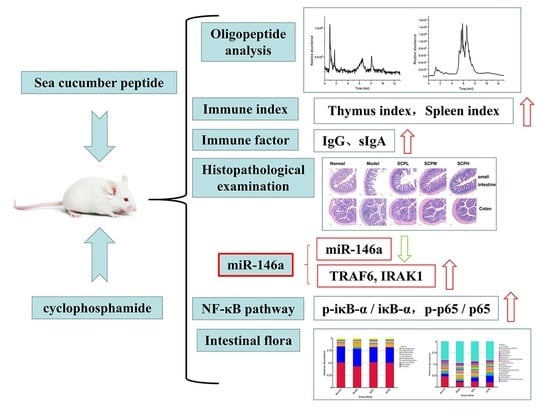
Sea Cucumber Hydrolysate Alleviates Immunosuppression and Gut Microbiota Imbalance Induced by Cyclophosphamide in Balb/c Mice through the NF-κB Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of SCH
2.3. Determination of the Basic Constituents of SCH
2.4. Determination of the Amino Acid Composition of SCH
2.5. Identification of Oligopeptides in SCH by LC-MS
2.6. Animals and Experimental Design
2.7. Immune Organ Index
2.8. Detection of Biochemical Indexes
2.9. Histopathological Examination
2.10. Western Blotting
2.11. Gut Microbiota Analysis
2.12. Statistical Analysis
3. Results
3.1. Amino Acid Composition and Oligopeptide Sequence Analysis of SCH
3.2. Effect of SCH on the Immune Organ Index in Mice
3.3. Effects of SCH on the Biochemical Indices in Mice
3.4. Effect of SCH on Histological Changes of the Small Intestine and Colon
3.5. Effect of SCH on the Expression of NF-κB Pathway Proteins in Mice
3.6. Effect of SCH on the Mice Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.C.; Neumann, M.; Desai, M.S. Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat. Rev. Microbiol. 2018, 16, 457–470. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Nunez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Sorbara, M.T.; Kim, S.G.; Littmann, E.L.; Dong, Q.; Walsh, G.; Wright, R.; Amoretti, L.; Fontana, E.; Hohl, T.M.; et al. Rapid transcriptional and metabolic adaptation of intestinal microbes to host immune activation. Cell Host Microbe 2021, 29, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, T.W. Role of the microbiota in immunity and inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef] [Green Version]
- Madondo, M.T.; Quinn, M.; Plebanski, M. Low dose cyclophosphamide: Mechanisms of T cell modulation. Cancer Treat. Rev. 2016, 42, 3–9. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharm. 2016, 78, 661–671. [Google Scholar] [CrossRef]
- Sistigu, A.; Viaud, S.; Chaput, N.; Bracci, L.; Proietti, E.; Zitvogel, L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin. Immunopathol. 2011, 33, 369–383. [Google Scholar] [CrossRef]
- Chen, D.; Chen, G.; Ding, Y.; Wan, P.; Peng, Y.; Chen, C.; Ye, H.; Zeng, X.; Ran, L. Polysaccharides from the flowers of tea (Camellia sinensis L.) modulate gut health and ameliorate cyclophosphamide-induced immunosuppression. J. Funct. Foods 2019, 61, 103470. [Google Scholar] [CrossRef]
- Han, H.S.; Shin, J.S.; Song, Y.R.; Rhee, Y.K.; Cho, C.W.; Ryu, J.H.; Inn, K.S.; Hong, H.D.; Lee, K.T. Immunostimulatory effects of polysaccharides isolated from young barley leaves (Hordeum vulgare L.) with dual activation of Th1 and Th2 in splenic T cells and cyclophosphamide-induced immunosuppressed mice. Int. J. Biol. Macromol. 2020, 147, 954–964. [Google Scholar] [CrossRef]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillere, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Huang, J.; Li, Y.; Wang, Y.; Wang, F.; Qiu, X.; Liu, X.; Li, H. Sodium Alginate Modulates Immunity, Intestinal Mucosal Barrier Function, and Gut Microbiota in Cyclophosphamide-Induced Immunosuppressed BALB/c Mice. J. Agric. Food Chem. 2021, 69, 7064–7073. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Q.; Zheng, L.; Zhao, M.; Fan, J.; Liu, S. Preparation of sea cucumber (Stichopus variegates) peptide fraction with desired organoleptic property and its anti-aging activity in fruit flies and D-galactose-induced aging mice. J. Funct. Foods 2020, 69, 103954. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, L.; Zhao, T.; Zhang, Q.; Liu, Z.; Liu, X.; Zhao, M. Anti-diabetic effects of sea cucumber (Holothuria nobilis) hydrolysates in streptozotocin and high-fat-diet induced diabetic rats via activating the PI3K/Akt pathway. J. Funct. Foods 2020, 75, 104224. [Google Scholar] [CrossRef]
