Current Innovations in the Development of Functional Gummy Candies
Abstract
:1. Introduction
2. Preparation of GCs Based on Novel Formulations
2.1. Alternative Gelling Agents
2.2. Alternative Sweeteners
2.3. Natural Flavors and Colorants
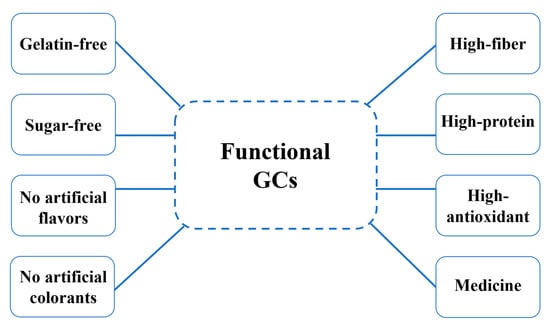
3. Development of GCs as a Functional Food
3.1. High-Fiber GCs
3.2. High-Protein GCs
3.3. High-Antioxidant GCs
3.4. GCs As a Medicine
4. A Summary of Advantages and Disadvantages of Novel Functional GCs
5. Conclusions
Funding
Conflicts of Interest
References
- Khan, R.S.; Grigor, J.; Winger, R.; Win, A. Functional food product development—Opportunities and challenges for food manufacturers. Trends Food Sci. Technol. 2013, 30, 27–37. [Google Scholar] [CrossRef]
- Siro, I.; Kápolna, E.; Kápolna, B.; Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008, 51, 456–467. [Google Scholar] [CrossRef]
- Baker, M.T.; Lu, P.; Parrella, J.A.; Leggette, H.R. Consumer acceptance toward functional foods: A scoping review. Int. J. Environ. Res. Public Health 2022, 19, 1217. [Google Scholar] [CrossRef] [PubMed]
- GrandViewResearch. Confectionery Market Size, Share & Trends Analysis Report by Product (Chocolate, Sugar Confectionery, Cookies, Ice Cream), by Distribution Channel (Offline, Online), by Region, And Segment Forecasts, 2022–2028. 2022. Available online: https://www.grandviewresearch.com/industry-analysis/confectionery-market# (accessed on 16 May 2022).
- Gunes, R.; Palabiyik, I.; Konar, N.; Toker, O.S. Soft confectionery products: Quality parameters, interactions with processing and ingredients. Food Chem. 2022, 385, 132735. [Google Scholar] [CrossRef] [PubMed]
- GrandViewResearch. Gummy Market Size, Share & Trends Analysis Report by Product (Vitamins, Minerals, Dietary Fibers), by Ingredient (Gelatin, Plant-Based Gelatin Substitutes), by End-Use (Adults, Kids), by Distribution Channel, by Region, And Segment Forecasts, 2023–2030. 2022. Available online: https://www.grandviewresearch.com/industry-analysis/gummy-market-report# (accessed on 17 May 2022).
- Moghaddas Kia, E.; Ghaderzadeh, S.L.; Langroodi, A.M.; Ghasempour, Z.; Ehsani, A. Red beet extract usage in gelatin/gellan based gummy candy formulation introducing Salix aegyptiaca distillate as a flavouring agent. J. Food Sci. Technol. 2020, 57, 3355–3362. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.; Bañón, S. Determining the minimum drying time of gummy confections based on their mechanical properties. CyTA J. Food 2015, 13, 329–335. [Google Scholar] [CrossRef]
- Tarahi, M.; Fakhr-Davood, M.M.; Ghaedrahmati, S.; Roshanak, S.; Shahidi, F. Physicochemical and Sensory Properties of Vegan Gummy Candies Enriched with High-Fiber Jaban Watermelon Exocarp Powder. Foods 2023, 12, 1478. [Google Scholar] [CrossRef]
- Periche, A.; Heredia, A.; Escriche, I.; Andrés, A.; Castelló, M. Optical, mechanical and sensory properties of based-isomaltulose gummy confections. Food Biosci. 2014, 7, 37–44. [Google Scholar] [CrossRef]
- Gan, D.; Xu, M.; Chen, L.; Cui, S.; Deng, C.; Qiao, Q.; Guan, R.; Zhong, F. Intake of Sugar Substitute Gummy Candies Benefits the Glycemic Response in Healthy Adults: A Prospective Crossover Clinical Trial. Gels 2022, 8, 642. [Google Scholar] [CrossRef]
- Mandura, A.; Šeremet, D.; Ščetar, M.; Cebin, A.V.; Belščak-Cvitanović, A.; Komes, D. Physico-chemical, bioactive, and sensory assessment of white tea-based candies during 4-months storage. J. Food Process. Preserv. 2020, 44, e14628. [Google Scholar] [CrossRef]
- Pegg, A. The application of natural hydrocolloids to foods and beverages. In Natural Food Additives, Ingredients and Flavourings; Elsevier: Amsterdam, The Netherlands, 2012; pp. 175–196. [Google Scholar]
