Assessing Matrix Solid-Phase Dispersion Extraction Strategies for Determining Bisphenols and Phthalates in Gilthead Sea Bream Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents, Standards, and Materials
2.2. Samples
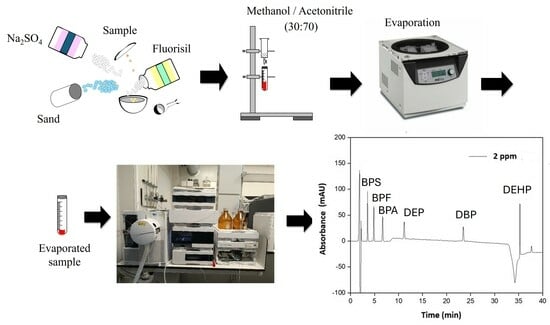
2.3. Sample Extraction Procedure
2.4. Chromatographic Analysis
2.5. Quality Assurance
3. Results and Discussion
3.1. Sample Extraction Procedure
3.2. Validation of the MSPD Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Wang, J.; Liu, X.; Qu, F.; Wang, X.; Wang, X.; Li, Y.; Sun, Y. Microplastics in the Environment: A Review of Analytical Methods, Distribution, and Biological Effects. TrAC Trends Anal. Chem. 2019, 111, 62–72. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J. Investigation of Microplastics in Aquatic Environments: An Overview of the Methods Used, from Field Sampling to Laboratory Analysis. TrAC Trends Anal. Chem. 2018, 108, 195–202. [Google Scholar] [CrossRef]
- Murphy, J. An Overview of Additives. In Additives for Plastics Handbook, 2nd ed.; Murphy, J., Ed.; Elsevier Advanced Technology: Oxford, UK, 2003; Chapter 1; pp. 1–3. [Google Scholar]
- Coleman, E.A. Plastics Additives. In Applied Plastics Engineering Handbook: Processing, Materials, and Applications, 2nd ed.; Kutz, M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Chapter 21; pp. 489–500. ISBN 9780323390408. [Google Scholar]
- Wang, R.; Huang, Y.; Dong, S.; Wang, P.; Su, X. The Occurrence of Bisphenol Compounds in Animal Feed Plastic Packaging and Migration into Feed. Chemosphere 2021, 265, 129022. [Google Scholar] [CrossRef] [PubMed]
- Üstündağ, Ü.V.; Ünal, İ.; Ateş, P.S.; Alturfan, A.A.; Yiğitbaşı, T.; Emekli-Alturfan, E. Bisphenol A and Di(2-Ethylhexyl) Phthalate Exert Divergent Effects on Apoptosis and the Wnt/β-Catenin Pathway in Zebrafish Embryos: A Possible Mechanism of Endocrine Disrupting Chemical Action. Toxicol. Ind. Health 2017, 33, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Huygh, J.; Clotman, K.; Malarvannan, G.; Covaci, A.; Schepens, T.; Verbrugghe, W.; Dirinck, E.; Van Gaal, L.; Jorens, P.G. Considerable Exposure to the Endocrine Disrupting Chemicals Phthalates and Bisphenol-A in Intensive Care Unit (ICU) Patients. Environ. Int. 2015, 81, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Mikołajewska, K.; Stragierowicz, J.; Gromadzińska, J. Bisphenol A—Application, Sources of Exposure and Potential Risks in Infants, Children and Pregnant Women. Int. J. Occup. Med. Environ. Health 2015, 28, 209–241. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Iacovidou, E.; Gerassimidou, S. An Overview of the Occurrence, Fate, and Human Risks of the Bisphenol—A Present in Plastic Materials, Components, and Products. Integr. Environ. Assess Manag. 2023, 19, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Bakirhan, N.K.; Ozkan, S.A. The Recent Electrochemical Studies on Bisphenol a Detection in Beverages. In Safety Issues in Beverage Production: Volume 18: The Science of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 309–333. ISBN 9780128166796. [Google Scholar]
