Development and Characterization of Edible Films Based on Cassava Starch Modified by Corona Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
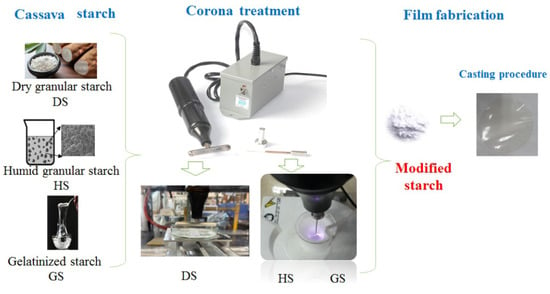
2.2. Sample Preparation for Plasma Treatment
Corona Treatment
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Determination of pH, Water Retention Capacity (WRC), and Solubility in Water (S)
2.5. Pasting Properties
2.6. Differential Scanning Calorimetry
2.7. Thermogravimetric Analysis
2.8. X-ray Diffraction Analysis (XRD)
2.9. Rheological Analysis
2.10. Film Fabrication
2.10.1. Characterization of the Films
Water Uptake Capacity and Soluble Matter Loss
Contact Angle and Water Vapor Permeability (WVP)
Mechanical Properties
2.10.2. Scanning Electron Microscopy (SEM)
2.11. Experimental and Statistical Analysis
3. Results and Discussion
3.1. Starch Characterization
3.1.1. FTIR
3.1.2. pH, Water Retention Capacity (WRC), and Solubility in Water (S)
3.1.3. Pasting Properties
3.1.4. Gelatinization Properties
3.1.5. Thermogravimetric Analysis
3.1.6. RX Diffraction
3.1.7. Rheological Properties of Starch Paste
3.2. Characterization of the Films
3.2.1. Water Vapor Permeability, Water Uptake Capacity, and Contact Angle of Films
3.2.2. Physical, Mechanical, and Morphological Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch-Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Bashir, K.; Aggarwal, M. Physicochemical, structural and functional properties of native and irradiated starch: A review. J. Food Sci. Technol. 2019, 56, 513–523. [Google Scholar] [CrossRef]
- BeMiller, J.N.; Huber, K.C. Physical modification of food starch functionalities. Annu. Rev. Food Sci. Technol. 2015, 6, 19–69. [Google Scholar] [CrossRef]
- Xiao, H.; Lin, Q.; Liu, G.Q.; Wu, Y.; Tian, W.; Wu, W.; Fu, X. Physicochemical properties of chemically modified starches from different botanical origin. Sci. Res. Essays 2011, 6, 4517–4525. [Google Scholar] [CrossRef]
- Zhu, F. Plasma modification of starch. Food Chem. 2017, 232, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Thirumdas, R.; Sarangapani, C.; Annapure, U.S. Cold plasma: A novel non-thermal technology for food processing. Food Biophys. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Otalora, C.M.; Flores, S.K.; Basanta, M.F.; Gerschenson, L.N. Effect of beetroot (Beta vulgaris L. var conditiva) fiber filler and corona treatment on cassava starch films properties. Food Packag. Shelf Life 2020, 26, 100605. [Google Scholar] [CrossRef]
- Bogaerts, A.; Neyts, E.; Gijbels, R.; Van der Mullen, J. Plasmas de descarga de gases y sus aplicaciones. Spectrochim. Acta Part B Espectroscopía Atómica 2002, 57, 609–658. [Google Scholar] [CrossRef]
- Khorram, S.; Zakerhamidi, M.S.; Karimzadeh, Z. Polarity functions’ characterization and the mechanism of starch modification by DC glow discharge plasma. Carbohydr. Polym. 2015, 127, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Sun, N.N.; Chau, C.F. Application of corona electrical discharge plasma on modifying the physicochemical properties of banana starch indigenous to Taiwan. J. Food Drug Anal. 2018, 26, 244–251. [Google Scholar] [CrossRef]