- Xu, X.; Liang, R.; Li, D.; Jiang, C.; Lin, S. Evaluation of sea cucumber peptides-assisted memory activity and acetylation modification in hippocampus of test mice based on scopolamine-induced experimental animal model of memory disorder. J. Funct. Foods 2020, 68, 103909. [Google Scholar] [CrossRef]
- Cai, N.; Luo, W.; Yao, L.; Li, X.; Wang, Z.; Xu, H.; Li, H.; Hu, Z.; Bao, W.; Xu, X. Activation of murine RAW264.7 macrophages by oligopeptides from sea cucumber (Apostichopus japonicus) and its molecular mechanisms. J. Funct. Foods 2020, 75, 104229. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, L.; Xu, J.; Zeng, M.; Zhao, Y. Amino acid composition and digestibility of Pacific oyster (Crassostrea gigas) proteins isolated from different parts. Lwt 2019, 116, 108591. [Google Scholar] [CrossRef]
- Zhao, F.; Ye, N.; Qiu, X.; Qian, J.; Wang, D.; Yue, W.; Zuo, Z.; Chen, M. Identification and comparison of oligopeptides during withering process of White tea by ultra-high pressure liquid chromatography coupled with quadrupole-orbitrap ultra-high resolution mass spectrometry. Food Res. Int. 2019, 121, 825–834. [Google Scholar] [CrossRef]
- Wen, J.; Hu, C.; Fan, S. Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agric. 2010, 90, 2469–2474. [Google Scholar] [CrossRef]
- Lu, Z.; Sun, N.; Dong, L.; Gao, Y.; Lin, S. Production of Bioactive Peptides from Sea Cucumber and Its Potential Health Benefits: A Comprehensive Review. J. Agric. Food Chem. 2022, 70, 7607–7625. [Google Scholar] [CrossRef]
- Zhao, S.; Peng, X.; Zhou, Q.Y.; Huang, Y.Y.; Rao, X.; Tu, J.L.; Xiao, H.Y.; Liu, D.M. Bacillus coagulans 13002 and fructo-oligosaccharides improve the immunity of mice with immunosuppression induced by cyclophosphamide through modulating intestinal-derived and fecal microbiota. Food Res. Int. 2021, 140, 109793. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Chen, D.; Ran, L.; Mi, J.; Lu, L.; Jing, B.; Li, X.; Zeng, X.; Cao, Y. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019, 10, 3671–3683. [Google Scholar] [CrossRef]
- Liu, D.; Ge, L.; Wang, Q.; Su, J.; Chen, X.; Wang, C.; Huang, K. Low-level contamination of deoxynivalenol: A threat from environmental toxins to porcine epidemic diarrhea virus infection. Environ. Int. 2020, 143, 105949. [Google Scholar] [CrossRef]
- Zinatizadeh, M.R.; Schock, B.; Chalbatani, G.M.; Zarandi, P.K.; Jalali, S.A.; Miri, S.R. The Nuclear Factor Kappa B (NF-kB) signaling in cancer development and immune diseases. Genes Dis. 2021, 8, 287–297. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Zhang, L.; van Dam, H.; ten Dijke, P. UBE2O negatively regulates TRAF6-mediated NF-kappaB activation by inhibiting TRAF6 polyubiquitination. Cell Res. 2013, 23, 366–377. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Qiao, Q.; Ferrao, R.; Shen, C.; Hatcher, J.M.; Buhrlage, S.J.; Gray, N.S.; Wu, H. Crystal structure of human IRAK1. Proc. Natl. Acad. Sci. USA 2017, 114, 13507–13512. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Li, C.; Zhao, F.; Cao, J.; Zhang, X.; Shen, X. Effects of co-fermented collagen peptide-jackfruit juice on the immune response and gut microbiota in immunosuppressed mice. Food Chem. 2021, 365, 130487. [Google Scholar] [CrossRef]