- DeMars, L.L.; Ziegler, G.R. Texture and structure of gelatin/pectin-based gummy confections. Food Hydrocoll. 2001, 15, 643–653. [Google Scholar] [CrossRef]
- Renaldi, G.; Junsara, K.; Jannu, T.; Sirinupong, N.; Samakradhamrongthai, R.S. Physicochemical, textural, and sensory qualities of pectin/gelatin gummy jelly incorporated with Garcinia atroviridis and its consumer acceptability. Int. J. Gastron. Food Sci. 2022, 28, 100505. [Google Scholar] [CrossRef]
- Tahmouzi, S.; Meftahizadeh, H.; Eyshi, S.; Mahmoudzadeh, A.; Alizadeh, B.; Mollakhalili-Meybodi, N.; Hatami, M. Application of guar (Cyamopsis tetragonoloba L.) gum in food technologies: A review of properties and mechanisms of action. Food Sci. Nutr. 2023, 11, 4869–4897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, F.; Yuan, R. Applications of natural polymer-based hydrogels in the food industry. In Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 357–410. [Google Scholar]
- Xu, X.; Ye, S.; Zuo, X.; Fang, S. Impact of Guar Gum and Locust Bean Gum Addition on the Pasting, Rheological Properties, and Freeze–Thaw Stability of Rice Starch Gel. Foods 2022, 11, 2508. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Kumar, R.; Sudhakar, V.; Sairagul, G.; Manoj, J.J.B. Studies on the Consistency of Jaggery-Based Hard-Boiled Candy by Incorporating Thickening and Gelling Agents. Sugar Tech 2022, 24, 1617–1623. [Google Scholar] [CrossRef]
- Matos, Â.P.; Novelli, E.; Tribuzi, G. Algae as food and ingredient: From production to consumer acceptance. Front. Food Sci. Technol. 2023, 3, 1220050. [Google Scholar] [CrossRef]
- Ge, H.; Wu, Y.; Woshnak, L.L.; Mitmesser, S.H. Effects of hydrocolloids, acids and nutrients on gelatin network in gummies. Food Hydrocoll. 2021, 113, 106549. [Google Scholar] [CrossRef]
- Song, X.; Chiou, B.S.; Xia, Y.; Chen, M.; Liu, F.; Zhong, F. The improvement of texture properties and storage stability for kappa carrageenan in developing vegan gummy candies. J. Sci. Food Agric. 2022, 102, 3693–3702. [Google Scholar] [CrossRef]
- Hamka, N. Pengaruh Sifat Kimia dan Organoleptik Permen Jelly Buah Naga (Hylocereus polyrhizus) dengan Penambahan Karagenan Sebagai Gelling Agent. Bul. Loupe 2020, 16, 8–13. [Google Scholar]
- Ali, M.R.; Mohamed, R.M.; Abedelmaksoud, T.G. Functional strawberry and red beetroot jelly candies rich in fibers and phenolic compounds. Food Syst. 2021, 4, 82–88. [Google Scholar] [CrossRef]
- de Avelar, M.H.M.; Efraim, P. Alginate/pectin cold-set gelation as a potential sustainable method for jelly candy production. LWT 2020, 123, 109119. [Google Scholar] [CrossRef]
- Tarahi, M.; Shahidi, F.; Hedayati, S. A Novel starch from bitter vetch (Vicia ervilia) seeds: A comparison of its physicochemical, structural, thermal, rheological and pasting properties with conventional starches. Int. J. Food Sci. Technol. 2022, 57, 6833–6842. [Google Scholar] [CrossRef]
- Tarahi, M.; Shahidi, F.; Hedayati, S. Physicochemical, pasting, and thermal properties of native corn starch–mung bean protein isolate composites. Gels 2022, 8, 693. [Google Scholar] [CrossRef] [PubMed]
- Marfil, P.H.; Anhê, A.C.; Telis, V.R. Texture and microstructure of gelatin/corn starch-based gummy confections. Food Biophys. 2012, 7, 236–243. [Google Scholar] [CrossRef]
- Pereira, D.G.; de Toledo Benassi, M.; Beleia, A.D.P. Gummy candies produced with acid-thinned Cassava starch: Physical and sensory evaluation. J. Food Process. Preserv. 2022, 46, e16661. [Google Scholar] [CrossRef]
- Peteliuk, V.; Rybchuk, L.; Bayliak, M.; Storey, K.B.; Lushchak, O. Natural sweetener Stevia rebaudiana: Functionalities, health benefits and potential risks. EXCLI J. 2021, 20, 1412. [Google Scholar] [PubMed]