- Kose, O.; Rachidi, W.; Beal, D.; Erkekoglu, P.; Fayyad-Kazan, H.; Kocer Gumusel, B. The Effects of Different Bisphenol Derivatives on Oxidative Stress, DNA Damage and DNA Repair in RWPE-1 Cells: A Comparative Study. J. Appl. Toxicol. 2020, 40, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Cunha, S.C.; Monteiro, C.; Fernandes, J.O.; Guilhermino, L. Bisphenol A and Its Analogs in Muscle and Liver of Fish from the North East Atlantic Ocean in Relation to Microplastic Contamination. Exposure and Risk to Human Consumers. J. Hazard Mater. 2020, 393, 122419. [Google Scholar] [CrossRef]
- Greene, J.P. Commodity Plastics. In Automotive Plastics and Composites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 83–105. [Google Scholar]
- Darbre, P.D. Endocrine Disrupters in Air. In Endocrine Disruption and Human Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 445–461. [Google Scholar]
- Jiménez-Díaz, I.; Vela-Soria, F.; Rodríguez-Gómez, R.; Zafra-Gómez, A.; Ballesteros, O.; Navalón, A. Analytical Methods for the Assessment of Endocrine Disrupting Chemical Exposure during Human Fetal and Lactation Stages: A Review. Anal. Chim. Acta 2015, 892, 27–48. [Google Scholar] [CrossRef]
- Langer, E.; Bortel, K.; Waskiewicz, S.; Lenartowicz-Klik, M. Classification of Plasticizers. In Plasticizers Derived from Post-Consumer PET; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–44. [Google Scholar]
- Gu, Y.Y.; Yu, X.J.; Peng, J.F.; Chen, S.B.; Zhong, Y.Y.; Yin, D.Q.; Hu, X.L. Simultaneous Solid Phase Extraction Coupled with Liquid Chromatography Tandem Mass Spectrometry and Gas Chromatography Tandem Mass Spectrometry for the Highly Sensitive Determination of 15 Endocrine Disrupting Chemicals in Seafood. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2014, 965, 164–172. [Google Scholar] [CrossRef]
- APROMAR OPP30. La Acuicultura En España. 2021. Available online: https://apromar.es/wp-content/uploads/2021/12/La-Acuicultura-en-Espana-2021.pdf (accessed on 13 January 2023).
- Akhbarizadeh, R.; Russo, G.; Rossi, S.; Golianova, K.; Moore, F.; Guida, M.; De Falco, M.; Grumetto, L. Emerging Endocrine Disruptors in Two Edible Fish from the Persian Gulf: Occurrence, Congener Profile, and Human Health Risk Assessment. Mar. Pollut. Bull. 2021, 166, 112241. [Google Scholar] [CrossRef]
- Petrarca, M.H.; Menezes-Sousa, D.; Ferreira, R.; Fernandes, J.O.; Vieira, L.R.; Guilhermino, L.; Cunha, S.C. Occurrence and Risk Assessment of Endocrine-Disrupting Compounds in Fish Muscle: The Case Study of the Douro River Estuary (North East Atlantic Ocean). Environ. Res. 2022, 215, 114236. [Google Scholar] [CrossRef]
- Seetha, B.S.; Ganneru, S.; Thati, R.; Mudiam, M.K.R. Experimental Design of Non-Ionic Hydrophobic DES-DLLME Coupled with Injector Port Silylation-GC–MS/MS for the Quantitative Determination of 13 Bisphenols in Food Samples. Food Chem. 2023, 405, 134778. [Google Scholar] [CrossRef]
- Lu, I.-C.; Chao, H.-R.; Mansor, W.-N.-W.; Peng, C.-W.; Hsu, Y.-C.; Yu, T.-Y.; Chang, W.-H.; Fu, L.-M. Levels of Phthalates, Bisphenol-A, Nonylphenol, and Miroplastics in Fish in the Estuaries of Northern Taiwan and the Impact on Human Health. Toxics 2021, 9, 246. [Google Scholar] [CrossRef]