- Gao, S.; Liu, H.; Sun, L.; Liu, N.; Wang, J.; Huang, Y.; Wang, F.; Cao, J.; Fan, R.; Zhang, X.; et al. The effects of dielectric barrier discharge plasma on physicochemical and digestion properties of starch. Int. J. Biol. Macromol. 2019, 138, 819–830. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; Milosavljević, V.; O’Donnell, C.P.; Bourke, P.; Keener, K.M.; Cullen, P.J. Applications of cold plasma technology in food packaging. Trends Food Sci. Technol. 2014, 35, 5–17. [Google Scholar] [CrossRef]
- Misra, N.N.; Kaur, S.; Tiwari, B.K.; Kaur, A.; Singh, N.; Cullen, P.J. Atmospheric pressure cold plasma (ACP) treatment of wheat flour. Food Hydrocoll. 2015, 44, 115–121. [Google Scholar] [CrossRef]
- Thirumdas, R.; Trimukhe, A.; Deshmukh, R.R.; Annapure, U.S. Functional and rheological properties of cold plasma treated rice starch. Carbohydr. Polym. 2017, 157, 1723–1731. [Google Scholar] [CrossRef]
- Wongsagonsup, R.; Deeyai, P.; Chaiwat, W.; Horrungsiwat, S.; Leejariensuk, K.; Suphantharika, M.; Fuongfuchat, A.; Dangtip, S. Modification of tapioca starch by non-chemical route using jet atmospheric argon plasma. Carbohydr. Polym. 2014, 102, 790–798. [Google Scholar] [CrossRef]
- Romani, V.P.; Olsen, B.; Collares, M.P.; Oliveira, J.R.M.; Prentice, C.; Martins, V.G. Plasma technology as a tool to decrease the sensitivity to water of fish protein films for food packaging. Food Hydrocoll. 2019, 94, 210–216. [Google Scholar] [CrossRef]
- Bahrami, R.; Zibaei, R.; Hashami, Z.; Hasanvand, S.; Garavand, F.; Rouhi, M.; Jafari, S.M.; Mohammadi, R. Modification and improvement of biodegradable packaging films by cold plasma; a critical review. Crit. Rev. Food Sci. Nutr. 2022, 62, 1936–1950. [Google Scholar] [CrossRef]
- Alzate, P.; Gerschenson, L.; Flores, S. Ultrasound application for production of nano-structured particles from esterified starches to retain potassium sorbate. Carbohydr. Polym. 2020, 247, 116759. [Google Scholar] [CrossRef]
- Otálora, C.M.; Alvarez Castillo, E.; Flores, S.; Gerschenson, L.N.; Bengoechea, C. Effect of plasticizer composition on the properties of injection molded cassava starch-based bioplastics. Food Packag. Shelf Life 2023, 40, 101218. [Google Scholar] [CrossRef]
- ASTM D570-00; Standard Method for Water Absorption of Plastics. American Society for Testing and Materials: Philadelphia, PA, USA,, 2002. [CrossRef]
- ASTM E96; Standard Test Method for Water Vapour Transmission of Materials. American Society for Testing and Materials: Philadelphia, PA, USA, 2000. [CrossRef]
- Gennadios, A.; Weller, C.L.; Gooding, C.H. Measurement errors in water vapor permeability of highly permeable, hydrophilic edible films. J. Food Eng. 1994, 21, 395–409. [Google Scholar] [CrossRef]
- ASTM D882-02; Standard Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: Philadelphia, PA, USA, 2002. [CrossRef]