- Li, Z.; An, L.; Zhang, S.; Shi, Z.; Bao, J.; Tuerhong, M.; Abudukeremu, M.; Xu, J.; Guo, Y. Structural elucidation and immunomodulatory evaluation of a polysaccharide from Stevia rebaudiana leaves. Food Chem. 2021, 364, 130310. [Google Scholar] [CrossRef]
- Rivero, R.; Archaina, D.; Sosa, N.; Schebor, C. Sensory characterization, acceptance, and stability studies on low calories fruit jelly candies. J. Food Sci. Technol. 2023, 60, 2204–2212. [Google Scholar] [CrossRef]
- Aranda-Gonzalez, I.; Tamayo-Dzul, O.; Barbosa-Martin, E.; Segura-Campos, M.; Moguel-Ordonez, Y.; Betancur-Ancona, D. Development of a gummy candy reduced in calories by sugar substitution with Stevia rebaudiana B. Nutr. Hosp. 2014, 31, 334–340. [Google Scholar]
- Samakradhamrongthai, R.S.; Jannu, T. Effect of stevia, xylitol, and corn syrup in the development of velvet tamarind (Dialium indum L.) chewy candy. Food Chem. 2021, 352, 129353. [Google Scholar] [CrossRef]
- Le, H.; Wang, X.; Wei, Y.; Zhao, Y.; Zhang, J.; Zhang, L. Making Polyol Gummies by 3D Printing: Effect of Polyols on 3D Printing Characteristics. Foods 2022, 11, 874. [Google Scholar] [CrossRef] [PubMed]
- Wölnerhanssen, B.K.; Meyer-Gerspach, A.C.; Beglinger, C.; Islam, M.S. Metabolic effects of the natural sweeteners xylitol and erythritol: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1986–1998. [Google Scholar] [CrossRef] [PubMed]
- Gasmi Benahmed, A.; Gasmi, A.; Arshad, M.; Shanaida, M.; Lysiuk, R.; Peana, M.; Pshyk-Titko, I.; Adamiv, S.; Shanaida, Y.; Bjørklund, G. Health benefits of xylitol. Appl. Microbiol. Biotechnol. 2020, 104, 7225–7237. [Google Scholar] [CrossRef] [PubMed]
- Čižauskaitė, U.; Jakubaitytė, G.; Žitkevičius, V.; Kasparavičienė, G. Natural ingredients-based gummy bear composition designed according to texture analysis and sensory evaluation in vivo. Molecules 2019, 24, 1442. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, V.; Macho, M.; Ewe, D.; Singh, M.; Saha, S.; Saurav, K. Biological and pharmacological potential of xylitol: A molecular insight of unique metabolism. Foods 2020, 9, 1592. [Google Scholar] [CrossRef] [PubMed]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Bakar, M.F.A. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.; Archaina, D.; Sosa, N.; Leiva, G.; Coronel, B.B.; Schebor, C. Development of healthy gummy jellies containing honey and propolis. J. Sci. Food Agric. 2020, 100, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Kamran, H.; Khalid, S.; Jabeen, S.; Aslam, M. Glycemic response of natural sweeteners like sugarcane juice, honey and jaggery in healthy individuals. EAS J. Humanit. Cult. Stud. 2020, 2, 279–281. [Google Scholar] [CrossRef]
- Gok, S.; Toker, O.S.; Palabiyik, I.; Konar, N. Usage possibility of mannitol and soluble wheat fiber in low calorie gummy candies. LWT 2020, 128, 109531. [Google Scholar] [CrossRef]
- Jiamjariyatam, R. Influence of gelatin and isomaltulose on gummy jelly properties. Int. Food Res. J. 2018, 25, 776–783. [Google Scholar]
- Ünal, M.H.; Arslan, D. Single and combined use of isomalt, polydextrose, and inulin as sugar substitutes in production of pectin jelly. J. Food Process. Preserv. 2022, 46, e17174. [Google Scholar] [CrossRef]
- Kurt, A.; Bursa, K.; Toker, O.S. Gummy candies production with natural sugar source: Effect of molasses types and gelatin ratios. Food Sci. Technol. Int. 2022, 28, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Korem, T.; Zeevi, D.; Zilberman-Schapira, G.; Thaiss, C.A.; Maza, O.; Israeli, D.; Zmora, N.; Gilad, S.; Weinberger, A. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014, 514, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Otálora, M.C.; de Jesús Barbosa, H.; Perilla, J.E.; Osorio, C.; Nazareno, M.A. Encapsulated betalains (Opuntia ficus-indica) as natural colorants. Case study: Gummy candies. LWT 2019, 103, 222–227. [Google Scholar] [CrossRef]