- Tian, L.; Zheng, J.; Pineda, M.; Yargeau, V.; Furlong, D.; Chevrier, J.; Bornman, R.; Obida, M.; Gates Goodyer, C.; Bayen, S. Targeted Screening of 11 Bisphenols and 7 Plasticizers in Food Composites from Canada and South Africa. Food Chem. 2022, 385, 132675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xiang, L.; Li, J.; Yang, Z.; Fang, J.; Zhao, C.; Xu, S.; Cai, Z. Investigation on Fragmentation Pathways of Bisphenols by Using Electrospray Ionization Orbitrap Mass Spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 1901–1913. [Google Scholar] [CrossRef] [PubMed]
- Naiara, P.-M.; Francesca, S.; Oscar, N. Determination of Phthalic Acid Esters in Drinking Water and Olive Oil by Ultra-High Performance Liquid Chromatography-Electrospray-Tandem Mass Spectrometry: Study of Phthalate Migration from Plastic Bottles to Drinking Water at Different Domestic Exposure Conditions. Trends Chromatogr. 2017, 11, 27–48. [Google Scholar]
- Petrarca, M.H.; Fernandes, J.O.; Marmelo, I.; Marques, A.; Cunha, S.C. Multi-Analyte Gas Chromatography-Mass Spectrometry Method to Monitor Bisphenols, Musk Fragrances, Ultraviolet Filters, and Pesticide Residues in Seafood. J. Chromatogr. A 2022, 1663, 462755. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Yan, P.; Chu, M.; Gao, Y.Q.; Li, W.H.; Yang, X.L. A Rapid and Simple HPLC–FLD Screening Method with QuEChERS as the Sample Treatment for the Simultaneous Monitoring of Nine Bisphenols in Milk. Food Chem. 2018, 244, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Casero, N.; Rubio, S. Comprehensive Supramolecular Solvent-Based Sample Treatment Platform for Evaluation of Combined Exposure to Mixtures of Bisphenols and Derivatives by Liquid Chromatography-Tandem Mass Spectrometry. Anal. Chim. Acta 2021, 1144, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Serrano, M.; Borrull, F.; Pocurull, E.; Marcé, R.M. Pressurised Liquid Extraction and Liquid Chromatography-High Resolution Mass Spectrometry for the Simultaneous Determination of Phthalate Diesters and Their Metabolites in Seafood Species. Food Anal. Methods 2020, 13, 1442–1453. [Google Scholar] [CrossRef]
- Pérez, R.A.; Albero, B.; Tadeo, J.L. Matrix Solid Phase Dispersion. In Solid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 531–549. ISBN 9780128169063. [Google Scholar]
- Anfaco-Cecopesca, Centro técnico nacional de conservación de productos de la pesca y la acuicultura. Guia de Las Cualidades Nutricionales de Los Productos Procedentes de La Pesca Extractiva y de La Acuicultura: Binomio Riesgo-Beneficio; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2012; NIPO: 280-12-178-4. [Google Scholar]
- Cañadas, R.; Garrido Gamarro, E.; Garcinuño Martínez, R.M.; Paniagua González, G.; Fernández Hernando, P. Occurrence of Common Plastic Additives and Contaminants in Mussel Samples: Validation of Analytical Method Based on Matrix Solid-Phase Dispersion. Food Chem. 2021, 349, 129169. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. VICH Topic GL49: Studies to Evaluate the Metabolism and Residue Kinetics of Veterinary Drugs in Food Producing Animals: Validation of Analytical Methods Used in Residue Depletion Studies: EMA/CVMP/VICH/463202/2009; European Medicines Agency: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Cerkvenik-Flajs, V.; Šturm, S. A Rapid Analysis of Bisphenol A Using MISPE Coupled with HPLC-FLD in Tissues of Food-Producing Animals. MethodsX 2021, 8, 101351. [Google Scholar] [CrossRef]




