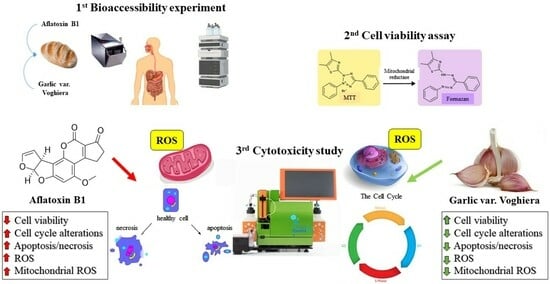
Allium sativum L. var. Voghiera Reduces Aflatoxin B1 Bioaccessibility and Cytotoxicity In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Maize Flour Contamination and Mycotoxin Production
2.3. Bread Ingredients, Bread Making, and Baking
2.4. In Vitro Static Digestion Model
2.5. AFB1 Extraction from Bread and Digest Samples
2.6. Quantitative Determination of AFB1 by HPLC-FLD
2.7. Quantitative Determination Method Validation
2.8. Bioaccessibility Study
2.9. Cell Culture
2.10. Cell Viability Assay
2.11. Cell Cycle Analysis
2.12. Apoptosis/Necrosis Pathway Analysis
2.13. ROS Analysis
2.14. Mitochondrial ROS Analysis
2.15. Mitochondrial Mass Analysis
2.16. Cytometer Settings
2.17. Statistical Analysis of the Data
3. Results
3.1. AFB1 Bioaccessibility
3.2. Cell Viability
3.3. Flow Cytometry Analysis
3.3.1. Cell Cycle
3.3.2. Apoptosis/Necrosis after Intestinal Digest Exposure
3.3.3. Effect of Intestinal Digest Exposure on ROS
3.3.4. Mitochondrial Mass
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.; Leblanc, J.; et al. Risk Assessment of Aflatoxins in Food. EFS2 2020, 18, e06040. [Google Scholar] [CrossRef]
- IARC (International Agency for Research on Cancer). Aflatoxins. Chemical Agents and Related Occupations. A review of Human Carcinogens. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2012; Volume 100F, pp. 225–248. [Google Scholar]
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of Mycotoxins in vivo on Vertebrate Organisms: A Review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar] [CrossRef] [PubMed]
- Frangiamone, M.; Cimbalo, A.; Alonso-Garrido, M.; Vila-Donat, P.; Manyes, L. In vitro and in vivo Evaluation of AFB1 and OTA-Toxicity through Immunofluorescence and Flow Cytometry Techniques: A Systematic Review. Food Chem. Toxicol. 2022, 160, 112798. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of Aflatoxin B1, Deoxynivalenol and Zearalenone in Feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Khodaei, D.; Javanmardi, F.; Khaneghah, A.M. The global overview of the occurrence of mycotoxins in cereals: A three-year survey. Curr. Opin. Food Sci. 2021, 39, 36–42. [Google Scholar] [CrossRef]
- Saladino, F.; Posarelli, E.; Luz, C.; Luciano, F.B.; Rodriguez-Estrada, M.T.; Mañes, J.; Meca, G. Influence of Probiotic Microorganisms on Aflatoxins B1 and B2 Bioaccessibility Evaluated with a Simulated Gastrointestinal Digestion. J. Food Compos. Anal. 2018, 68, 128–132. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Rabbani, T.; Asi, M.R.; Jinap, S. Assessment of Aflatoxins, Ochratoxin A and Zearalenone in Breakfast Cereals. Food Chem. 2014, 157, 257–262. [Google Scholar] [CrossRef]