- Sokal, R.; Rohlf, J. Biometry. In The Principles and Practice of Statistics in Biological Research; W. H. Freeman and Company: San Francisco, CA, USA, 2000. [Google Scholar]
- Nemtanu, M.R.; Braşoveanu, M. Exposure of starch to combined physical treatments based on corona electrical discharges and ionizing radiation. Impact on physicochemical properties. Radiat. Phys. Chem. 2021, 184, 109480. [Google Scholar] [CrossRef]
- Carvalho, A.P.M.G.; Barros, D.R.; da Silva, L.S.; Sanches, E.A.; Pinto, C.d.C.; de Souza, S.M.; Clerici, M.T.P.S.; Rodrigues, S.; Fernandes, F.A.N.; Campelo, P.H. Dielectric barrier atmospheric cold plasma applied to the modification of Ariá (Goeppertia allouia) starch: Effect of plasma generation voltage. Int. J. Biol. Macromol. 2021, 182, 1618–1627. [Google Scholar] [CrossRef]
- Zou, J.J.; Liu, C.J.; Eliasson, B. Modification of starch by glow discharge plasma. Carbohydr. Polym. 2004, 55, 23–26. [Google Scholar] [CrossRef]
- Guo, D.; Liu, H.; Zhou, L.; Xie, J.; He, C. Plasma-activated water production and its application in agriculture. J. Sci. Food Agric. 2021, 101, 4891–4899. [Google Scholar] [CrossRef] [PubMed]
- Nemtanu, M.R.; Minea, R. Functional properties of corn starch treated with corona electrical discharges. In Macromolecular Symposia; Wiley-VCH Verlag: Weinheim, Germany, 2006; Volume 245, pp. 525–528. [Google Scholar] [CrossRef]
- Okyere, A.Y.; Rajendran, S.; Annor, G.A. Cold plasma technologies: Their effect on starch properties and industrial scale-up for starch modification. Curr. Res. Food Sci. 2022, 5, 451–463. [Google Scholar] [CrossRef]
- Kumar, R.; Khatkar, B.S. Thermal, pasting and morphological properties of starch granules of wheat (Triticum aestivum L.) varieties. J. Food Sci. Technol. 2017, 54, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Fukai, S.; Bhandari, B. Effect of different cooking conditions on the pasting properties of flours of glutinous rice varieties from Lao people’s democratic republic. Int. J. Food Prop. 2016, 19, 2026–2040. [Google Scholar] [CrossRef]
- Chaiwat, W.; Wongsagonsup, R.; Tangpanichyanon, N.; Jariyaporn, T.; Deeyai, P.; Suphantharika, M.; Fuongfuchat, A.; Nisoa, M.; Dangtip, S. Argon plasma treatment of tapioca starch using a semi-continuous downer reactor. Food Bioprocess Technol. 2016, 9, 1125–1134. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, L.; Li, X.; Li, L.; Zhang, H. Understanding the multi-scale structure and functional properties of starch modulated by glow-plasma: A structure-functionality relationship. Food Hydrocoll. 2015, 50, 228–236. [Google Scholar] [CrossRef]
- Bie, P.; Li, X.; Xie, F.; Chen, L.; Zhang, B.; Li, L. Supramolecular structure and thermal behavior of cassava starch treated by oxygen and helium glow-plasmas. Innov. Food Sci. Emerg. Technol. 2016, 34, 336–343. [Google Scholar] [CrossRef]
- Wang, L.; Xie, B.; Shi, J.; Xue, S.; Deng, Q.; Wei, Y.; Tian, B. Physicochemical properties and structure of starches from Chinese rice cultivars. Food Hydrocoll. 2010, 24, 208–216. [Google Scholar] [CrossRef]
