Lipid Peroxidation in Muscle Foods: Impact on Quality, Safety and Human Health
Abstract
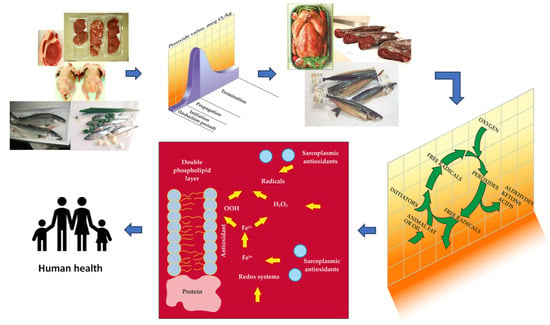
:1. Introduction
2. The Essence of lipid Peroxidation in Muscle Foods
2.1. Phases of Lipid Peroxidation
2.2. The Cyclical Nature of Lipid Peroxidation
3. Mechanisms of Free Radical Initiation in the Lipid Fraction of Muscle Foods
3.1. Non-Enzymatic Initiation of Lipid Peroxidation in Meat Products
- (1)
- By oxidases, such as cytochrome oxidase, catalysing the transfer of electrons from cytochrome to oxygen;
- (2)
- Through auto-oxidation of oxy-myoglobin and oxyhaemoglobin: (both containing Fe2+ in the oxidised state) with the formation of superoxyl anion radical (O2−·) and metmyoglobin or methaemoglobin (both containing Fe3+ in the oxidised state);
- (3)
- Via free iron ions capable of participating in transfer reactions with molecular O2, leading to the generation of superoxyl anion radical (O2−·) [10].
3.2. Enzymatic Initiation of Lipid Peroxidation in Meat Products
4. Free Radical Chain Mechanisms for Propagation of Oxidative Processes in Muscle Foods
5. Kinetics of Oxidation Processes
5.1. Electron Structure of Oxygen
| Type | π*a | π*b | Energy, kJ |
| 1Σg |  |  | 155 |
| 1Δg |  |  | 92 |
| 3Σg |  |  | 0—basic state |
5.2. Superoxyl Anion Radical
5.3. Hydrogen Peroxide
5.4. Hydroxyl Radical
5.5. Iron Ions and Iron Complexes
5.6. Singlet Oxygen
5.7. Haemoproteins
- (1)
- By forming a radical of PUFA, for instance, through a reaction catalysed by lipoxygenase, involving the transfer of one electron or the evolution of hydrogen;
- (2)
- By generating a superoxyl anion radical (O2−·) and indirectly interacting with triplet oxygen, thus forming a more reactive oxygen species;
- (3)
- By oxidising phospholipid flavin cofactors, they activate oxygen by adopting a semiquinone radical state, indirectly participating in the generation of an oxygen species;
- (4)
- By interacting with oxygen (using cytochrome P450) or with peroxides (via peroxidase, catalase, myoglobin, haemoglobin, or cyclooxygenase).
- (1)
- Direct initiation by Fenton-type reactions;
- (2)
- Indirect initiation, via hypervalent iron complexes;
- (3)
- Indirect initiation and propagation, via iron-catalysed degradation of hydroperoxides to peroxyl radicals (LOO·) and abstraction of hydrogen from unsaturated fatty acids.
- (1)
- Fe3+ abstracts H+ from unsaturated fatty acids and forms an alkyl radical (L·);
- (2)
- Fe2+ forms metal–oxygen transfer complexes, generating reactive oxygen species in non-polar solvents—an unlikely mechanism for meat products;
- (3)
6. Oxidative Stability of Muscle Lipids
6.1. Background
6.2. Type of Oxidised Substrates
6.3. Prooxidant Factors
- -
- Deboning, tendon removal, shaping, chopping, grinding, mincing, and cutting, are processes in which lipid peroxidation catalysts and substrates are mixed [138]. Consequently, oxygen enters the anaerobic muscle tissue;
- -
- -
- Heat treatments such as surface hot drying, roasting, hot smoking, steaming, boiling, grilling, baking, and frying [1,13,15,138] destroy the cellular organisation of muscles, leading to protein and enzyme denaturation. This, in turn, affects the antioxidant enzymatic activity, which is partially or completely lost, releasing iron connected with proteins.