- Noroozi, R.; Kobarfard, F.; Rezaei, M.; Ayatollahi, S.A.; Paimard, G.; Eslamizad, S.; Razmjoo, F.; Sadeghi, E. Occurrence and Exposure Assessment of Aflatoxin B1 in Iranian Breads and Wheat-Based Products Considering Effects of Traditional Processing. Food Control 2022, 138, 108985. [Google Scholar] [CrossRef]
- Rahman, M.; Islam, R.; Hasan, S.; Zzaman, W.; Rana, M.R.; Ahmed, S.; Roy, M.; Sayem, A.; Matin, A.; Raposo, A.; et al. A Comprehensive Review on Bio-Preservation of Bread: An Approach to Adopt Wholesome Strategies. Foods 2022, 11, 319. [Google Scholar] [CrossRef]
- Escrivá, L.; Agahi, F.; Vila-Donat, P.; Mañes, J.; Meca, G.; Manyes, L. Bioaccessibility Study of Aflatoxin B1 and Ochratoxin A in Bread Enriched with Fermented Milk Whey and/or Pumpkin. Toxins 2021, 14, 6. [Google Scholar] [CrossRef]
- Ferrer, M.; Manyes, L.; Mañes, J.; Meca, G. Influence of Prebiotics, Probiotics and Protein Ingredients on Mycotoxin Bioaccessibility. Food Funct. 2015, 6, 987–994. [Google Scholar] [CrossRef]
- Kabak, B.; Ozbey, F. Assessment of the Bioaccessibility of Aflatoxins from Various Food Matrices Using an in vitro Digestion Model, and the Efficacy of Probiotic Bacteria in Reducing Bioaccessibility. J. Food Compos. Anal. 2012, 27, 21–31. [Google Scholar] [CrossRef]
- Llorens Castelló, P.; Juan-García, A.; Cortés, J.C.M.; Mañes Vinuesa, J.; Juan García, C. Application of an in vitro Digestion Model for Wheat and Red Beetroot Bread to Assess the Bioaccessibility of Aflatoxin B1, Ochratoxin A and Zearalenone and Betalains. Toxins 2022, 14, 540. [Google Scholar] [CrossRef]
- Saladino, F.; Quiles, J.M.; Mañes, J.; Fernández-Franzón, M.; Luciano, F.B.; Meca, G. Dietary Exposure to Mycotoxins through the Consumption of Commercial Bread Loaf in Valencia, Spain. LWT 2017, 75, 697–701. [Google Scholar] [CrossRef]
- Pauletto, M.; Giantin, M.; Tolosi, R.; Bassan, I.; Barbarossa, A.; Zaghini, A.; Dacasto, M. Curcumin Mitigates AFB1-Induced Hepatic Toxicity by Triggering Cattle Antioxidant and Anti-Inflammatory Pathways: A Whole Transcriptomic in vitro Study. Antioxidants 2020, 9, 1059. [Google Scholar] [CrossRef] [PubMed]
- Vila-Donat, P.; Fernández-Blanco, C.; Sagratini, G.; Font, G.; Ruiz, M.-J. Effects of Soyasaponin I and Soyasaponins-Rich Extract on the Alternariol-Induced Cytotoxicity on Caco-2 Cells. Food Chem. Toxicol. 2015, 77, 44–49. [Google Scholar] [CrossRef]
- Bongiorno, P.B.; Fratellone, P.M.; LoGiudice, P. Potential Health Benefits of Garlic (Allium sativum): A Narrative Review. J. Complement. Integr. Med. 2008, 2. [Google Scholar] [CrossRef]
- Lanzotti, V.; Barile, E.; Antignani, V.; Bonanomi, G.; Scala, F. Antifungal Saponins from Bulbs of Garlic, Allium Sativum L. Var. Voghiera. Phytochemistry 2012, 78, 126–134. [Google Scholar] [CrossRef]