- Bagautdinov, I.; Gusev, A.; Nigmatzyanov, A.; Chernenkov, E.; Kaluzhina, O. Development of Confectionery Products with Functional Properties Using Non-Traditional Plant Raw Materials. J. Culin. Sci. Technol. 2023, 1–20. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Oliveira, M.B.P.; Barros, L.; Ferreira, I.C. Could fruits be a reliable source of food colorants? Pros and cons of these natural additives. Crit. Rev. Food Sci. Nutr. 2021, 61, 805–835. [Google Scholar] [CrossRef]
- Lonez, H.; Banwa, T. Butterfly Pea (Clitoria ternatea): A natural colorant for soft candy (Gummy Candy). Indian. J. Sci. Technol. 2021, 14, 239–244. [Google Scholar] [CrossRef]
- Casas-Forero, N.; Trujillo-Mayol, I.; Zúñiga, R.N.; Petzold, G.; Orellana-Palma, P. Effects of cryoconcentrated blueberry juice as functional ingredient for preparation of commercial confectionary hydrogels. Gels 2022, 8, 217. [Google Scholar] [CrossRef]
- de Oliveira Nishiyama-Hortense, Y.P.; de Paula Rossi, M.J.; Shimizu-Marin, V.D.; Janzantti, N.S.; Gómez-Alonso, S.; Da-Silva, R.; Lago-Vanzela, E.S. Jelly candy enriched with BRS Violeta grape juice: Anthocyanin retention and sensory evaluation. Fut. Foods 2022, 6, 100179. [Google Scholar] [CrossRef]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef]
- Ramesh, M.; Muthuraman, A. Flavoring and coloring agents: Health risks and potential problems. In Natural and Artificial Flavoring Agents and Food Dyes; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–28. [Google Scholar]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.; Weickert, M.O. The health benefits of dietary fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Ktenioudaki, A.; Gallagher, E. Recent advances in the development of high-fibre baked products. Trends Food Sci. Technol. 2012, 28, 4–14. [Google Scholar] [CrossRef]
- Cappa, C.; Lavelli, V.; Mariotti, M. Fruit candies enriched with grape skin powders: Physicochemical properties. LWT-Food Sci. Technol. 2015, 62, 569–575. [Google Scholar] [CrossRef]
- Hariadi, H. The influence of carambola starfruit (Averrhoa bilimbi) and Papaya (Carica papaya) on the quality of the organoleptic properties, vitamin C content, and fiber at jelly candies. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Mohammadi, F.; Shiri, A.; Tahmouzi, S.; Mollakhalili-Meybodi, N.; Nematollahi, A. Application of inulin in bread: A review of technological properties and factors affecting its stability. Food Sci. Nutr. 2023, 11, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Delgado, P.; Bañón, S. Effects of replacing starch by inulin on the physicochemical, texture and sensory characteristics of gummy jellies. CyTA J. Food 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Cedeño-Pinos, C.; Martínez-Tomé, M.; Murcia, M.A.; Jordán, M.J.; Bañón, S. Assessment of rosemary (Rosmarinus officinalis L.) extract as antioxidant in jelly candies made with fructan fibres and stevia. Antioxidants 2020, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.C.S.; Muxfeldt, L.; Motta, N.G.; Skonieski, C.; Fagundes, K.R.; Sandri, G.; de Chaves, D.B.; Suthovski, G.; Gallina, A.L.; Araujo, S.M. Gummies candy enriched with Konjac glucomannan reduces hunger intensity and waist circumference of overweight individuals. Int. J. Biol. Macromol. 2023, 226, 72–76. [Google Scholar] [CrossRef]
- Tarahi, M.; Hedayati, S.; Shahidi, F. Effects of mung bean (Vigna radiata) protein isolate on rheological, textural, and structural properties of native corn starch. Polymers 2022, 14, 3012. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Perkowska, K.; Rzymski, P. Food Fortification: What’s in It for the Malnourished World? In Vitamins and Minerals Biofortification of Edible Plants; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 27–44. [Google Scholar]