6.4. Antioxidant Factors
6.4.1. Own Endogenous Antioxidant Systems
6.4.2. Exogenous Antioxidants
- (1)
- Inhibition of Fenton’s reaction: nitric oxide can inhibit Fenton’s reaction, which involves the formation of ferrous ions and contributes to oxidative stress [335];
- (1)
- Interaction with iron: nitric oxide can interact with both non-heme and heme iron, preventing these metals from catalysing oxidative reactions; [336];
- (2)
- Radical acceptance: nitrogen oxide, nitrogen–oxide complexes, and S-nitrosothiols formed from nitrites act as radical acceptors, neutralising free radicals and interrupting chain reactions [338];
- (3)
- Protection of porphyrin: nitrogen oxide complexes with haem proteins protect porphyrin from releasing iron when exposed to hydrogen peroxide and hydroperoxides [339];
- (4)
- Stabilisation of lipids: nitrogen oxides formed outside the membranes during the smoking of meat products can stabilise unsaturated lipids [340].
6.4.3. Technological Methods for Inhibition of Lipid Peroxidation in Muscle Foods
- (1)
- (2)
- Modified atmosphere packaging (MAP) involves changing the composition of the air inside the package to slow down oxidative reactions. Fresh sea fish, for example, benefit from MAP. While MAP slows down lipid peroxidation compared to storage in the air, it may result in higher levels of TBARS compared to vacuum packaging.
7. The Quality of Muscle Foods Affected by Oxidative Processes
7.1. Effect of Muscle Fibre Type on Lipid Peroxidation-Induced Alterations in Pork Leg Ham Flavour
7.2. The Aroma of Roast Meat Related to the Maillard Reaction Affected by Lipid Peroxidation
7.3. Effect of Derivatives of Lipid Hydroperoxide Degradation on Meat Aroma
7.4. Negative Influence of Lipid Peroxidation on the Warmed-Over Flavour
7.5. Lipid Peroxidation and Meat Taste
7.6. Lipid Peroxidation and Meat Colour
7.7. Lipid Peroxidation and Meat Texture
7.8. Lipid Peroxidation and Nutritional Value of Meat
8. Effect of Oxidised Muscle Foods on Human Health
8.1. Introduction
8.2. Physiological Effects of Lipid Peroxidation Derivatives
8.3. Autooxidation of Cholesterol and Human Health
8.4. Coronary Cardiovascular Diseases
8.5. Stroke
8.6. Neurodegenerative Diseases and Oxidative Stress
8.7. Influenza Virus Infection and Oxidative Stress
8.8. Rheumatoid Arthritis and Oxidative Stress
8.9. Kidney Diseases and Oxidative Stress
8.10. Liver Disease and Oxidative Stress
8.11. Disorders in Erythropoiesis, Leukaemia and Oxidative Stress
8.12. Carcinogenesis
9. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | Adenosine diphosphate |
| ATP | Adenosine triphosphate |
| DNA | Deoxyribonucleic acid |
| DFD | Darkish red, firm, dry meat |
| DTPA | Diethylenetriamine pentaacetate |
| FAD | Flavin adenine dinucleotide |
| EDTA | Ethylenediaminetetraacetic acid |
| FMH | Flavin mononucleotide |
| HDL | High-density lipoproteins |
| HMG-CoA reductase | 3-OH-3-CH3-glutaryl coenzyme A reductase |
| 4-HNE | 4-Hydroxynonenal |
| LDL | Low-density lipoproteins |
| MAP | Modified atmosphere packaging |
| MDA | Malondialdehyde |
| mtDNA | Mitochondrial DNA |
| NAD | Nicotinamide adenine dinucleotide |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| PSE | Pinkish pale, soft, exudative meat |
| PUFA | Polyunsaturated fatty acids |
| TBARS | 2-Thiobarituric acid reactive substances |