- Brugnoli, F.; Tedeschi, P.; Grassilli, S.; Maietti, A.; Brandolini, V.; Bertagnolo, V. Ethanol-Based Garlic Extract Prevents Malignant Evolution of Non-Invasive Breast Tumor Cells Induced by Moderate Hypoxia. Biomed. Pharmacother. 2021, 142, 112052. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health; Springer: Cham, Szwitzerland, 2015; pp. 103–111. [Google Scholar]
- Chen, J.-L.; Nong, G.-M. Advances in application of Jurkat cell model in research on infectious diseases. Zhongguo dang dai er ke za zhi. Chin. J. Contemp. Pediatr. 2018, 20, 236–242. [Google Scholar] [CrossRef]
- Gao, Y.; Bao, X.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. Aflatoxin B1 and Aflatoxin M1 Induce Compromised Intestinal Integrity through Clathrin-Mediated Endocytosis. Toxins 2021, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zheng, N.; Liu, J.; Li, F.D.; Li, S.L.; Wang, J.Q. Aflatoxin B1 and Aflatoxin M1 Induced Cytotoxicity and DNA Damage in Differentiated and Undifferentiated Caco-2 Cells. Food Chem. Toxicol. 2015, 83, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Engdal, S.; Nilsen, O.G. Inhibition of P-Glycoprotein in Caco-2 Cells: Effects of Herbal Remedies Frequently Used by Cancer Patients. Xenobiotica 2008, 38, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, A.; Murakami, A.; Ohigashi, H. Novel Bioassay System for Evaluating Anti-Oxidative Activities of Food Items: Use of Basolateral Media from Differentiated Caco-2 Cells. Free. Radic. Res. 2005, 39, 1367–1375. [Google Scholar] [CrossRef]
- Bhatt, A.; Patel, V. Antioxidant Activity of Garlic Using Conventional Extraction and in vitro Gastrointestinal Digestion. Free Radic. Antioxid. 2013, 3, 30–34. [Google Scholar] [CrossRef]
- Zhu, L.; Gao, J.; Huang, K.; Luo, Y.; Zhang, B.; Xu, W. miR-34a Screened by miRNA Profiling Negatively Regulates Wnt/β-Catenin Signaling Pathway in Aflatoxin B1 Induced Hepatotoxicity. Sci. Rep. 2015, 5, 16732. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Wu, J.; Ji, X.; Xu, Q. Research Progress in Toxicological Effects and Mechanism of Aflatoxin B1 Toxin. PeerJ 2022, 10, e13850. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Q.; Wang, L.; Gao, X.; Zhu, W.; Mu, P.; Deng, Y. Aflatoxin B1 Induces Neurotoxicity through Reactive Oxygen Species Generation, DNA Damage, Apoptosis, and S-Phase Cell Cycle Arrest. IJMS 2020, 21, 6517. [Google Scholar] [CrossRef]
- Liu, C.; Yu, H.; Zhang, Y.; Li, D.; Xing, X.; Chen, L.; Zeng, X.; Xu, D.; Fan, Q.; Xiao, Y.; et al. Upregulation of miR-34a-5p Antagonizes AFB1-Induced Genotoxicity in F344 Rat Liver. Toxicon 2015, 106, 46–56. [Google Scholar] [CrossRef]