- Carmona-Garcia, R.; Sanchez-Rivera, M.M.; Méndez-Montealvo, G.; Garza-Montoya, B.; Bello-Pérez, L.A. Effect of the cross-linked reagent type on some morphological, physicochemical and functional characteristics of banana starch (Musa paradisiaca). Carbohydr. Polym. 2009, 76, 117–122. [Google Scholar] [CrossRef]
- Singh, A.V.; Nath, L.K. Synthesis and evaluation of physicochemical properties of cross-linked sago starch. Int. J. Biol. Macromol. 2012, 50, 14–18. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Thermoplastic starch blown films with improved mechanical and barrier properties. Int. J. Biol. Macromol. 2021, 188, 290–299. [Google Scholar] [CrossRef]
- Sarangapani, C.; Devi, Y.; Thirundas, R.; Annapure, U.S.; Deshmukh, R.R. Effect of low-pressure plasma on physico-chemical properties of parboiled rice. LWT-Food Sci. Technol. 2015, 63, 452–460. [Google Scholar] [CrossRef]
- Heidemann, H.M.; Dotto, M.E.; Laurindo, J.B.; Carciofi, B.A.; Costa, C. Cold plasma treatment to improve the adhesion of cassava starch films onto PCL and PLA surface. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 580, 123739. [Google Scholar] [CrossRef]
- Lyytikäinen, J.; Ovaska, S.-S.; Soboleva, E.; Rinkunas, R.; Lozovski, T.; Maldzius, R.; Sidaravicius, J.; Johansson, L.-S.; Backfolk, K. Optimizing electric corona treatment for hydroxypropylated starch-based coatings. Carbohydr. Polym. 2018, 197, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, S.K.; Bueno-Ferrer, C.; Misra, N.N.; O’Neill, L.; Tiwari, B.K.; Bourke, P.; Cullen, P.J. Dielectric barrier discharge atmospheric air plasma treatment of high amylose corn starch films. LWT-Food Sci. Technol. 2015, 63, 1076–1082. [Google Scholar] [CrossRef]
- Bastos, D.C.; Santos, A.E.; da Silva, M.L.; Simão, R.A. Hydrophobic corn starch thermoplastic films produced by plasma treatment. Ultramicroscopy 2009, 109, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Gou, Q.; Yang, L.; Yu, Q.L.; Han, L. Dielectric barrier discharge plasma: A green method to change structure of potato starch and improve physicochemical properties of potato starch films. Food Chem. 2022, 370, 130992. [Google Scholar] [CrossRef] [PubMed]







| Sample | pH | WRC (g/g) | S (%) |
|---|---|---|---|
| DS-U | 6.49 ± 0.04 a | 18.8 ± 0.8 a | 26 ± 1 a |
| DS-T | 4.87 ± 0.06 b | 9.10 ± 0.4 b | 6.2 ± 0.4 b |
| HS-U | 5.67 ± 0.04 a | 18.2 ± 0.5 a | 14 ± 1 a |
| HS-T | 3.45 ± 0.07 b | 22.0 ± 1.0 b | 20 ± 2 b |
| GS-U | 5.68 ± 0.03 a | 11.5 ± 0.6 a | 42 ± 2 a |
| GS-T | 3.89 ± 0.02 b | 9.50 ± 0.6 b | 37 ± 1 b |
| Samples | Pasting Properties | Gelatinization Properties | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PT (°C) | PV × 103 (Pa·s) | BV × 103 (Pa·s) | FV × 103 (Pa·s) | SV × 103 (Pa·s) | To (°C) | Tp (°C) | Tc (°C) | ∆H (J/g) | |
| DS-U | 67.9 ± 0.5 a | 534 ± 1 a | 156.4 ± 0.8 a | 625 ± 1 a | 91.1 ± 0.8 a | 61.3 ± 0.5 a | 69.4 ± 0.7 a | 84.7 ± 0.9 a | 81 ± 1 a |