- Tarahi, M.; Ahmed, J. Recent advances in legume protein-based colloidal systems. Legume Sci. 2023, e185. [Google Scholar] [CrossRef]
- Siegwein, A.M.; Vodovotz, Y.; Fisher, E.L. Concentration of soy protein isolate affects starch-based confections’ texture, sensory, and storage properties. J. Food Sci. 2011, 76, E422–E428. [Google Scholar] [CrossRef]
- Bartkiene, E.; Sakiene, V.; Bartkevics, V.; Wiacek, C.; Rusko, J.; Lele, V.; Ruzauskas, M.; Juodeikiene, G.; Klupsaite, D.; Bernatoniene, J. Nutraceuticals in gummy candies form prepared from lacto-fermented lupine protein concentrates, as high-quality protein source, incorporated with Citrus paradise L. essential oil and xylitol. Int. J. Food Sci. Technol. 2018, 53, 2015–2025. [Google Scholar] [CrossRef]
- Paternina, L.P.R.; Moraes, L.; Santos, T.D.; de Morais, M.G.; Costa, J.A.V. Spirulina and açai as innovative ingredients in the development of gummy candies. J. Food Process. Preserv. 2022, 46, e17261. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.; Azeez, R.F.A. A review on natural antioxidants. In Traditional and Complementary Medicine; Intech Open: London, UK, 2019; pp. 1–24. [Google Scholar]
- Pokorný, J.; Schmidt, S. Natural antioxidant functionality during food processing. In Antioxidants in Food: Practical Applications; Woodhead Publishing: Cambridge, UK, 2001; pp. 331–354. [Google Scholar]
- Charoen, R.; Savedboworn, W.; Phuditcharnchnakun, S.; Khuntaweetap, T. Development of antioxidant gummy jelly candy supplemented with Psidium guajava leaf extract. Appl. Sci. Eng. Prog. 2015, 8, 145–151. [Google Scholar] [CrossRef]
- Archaina, D.; Sosa, N.; Rivero, R.; Schebor, C. Freeze-dried candies from blackcurrant (Ribes nigrum L.) and yoghurt. Physicochemical and sensorial characterization. LWT 2019, 100, 444–449. [Google Scholar] [CrossRef]
- Altamash, A.; Peter, E.S.; Nautiyal, H. Studies on the preparation of gummy candy from a blend of pineapple and beetroot juice. Pharma Innov. J. 2022, 11, 1708–1713. [Google Scholar]
- A Abd EL Latif, M.; A Abd El Aziz, H.; A Kamal El Deen, A. Utilization of some natural plants sources in producing new product (gummy jelly candy). Int. J. Fam. Stud. Food Sci. Nutr. Health 2022, 3, 40–63. [Google Scholar] [CrossRef]
- Gramza-Michalowska, A.; Regula, J. Use of tea extracts (Camelia sinensis) in jelly candies as polyphenols sources in human diet. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. S1), 43–46. [Google Scholar]
- Suman, K.M.; Gupta, A.; Vaidya, D.; Ranjan, K. Standardization of formulation for the preparation of ginger supplemented jelly candies. Pharma Innov. J. 2021, 10, 608–613. [Google Scholar]
- Sarabandi, K.; Mohammadi, A. Stabilization of peppermint polyphenols within crystalline sucrose matrix: Fortification of gummy candy as a food model system. J. Food Process. Preserv. 2022, 46, e16720. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cristea, E.; Sturza, R.; Niculaua, M.; Patras, A. Synthetic dye’s substitution with chokeberry extract in jelly candies. J. Food Sci. Technol. 2020, 57, 4383–4394. [Google Scholar] [CrossRef] [PubMed]
- Karaiskou, S.G.; Kouskoura, M.G.; Markopoulou, C.K. Modern pediatric formulations of the soft candies in the form of a jelly: Determination of metoclopramide content and dissolution. Pharm. Dev. Technol. 2020, 25, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Crawford, E.B.; Coco, T.; Gaines, L.; Shah, N.; Slattery, A. Pediatric ingestions with gummy formulated medications: A retrospective study. Clin. Toxicol. 2021, 59, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Bagheri, F.; Mohammadi, G.; Azami, M.; Tahvilian, R. Design and preparation of oral jelly candies of acetaminophen and its nanoparticles. Appl. Nanosci. 2022, 12, 101–107. [Google Scholar] [CrossRef]