- Kolodziejczyk, A.A.; Zheng, D.; Elinav, E. Diet-microbiota interactions and personalized nutrition. Nat. Rev. Microbiol. 2019, 17, 742–753. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, J.; Zhang, M.; Chen, Z.; Ma, Q.; Liu, H.; Nie, C.; Zhang, Z.; An, W.; Li, J. Lycium ruthenicum Anthocyanins Attenuate High-Fat Diet-Induced Colonic Barrier Dysfunction and Inflammation in Mice by Modulating the Gut Microbiota. Mol. Nutr. Food Res. 2021, 65, 2000745. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-H.; Wang, J.; Zhang, C.-Y.; Zhao, L.; Sheng, Y.-Y.; Tao, G.-S.; Xue, Y.-Z. Gut microbial characteristical comparison reveals potential anti-aging function of Dubosiella newyorkensis in mice. Front. Endocrinol. 2023, 14, 1133167. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Yang, Y.; Wang, H.; Liu, N.; Yang, Z.; Li, S. Unsaturated alginate oligosaccharides (UAOS) protects against dextran sulfate sodium-induced colitis associated with regulation of gut microbiota. J. Funct. Foods 2021, 83, 104536. [Google Scholar] [CrossRef]
- Wang, D.; Cai, M.; Wang, T.; Liu, T.; Huang, J.; Wang, Y.; Granato, D. Ameliorative effects of L-theanine on dextran sulfate sodium induced colitis in C57BL/6J mice are associated with the inhibition of inflammatory responses and attenuation of intestinal barrier disruption. Food Res. Int. 2020, 137, 109409. [Google Scholar] [CrossRef]
- Hu, S.; Li, S.; Liu, Y.; Sun, K.; Luo, L.; Zeng, L. Aged Ripe Pu-erh Tea Reduced Oxidative Stress-Mediated Inflammation in Dextran Sulfate Sodium-Induced Colitis Mice by Regulating Intestinal Microbes. J. Agric. Food Chem. 2021, 69, 10592–10605. [Google Scholar] [CrossRef]
- Guerrero-Sanchez, M.; Passot, S.; Campoy, S.; Olivares, M.; Fonseca, F. Ligilactobacillus salivarius functionalities, applications, and manufacturing challenges. Appl. Microbiol. Biotechnol. 2022, 106, 57–80. [Google Scholar] [CrossRef]
- Wang, K.; Jin, X.; Li, Q.; Sawaya, A.; Le Leu, R.K.; Conlon, M.A.; Wu, L.; Hu, F. Propolis from Different Geographic Origins Decreases Intestinal Inflammation and Bacteroides spp. Populations in a Model of DSS-Induced Colitis. Mol. Nutr. Food Res. 2018, 62, 1800080. [Google Scholar] [CrossRef]
- Fan, S.; Raychaudhuri, S.; Page, R.; Shahinozzaman, M.; Obanda, D.N. Metagenomic insights into the effects of Urtica dioica vegetable on the gut microbiota of C57BL/6J obese mice, particularly the composition of Clostridia. J. Nutr. Biochem. 2021, 91, 108594. [Google Scholar] [CrossRef]
- Wan, F.; Zhong, R.; Wang, M.; Zhou, Y.; Chen, Y.; Yi, B.; Hou, F.; Liu, L.; Zhao, Y.; Chen, L.; et al. Caffeic Acid Supplement Alleviates Colonic Inflammation and Oxidative Stress Potentially Through Improved Gut Microbiota Community in Mice. Front. Microbiol. 2021, 12, 784211. [Google Scholar] [CrossRef]








| Amino Acid | Content (%) |
|---|---|
| Gly | 7.77 ± 0.07 |
| Glu | 6.49 ± 0.19 |
| Ala | 3.81 ± 0.07 |
| Pro | 3.41 ± 0.07 |
| Asp | 3.25 ± 0.10 |
| Arg | 2.92 ± 0.05 |
| Thr | 1.67 ± 0.04 |
| Val | 1.58 ± 0.04 |
| Phe | 1.47 ± 0.15 |
| Ser | 1.47 ± 0.06 |
| Leu | 1.34 ± 0.10 |
| Lys | 1.04 ± 0.19 |
| Tyr | 0.86 ± 0.07 |
| His | 0.64 ± 0.22 |
| Ile | 0.62 ± 0.03 |