| DS-T | 66.5 ± 0.2 a | 510 ± 2 b | 135 ± 2 b | 604 ± 2 b | 93.7 ± 0.1 b | 82.0 ± 1.0 b | 85.0 ± 2.0 b | 96.0 ± 1.0 b | 34 ± 2 b |
| HS-U | 68.5 ± 0.4 a | 273.9 ± 0.3 a | 45.8 ± 0.4 a | 391 ± 1 a | 117.8 ± 0.7 a | 58.9 ± 0.4 a | 63.9 ± 0.7 a | 73.3 ± 0.5 a | 80.3 ± 0.6 a |
| HS-T | 67.9 ± 0.8 a | 415.7 ± 0.5 b | 84.4 ± 0.8 b | 592 ± 1 b | 176.7 ± 0.5 b | 62.6 ± 0.7 b | 68.8 ± 0.8 b | 76.8 ± 0.4 b | 0.63 ± 0.03 b |
| GS-U | ND | ND | 51.9 ± 0.5 a | 99.1 ± 0.7 a | 3.61 ± 0.04 a | ND | ND | ND | ND |
| GS-T | ND | ND | 257.4 ± 0.8 b | 94.1 ± 0.6 b | 216.7 ± 0.7 b | ND | ND | ND | ND |
| Samples | Steady Flow Test | Dynamic Viscoelastic Test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| η0(Pa·s) | η∞ (Pa·s) | λ (s) | n | a | R2 | G′1 Hz(Pa) | G″1 Hz (Pa) | tan δ1 Hz | |
| DS-U | 220.4 ± 0.7 a | 0.072 ± 0.001 a | 39.4 ± 0.8 a | 0.250 ± 0.07 a | 8.08 ± 0.3 a | 0.9975 | 3.2 ± 0.8 a | 2.1 ± 0.3 a | 34 ± 1 a |
| DS-T | 1992.7 ± 0.2 b | 0.123 ± 0.04 b | 220.0 ± 0.2 b | 0.146 ± 0.002 b | 24.3 ± 0.1 b | 0.9962 | 29 ± 0.3 b | 6.9 ± 0.3 b | 13.2 ± 0.2 b |
| HS-U | 157.3 ± 0.3 a | 0.048 ± 0.003 a | 19.9 ± 0.5 a | 0.27 ± 0.01 a | 1.52 ± 0.05 a | 0.9993 | 6.1 ± 0.4 a | 2.7 ± 0.1 a | 24.1 ± 0.6 a |
| HS-T | 1212.3 ± 0.5 b | 0.058 ± 0.005 a | 84.4 ± 0.3 b | 0.24 ± 0.01 a | 14.5 ± 0.5 b | 0.9997 | 21.7 ± 0.7 b | 5.6 ± 0.5 b | 14.4 ± 0.2 b |
| GS-U | 302.4 ± 0.2 a | 0.047 ± 0.006 a | 63.4 ± 0.5 a | 0.22 ± 0.02 a | 4.44 ± 0.4 a | 0.9998 | 6.2 ± 0.2 a | 2.2 ± 0.1 b | 20.3 ± 0.7 a |
| GS-T | 22.52 ± 0.8 b | 0.048 ± 0.001 a | 33.9 ± 0.3 b | 0.39 ± 0.06 b | 68.0 ± 0.1 b | 0.9983 | 1.5 ± 0.4 b | 1.3 ± 0.2 b | 41.3 ± 0.3 b |
| Physical Parameters | FDS-U | FGS-T | FDS-T |
|---|---|---|---|
| Moisture (% db) | 24.7 ± 0.2 a | 23 ± 1 a | 20.2 ± 0.1 b |
| SML (%) | 40.1 ± 0.7 a | 35 ± 2 b | 32 ± 1 b |
| Mechanical properties | |||
| εmax (mm/mm) | 1.25 ± 0.07 a | 1.1 ± 0.1 a | 1.4 ± 0.1 a |
| σmax (MPa) | 1.62 ± 0.03 a | 1.3 ± 0.1 b | 2.0 ± 0.2 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otálora González, C.M.; Felix, M.; Bengoechea, C.; Flores, S.; Gerschenson, L.N. Development and Characterization of Edible Films Based on Cassava Starch Modified by Corona Treatment. Foods 2024, 13, 468. https://doi.org/10.3390/foods13030468
Otálora González CM, Felix M, Bengoechea C, Flores S, Gerschenson LN. Development and Characterization of Edible Films Based on Cassava Starch Modified by Corona Treatment. Foods. 2024; 13(3):468. https://doi.org/10.3390/foods13030468
Chicago/Turabian StyleOtálora González, Carlos Mauricio, Manuel Felix, Carlos Bengoechea, Silvia Flores, and Lía Noemí Gerschenson. 2024. "Development and Characterization of Edible Films Based on Cassava Starch Modified by Corona Treatment" Foods 13, no. 3: 468. https://doi.org/10.3390/foods13030468
APA StyleOtálora González, C. M., Felix, M., Bengoechea, C., Flores, S., & Gerschenson, L. N. (2024). Development and Characterization of Edible Films Based on Cassava Starch Modified by Corona Treatment. Foods, 13(3), 468. https://doi.org/10.3390/foods13030468