- Hou, L.; Gan, F.; Zhou, X.; Zhou, Y.; Qian, G.; Liu, Z.; Huang, K. Immunotoxicity of Ochratoxin A and Aflatoxin B1 in Combination Is Associated with the Nuclear Factor Kappa B Signaling Pathway in 3D4/21 Cells. Chemosphere 2018, 199, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W. Aflatoxin B1 Impairs Mitochondrial Functions, Activates ROS Generation, Induces Apoptosis and Involves Nrf2 Signal Pathway in Primary Broiler Hepatocytes. Anim. Sci. J. 2016, 87, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Z.; Yu, C.; Xu, X. Effects of Aflatoxin B1 on Mitochondrial Respiration, ROS Generation and Apoptosis in Broiler Cardiomyocytes. Anim. Sci. J. 2017, 88, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Kotan, E.; Alpsoy, L.; Anar, M.; Aslan, A.; Agar, G. Protective Role of Methanol Extract of Cetraria Islandica (L.) against Oxidative Stress and Genotoxic Effects of AFB 1 in Human Lymphocytes in vitro. Toxicol. Ind. Health 2011, 27, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Khalil, R.H.; Diab, A.M.; Khallaf, M.A.; Abdel-Razek, N.; Abdel-Latif, H.M.R.; Khalifa, E. Dietary Garlic and Chitosan Enhanced the Antioxidant Capacity, Immunity, and Modulated the Transcription of HSP70 and Cytokine Genes in Zearalenone-Intoxicated European Seabass. Fish Shellfish. Immunol. 2021, 113, 35–41. [Google Scholar] [CrossRef] [PubMed]
- AL-Fayyadh, M.S.; Wadood, S.A. Effects of Red Cabbage and Garlic Extracts on Oxidative Stress Induced by Fumonisin B1. Iraqi J. Sci. 2021, 62, 1850–1861. [Google Scholar] [CrossRef]
- Kohda, K.; Goda, H.; Itoh, K.; Samejima, K.; Fukuuchi, T. Aged Garlic Extract Reduces ROS Production and Cell Death Induced by 6-Hydroxydopamine through Activation of the Nrf2-ARE Pathway in SH-SY5Y Cells. Pharmacol. Pharm. 2013, 04, 31–40. [Google Scholar] [CrossRef]
- Ilmawati, R.R.; Gofur, A.; Lestari, S.R. Single Bulb Garlic Oil Improves Interleukin-6 via Decreased Reactive Oxygen Species (ROS) in High-Fat Diet Male Mice. Universa Med. 2019, 38, 100–107. [Google Scholar] [CrossRef]
- Liu, Y.; Du, M.; Zhang, G. Proapoptotic Activity of Aflatoxin B1 and Sterigmatocystin in HepG2 Cells. Toxicol. Rep. 2014, 1, 1076–1086. [Google Scholar] [CrossRef]
- Chen, X.; Abdallah, M.F.; Grootaert, C.; Rajkovic, A. Bioenergetic Status of the Intestinal and Hepatic Cells after Short Term Exposure to Fumonisin B1 and Aflatoxin B1. IJMS 2022, 23, 6945. [Google Scholar] [CrossRef]
- Monteiro, L.D.B.; Davanzo, G.G.; De Aguiar, C.F.; Moraes-Vieira, P.M.M. Using Flow Cytometry for Mitochondrial Assays. MethodsX 2020, 7, 100938. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Qin, Y.; Hu, X.; Ren, L.; Zhang, C.; Wang, X.; Wang, W.; Zhang, Z.; Hao, J.; Guo, M.; et al. Melatonin Protects in vitro Matured Porcine Oocytes from Toxicity of Aflatoxin B1. J. Pineal Res. 2019, 66, e12543. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, Y.; Cao, Z.; Zhang, J.; Huang, W. AFB1-Induced Mice Liver Injury Involves Mitochondrial Dysfunction Mediated by Mitochondrial Biogenesis Inhibition. Ecotoxicol. Environ. Saf. 2021, 216, 112213. [Google Scholar] [CrossRef] [PubMed]






| Ingredient (g) | C | G | A | A + G | ||||