| Met | 0.58 ± 0.02 |
| Number | Observed Mass (m/z) | Calculated Mass (m/z) | Charges | Mass Error | RT (min) | Intensity | Activity Prediction Score | Sequence |
|---|---|---|---|---|---|---|---|---|
| 1 | 297.12674 | 296.11946 | 1 | −2.0494 | 6.77 | 1,906,100 | 1.00 | MF |
| 2 | 223.10772 | 222.10044 | 1 | −1.7651 | 3.65 | 72,543,000 | 0.99 | GF |
| 3 | 547.27758 | 546.2703 | 1 | 3.2459 | 6.04 | 96,065 | 0.99 | WRW |
| 4 | 161.59732 | 321.18009 | 1;2 | −0.9894 | 1.49 | 27,211,000 | 0.99 | FR |
| 5 | 181.10277 | 360.19099 | 2 | −1.2965 | 2.09 | 1,190,500 | 0.98 | WR |
| 6 | 539.23948 | 538.2322 | 1 | 1.6862 | 1.32 | 144,310 | 0.97 | GFRC |
| 7 | 207.07979 | 206.07251 | 1 | −3.0502 | 1.52 | 23,017,000 | 0.95 | GM |
| 8 | 279.17032 | 278.16304 | 1 | −1.1567 | 7.26 | 12,614,000 | 0.95 | IF |
| 9 | 294.14483 | 293.13756 | 1 | −1.9427 | 2.32 | 2,390,400 | 0.92 | FQ |
| 10 | 173.09207 | 172.08479 | 1 | −1.468 | 1.13 | 8,256,900 | 0.91 | GP |
| 11 | 165.10023 | 328.1859 | 2 | −1.0154 | 1.01 | 3,343,200 | 0.84 | PGR |
| 12 | 548.27218 | 547.2649 | 1 | −1.3542 | 1.97 | 18,687 | 0.82 | GCRR |
| 13 | 232.14042 | 231.13314 | 1 | −1.7049 | 1.01 | 51,228,000 | 0.77 | GR |
| 14 | 239.10263 | 238.09536 | 1 | −1.3903 | 2.08 | 17,041,000 | 0.74 | GY |
| 15 | 279.13393 | 278.12666 | 1 | 2.1445 | 2.52 | 1,674,600 | 0.74 | PY |
| 16 | 505.33845 | 504.33117 | 1 | −0.073093 | 6.8 | 4,023,000 | 0.70 | IIIF |
| 17 | 286.17613 | 285.16886 | 1 | −2.1445 | 5.87 | 7,544,800 | 0.69 | IGP |
| 18 | 194.6244 | 387.23425 | 2 | −1.0358 | 1.01 | 6,610,400 | 0.64 | RGR |
| 19 | 237.09035 | 236.08308 | 1 | −1.4734 | 1.46 | 9,146,500 | 0.63 | SM |
| 20 | 180.10551 | 358.19647 | 2 | −1.6217 | 1.11 | 11,547,000 | 0.62 | SPR |
| 21 | 295.12885 | 294.12157 | 1 | −4.0948 | 3.16 | 3,685,100 | 0.59 | FE |
| 22 | 173.11588 | 344.2172 | 2 | −0.90103 | 1.34 | 12,308,000 | 0.57 | GIR |
| 23 | 166.11367 | 330.21279 | 2 | 0.50389 | 1.01 | 2,633,900 | 0.57 | RR |
| 24 | 152.0924 | 302.17025 | 2 | −1.5916 | 1.12 | 77,656,000 | 0.55 | GAR |
| 25 | 519.22316 | 518.21588 | 1 | 2.0725 | 5.12 | 352,480 | 0.54 | NCPK |
| 26 | 189.12337 | 188.11609 | 1 | −1.1305 | 1.37 | 45,416,000 | 0.50 | IG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.; Li, S.; Fu, R.; Wang, Y.; Meng, J.; Jin, Y.; Wu, T.; Zhang, M. Sea Cucumber Hydrolysate Alleviates Immunosuppression and Gut Microbiota Imbalance Induced by Cyclophosphamide in Balb/c Mice through the NF-κB Pathway. Foods 2023, 12, 1604. https://doi.org/10.3390/foods12081604
Mao J, Li S, Fu R, Wang Y, Meng J, Jin Y, Wu T, Zhang M. Sea Cucumber Hydrolysate Alleviates Immunosuppression and Gut Microbiota Imbalance Induced by Cyclophosphamide in Balb/c Mice through the NF-κB Pathway. Foods. 2023; 12(8):1604. https://doi.org/10.3390/foods12081604
Chicago/Turabian StyleMao, Jing, Shunqin Li, RongRong Fu, Yijin Wang, Jing Meng, Yan Jin, Tao Wu, and Min Zhang. 2023. "Sea Cucumber Hydrolysate Alleviates Immunosuppression and Gut Microbiota Imbalance Induced by Cyclophosphamide in Balb/c Mice through the NF-κB Pathway" Foods 12, no. 8: 1604. https://doi.org/10.3390/foods12081604
APA StyleMao, J., Li, S., Fu, R., Wang, Y., Meng, J., Jin, Y., Wu, T., & Zhang, M. (2023). Sea Cucumber Hydrolysate Alleviates Immunosuppression and Gut Microbiota Imbalance Induced by Cyclophosphamide in Balb/c Mice through the NF-κB Pathway. Foods, 12(8), 1604. https://doi.org/10.3390/foods12081604