| Cleaning Solvent | % Recovery | |||||
|---|---|---|---|---|---|---|
| BPS | BPF | BPA | DEP | DBP | DHEP | |
| NaOH 0.5 M | 50.81 | 74.96 | 39.60 | 48.52 | 43.28 | 33.05 |
| Hexane | 76.05 | 80.24 | 51.34 | 52.75 | 39.93 | 24.92 |
| Dichloromethane | 91.77 | 87.23 | 70.06 | 85.23 | 79.54 | 70.55 |
| Petroleum ether | 76.58 | 75.26 | 74.22 | 57.53 | 60.85 | 45.59 |
| Diethyl ether | 58.32 | 21.62 | 51.10 | 1.860 | 16.77 | 16.90 |
| Analyte | Linearity | Spiking Level µg/kg | Recovery ± RSD % | LOD µg/kg | LOQ µg/kg | ||
|---|---|---|---|---|---|---|---|
| Concentration Range µg/kg | R2 | Inter-Day | Intra-Day | ||||
| BPS | 0.5–50 | 0.999 | 0.50 | 92.3 ± 6.21 | 90.5 ± 7.57 | 0.23 | 0.78 |
| 5.00 | 91.9 ± 5.03 | 92.5 ± 5.82 | |||||
| 10.0 | 92.0 ± 5.30 | 91.8 ± 5.92 | |||||
| BPF | 0.5–50 | 0.999 | 0.50 | 86.3 ± 7.22 | 84.9 ± 8.59 | 0.11 | 0.37 |
| 5.00 | 87.6 ± 6.75 | 88.2 ± 6.81 | |||||
| 10.0 | 83.4 ± 6.27 | 85.7 ± 6.94 | |||||
| BPA | 0.5–50 | 0.994 | 0.50 | 70.2 ± 4.50 | 69.9 ± 4.85 | 0.27 | 0.89 |
| 5.00 | 71.5 ± 4.14 | 70.9 ± 4.62 | |||||
| 10.0 | 70.9 ± 3.92 | 71.2 ± 4.50 | |||||
| DEP | 0.5–50 | 0.996 | 0.50 | 85.4 ± 3.87 | 81.3 ± 4.67 | 0.32 | 1.10 |
| 5.00 | 86.5 ± 3.05 | 87.7 ± 3.91 | |||||
| 10.0 | 86.3 ± 3.19 | 88.5 ± 4.10 | |||||
| DBP | 0.5–50 | 0.993 | 0.50 | 79.6 ± 7.14 | 77.5 ± 8.01 | 0.21 | 0.69 |
| 5.00 | 78.2 ± 7.22 | 78.9 ± 7.97 | |||||
| 10.0 | 79.1 ± 6.51 | 78.3 ± 7.15 | |||||
| DHEP | 0.5–50 | 0.996 | 0.50 | 71.1 ± 9.04 | 70.1 ± 10.0 | 0.68 | 2.28 |
| 5.00 | 72.4 ± 8.21 | 71.5 ± 9.54 | |||||
| 10.0 | 70.2 ± 8.08 | 73.5 ± 8.21 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliz, D.L.; Garcinuño, R.M.; Paniagua González, G.; Bravo, J.C.; Fernández Hernando, P. Assessing Matrix Solid-Phase Dispersion Extraction Strategies for Determining Bisphenols and Phthalates in Gilthead Sea Bream Samples. Foods 2024, 13, 413. https://doi.org/10.3390/foods13030413
Soliz DL, Garcinuño RM, Paniagua González G, Bravo JC, Fernández Hernando P. Assessing Matrix Solid-Phase Dispersion Extraction Strategies for Determining Bisphenols and Phthalates in Gilthead Sea Bream Samples. Foods. 2024; 13(3):413. https://doi.org/10.3390/foods13030413
Chicago/Turabian StyleSoliz, Dulce L., Rosa Ma Garcinuño, Gema Paniagua González, Juan Carlos Bravo, and Pilar Fernández Hernando. 2024. "Assessing Matrix Solid-Phase Dispersion Extraction Strategies for Determining Bisphenols and Phthalates in Gilthead Sea Bream Samples" Foods 13, no. 3: 413. https://doi.org/10.3390/foods13030413
APA StyleSoliz, D. L., Garcinuño, R. M., Paniagua González, G., Bravo, J. C., & Fernández Hernando, P. (2024). Assessing Matrix Solid-Phase Dispersion Extraction Strategies for Determining Bisphenols and Phthalates in Gilthead Sea Bream Samples. Foods, 13(3), 413. https://doi.org/10.3390/foods13030413