|---|---|---|---|---|---|---|---|---|
| E1 | E2 | E1 | E2 | E1 | E2 | E1 | E2 | |
| Wheat flour | 127 | 127 | 124 | 124 | 121.1 | 72.3 | 118.1 | 69.3 |
| C maize flour | - | - | - | - | 5.9 | 54.7 | 5.9 | 54.7 |
| Water | 66 | 66 | 65 | 65 | 66 | 66 | 65 | 65 |
| Salt | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 | 2.6 |
| Sugar | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Fresh yeast | 8 | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Garlic | - | - | 4 | 4 | - | - | 4 | 4 |
| Total quantity | 207.6 | 207.6 | 207.6 | 207.6 | 207.6 | 207.6 | 207.6 | 207.6 |
| Matrices | Linearity Range | r2 | Linear Regression Equation | LOD/LOQ (µg/L) |
|---|---|---|---|---|
| Maize flour | 0.05–5 mg/L | 0.9998 | y = 516.44x − 4.7063 | 0.25/0.75 |
| Bread | 0.05–0.5 mg/L | 0.9948 | y = 389.03 − 3.1217 | 0.25/0.75 |
| 0.05–5 mg/L | 0.9999 | y = 400.28x − 9.6478 | 0.25/0.75 | |
| Gastric digest | 5–200 µg/L | 0.9998 | y = 0.7611x − 0.3598 | 0.25/0.75 |
| 50–1500 µg/L | 0.9996 | y = 1134.4x − 14.942 | 0.25/0.75 | |
| Intestinal digest | 5–50 µg/L | 0.9984 | y = 0.8279x − 0.7944 | 0.25/0.75 |
| 10–1500 µg/L | 0.9994 | y = 633.28x − 4.3939 | 0.25/0.75 |
| Cell Viability (%) | ||||||
|---|---|---|---|---|---|---|
| Exposure Time | 24 h | 48 h | 72 h | |||
| Intestinal Digest | AFB1 | AFB1–Garlic | AFB1 | AFB1–Garlic | AFB1 | AFB1–Garlic |
| 1/32 | 79 ± 4 | 86 ± 2 | 74 ± 4 | 86 ± 4 * | 73 ± 1 | 87 ± 6 *** |
| 1/16 | 76 ± 5 | 84 ± 6 | 72 ± 5 | 85 ± 4 * | 72 ± 9 | 83 ± 2 ** |
| 1/8 | 76 ± 7 | 81 ± 3 | 72 ± 1 | 83 ± 5 ** | 66 ± 2 | 83 ± 3 * |
| 1/4 | 76 ± 3 | 80 ± 2 | 69 ± 4 | 80 ± 3 * | 64 ± 8 | 82 ± 7 *** |
| 1/2 | 73 ± 6 | 79 ± 7 | 68 ± 4 | 78 ± 4 ** | 63 ± 6 | 81 ± 3 * |
| No dilution | 70 ± 4 | 79 ± 6 * | 66 ± 5 | 77 ± 4 ** | 62 ± 4 | 79 ± 3 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lázaro, Á.; Frangiamone, M.; Maietti, A.; Cimbalo, A.; Vila-Donat, P.; Manyes, L. Allium sativum L. var. Voghiera Reduces Aflatoxin B1 Bioaccessibility and Cytotoxicity In Vitro. Foods 2024, 13, 487. https://doi.org/10.3390/foods13030487
Lázaro Á, Frangiamone M, Maietti A, Cimbalo A, Vila-Donat P, Manyes L. Allium sativum L. var. Voghiera Reduces Aflatoxin B1 Bioaccessibility and Cytotoxicity In Vitro. Foods. 2024; 13(3):487. https://doi.org/10.3390/foods13030487
Chicago/Turabian StyleLázaro, Álvaro, Massimo Frangiamone, Annalisa Maietti, Alessandra Cimbalo, Pilar Vila-Donat, and Lara Manyes. 2024. "Allium sativum L. var. Voghiera Reduces Aflatoxin B1 Bioaccessibility and Cytotoxicity In Vitro" Foods 13, no. 3: 487. https://doi.org/10.3390/foods13030487
APA StyleLázaro, Á., Frangiamone, M., Maietti, A., Cimbalo, A., Vila-Donat, P., & Manyes, L. (2024). Allium sativum L. var. Voghiera Reduces Aflatoxin B1 Bioaccessibility and Cytotoxicity In Vitro. Foods, 13(3), 487. https://doi.org/10.3390/foods13030487