0.03 a | 10.34 ± 0.05 d | 8.98 ± 0.03 b | 9.41 ± 0.03 c |
| L. plantarum | 7.86 ± 0.02 a | 7.91 ± 0.01 a | 10.38 ± 0.04 d | 9.77 ± 0.02 c | 9.28 ± 0.03 b |
| E. coli | 7.61 ± 0.02 a | 7.62 ± 0.08 a | 9.51 ± 0.03 b | 7.62 ± 0.05 a | 7.58 ± 0.03 a |
| S. enterica | 7.44 ± 0.04 a | 7.50 ± 0.04 a | 9.79 ± 0.08 b | 7.42 ± 0.31 a | 7.39 ± 0.01 a |
| Bacterium | SCFAs (mM) | No Sugar | 2% (w/v) FOSs | 2% (w/v) IMOs |
|---|---|---|---|---|
| B. longum | Lactic acid | 0.15 ± 0.01 a | 2.63 ± 0.02 c | 2.56 ± 0.01 b |
| Acetic acid | 0.07 ± 0.01 a | 3.53 ± 0.18 b | 6.55 ± 0.21 c | |
| Propionic acid | 0.14 ± 0.04 a | 0.55 ± 0.04 b | 1.11 ± 0.08 c | |
| Butyric acid | 0.06 ± 0.01 a | 0.21 ± 0.02 b | 0.40 ± 0.03 c | |
| L. plantarum | Lactic acid | 0.20 ± 0.01 a | 13.06 ± 0.02 c | 9.09 ± 0.02 b |
| Acetic acid | 0.07 ± 0.01 a | 1.13 ± 0.07 b | 2.65 ± 0.05 c | |
| Propionic acid | 0.15 ± 0.01 a | 0.55 ± 0.03 b | 0.89 ± 0.03 c | |
| Butyric acid | 0.06 ± 0.01 a | 0.14 ± 0.02 b | 0.33 ± 0.02 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiangpook, S.; Nhim, S.; Prangthip, P.; Pason, P.; Tachaapaikoon, C.; Ratanakhanokchai, K.; Waeonukul, R. Production of a Series of Long-Chain Isomaltooligosaccharides from Maltose by Bacillus subtilis AP-1 and Associated Prebiotic Properties. Foods 2023, 12, 1499. https://doi.org/10.3390/foods12071499
Tiangpook S, Nhim S, Prangthip P, Pason P, Tachaapaikoon C, Ratanakhanokchai K, Waeonukul R. Production of a Series of Long-Chain Isomaltooligosaccharides from Maltose by Bacillus subtilis AP-1 and Associated Prebiotic Properties. Foods. 2023; 12(7):1499. https://doi.org/10.3390/foods12071499
Chicago/Turabian StyleTiangpook, Suratsawadee, Sreyneang Nhim, Pattaneeya Prangthip, Patthra Pason, Chakrit Tachaapaikoon, Khanok Ratanakhanokchai, and Rattiya Waeonukul. 2023. "Production of a Series of Long-Chain Isomaltooligosaccharides from Maltose by Bacillus subtilis AP-1 and Associated Prebiotic Properties" Foods 12, no. 7: 1499. https://doi.org/10.3390/foods12071499
APA StyleTiangpook, S., Nhim, S., Prangthip, P., Pason, P., Tachaapaikoon, C., Ratanakhanokchai, K., & Waeonukul, R. (2023). Production of a Series of Long-Chain Isomaltooligosaccharides from Maltose by Bacillus subtilis AP-1 and Associated Prebiotic Properties. Foods, 12(7), 1499. https://doi.org/10.3390/foods12071499