- Handayani, N.A.; Krisanti, E.; Kartohardjono, S.; Mulia, K. Effect of iron fortification on gummy candies properties: Basic nutrient, microstructure, and texture during the storage period. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Constantino, A.B.T.; Garcia-Rojas, E.E. Microencapsulation of beta-carotene by complex coacervation using amaranth carboxymethyl starch and lactoferrin for application in gummy candies. Food Hydrocoll. 2023, 139, 108488. [Google Scholar] [CrossRef]
- Lele, V.; Ruzauskas, M.; Zavistanaviciute, P.; Laurusiene, R.; Rimene, G.; Kiudulaite, D.; Tomkeviciute, J.; Nemeikstyte, J.; Stankevicius, R.; Bartkiene, E. Development and characterization of the gummy–supplements, enriched with probiotics and prebiotics. CyTA J. Food 2018, 16, 580–587. [Google Scholar] [CrossRef]
- Bartkiene, E.; Ruzauskas, M.; Lele, V.; Zavistanaviciute, P.; Bernatoniene, J.; Jakstas, V.; Ivanauskas, L.; Zadeike, D.; Klupsaite, D.; Viskelis, P. Development of antimicrobial gummy candies with addition of bovine colostrum, essential oils and probiotics. Int. J. Food Sci. Technol. 2018, 53, 1227–1235. [Google Scholar] [CrossRef]
- Miranda, J.S.; BCosta, V.; de Oliveira, I.V.; de Lima, D.C.N.; Martins, E.M.F.; Júnior, B.R.D.C.L.; Benevenuto, W.C.A.D.N.; de Queiroz, I.C.; da Silva, R.R.; Martins, M.L. Probiotic jelly candies enriched with native Atlantic Forest fruits and Bacillus coagulans GBI-30 6086. LWT 2020, 126, 109275. [Google Scholar] [CrossRef]


| Novel GCs | Functional Ingredients | Advantages | Disadvantages |
|---|---|---|---|
| Gelatin-free | Pectin, guar gum, agar gum, carrageenan, carboxy methyl cellulose (CMC), alginate, corn starch, and cassava starch |
|
|
| Sugar-free | Stevia, corn syrup, erythritol, xylitol, honey, isomaltulose, maltitol, isomaltitol, and natural sugar sources (e.g., grape, mulberry, and carob molasses) |
|
|
| Artificial additives-free |
|
|
|
| High-fiber |
|
|
|
| High-protein | Soy protein isolate (SPI), lupine protein concentrate (LPC), and Spirulina biomass |
|
|
| High-antioxidant | Natural plant extracts (e.g., rosemary, Psidium guajava L. leaf, tea, peppermint, chokeberry, etc.), fresh fruits (e.g., strawberries and red beetroots), pineapple and beetroot juice, and ginger powder |
|
|
| Medicine | Metoclopramide hydrochloride, acetaminophen, ferrous gluconate (FeG), beta-carotene (β-C) probiotics (e.g., Lactobacillus paracasei LUHS244 and Lactobacillus plantarum LUHS135), prebiotics (e.g., psyllium husk fibers), and essential oils (e.g., Thymus vulgaris L., Citrus reticulate L., and Citrus paradise L.) |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarahi, M.; Tahmouzi, S.; Kianiani, M.R.; Ezzati, S.; Hedayati, S.; Niakousari, M. Current Innovations in the Development of Functional Gummy Candies. Foods 2024, 13, 76. https://doi.org/10.3390/foods13010076
Tarahi M, Tahmouzi S, Kianiani MR, Ezzati S, Hedayati S, Niakousari M. Current Innovations in the Development of Functional Gummy Candies. Foods. 2024; 13(1):76. https://doi.org/10.3390/foods13010076
Chicago/Turabian StyleTarahi, Mohammad, Sima Tahmouzi, Mohammad Reza Kianiani, Shiva Ezzati, Sara Hedayati, and Mehrdad Niakousari. 2024. "Current Innovations in the Development of Functional Gummy Candies" Foods 13, no. 1: 76. https://doi.org/10.3390/foods13010076
APA StyleTarahi, M., Tahmouzi, S., Kianiani, M. R., Ezzati, S., Hedayati, S., & Niakousari, M. (2024). Current Innovations in the Development of Functional Gummy Candies. Foods, 13(1), 76. https://doi.org/10.3390/foods13010076