| TVB-N | Total volatile basic nitrogen |
| UV | Ultraviolet |
| VLDL | Very low-density lipoprotein |
| WOF | Warmed-over flavour |
References
- Johnson, D.R.; Decker, E.A. The role of oxygen in lipid oxidation reactions: A review. Annu. Rev. Food Sci. Technol. 2015, 6, 171–190. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Pickova, J.; Ahmad, T.; Liaquat, M.; Farid, A.; Jahangir, M. Oxidation of lipids in foods. Sarhad J. Agric. 2016, 32, 230–238. [Google Scholar] [CrossRef]
- Bekhit, A.E.-D.A.; Hopkins, D.L.; Fahri, F.T.; Ponnampalam, E.N. Oxidative processes in muscle systems and fresh meat: Sources, markers, and remedies. Comp. Rev. Food Sci. Food Saf. 2013, 12, 565–597. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Xu, L.; Porter, N.A. Free radical lipid peroxidation: Mechanisms and analysis. Chem. Rev. 2011, 111, 5944–5972. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signalling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Long. 2014, 8, 360438. [Google Scholar] [CrossRef] [PubMed]
- Köckritz, A.; Blumenstein, M.; Martin, A. Catalytic cleavage of methyl oleate or oleic acid. Eur. J. Lipid Sci. Technol. 2010, 112, 58–63. [Google Scholar] [CrossRef]
- Kanner, J. Oxidative processes in meat and meat products: Quality implications. Meat Sci. 1994, 36, 169–189. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation: Physiological levels and dual biological effects. Free Rad. Biol. Med. 2009, 47, 469–484. [Google Scholar] [CrossRef]
- Shui, S.; Zhao, Z.; Wang, H.; Conrad, M.; Liu, G. Non-enzymatic lipid peroxidation initiated by photodynamic therapy drives a distinct ferroptosis-like cell death pathway. Redox Biol. 2021, 45, 102056. [Google Scholar] [CrossRef]
- Zhang, J.; Montine, T.J. Oxidative processes. In Primer on the Autonomic Nervous System, 2nd ed.; Robertson, D., Biaggioni, I., Burnstock, G., Low, P.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2004; Part V: Neuropathology; pp. 201–203. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of oxidative processes in meat and toxicity induced by postprandial degradation products: A review. Comp. Rev. Food Sci. Food Saf. 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Schaich, K.M. Lipid Oxidation: Theoretical Aspects. In Bailey’s Industrial Oil and Fat Products, 6th ed.; Shahidi, F., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; Volume 1, pp. 269–356. [Google Scholar] [CrossRef]
- Min, B.; Ahn, D.U. Mechanism of lipid peroxidation in meat and meat products—A review. Food Sci. Biotechnol. 2005, 14, 152–163. [Google Scholar]
- Frankel, E.N. (Ed.) Foods. In Lipid Oxidation, 2nd ed.; Woodhead Publishing: Sawston/Cambridge, UK, 2005; pp. 299–354. Available online: https://shop.elsevier.com/books/lipid-oxidation/frankel/978-0-9531949-8-8 (accessed on 1 February 2005).
- Raghavan, S.; Kristinsson, H.G. Influence of processing on lipids and lipid oxidation in aquatic foods. In Antioxidants and Functional Components in Aquatic Foods, 1st ed.; Kristinsson, H.G., Ed.; John Wiley & Sons, Ltd.: Chichester/West Sussex, UK, 2014; pp. 43–94. [Google Scholar] [CrossRef]
- Wu, H.; Tatiyaborworntham, N.; Hajimohammadi, M.; Decker, E.A.; Richards, M.P.; Undeland, I. Model systems for studying lipid oxidation associated with muscle foods: Methods, challenges, and prospects. Crit. Rev. Food Sci. Nutr. 2022, 8, 2105302. [Google Scholar] [CrossRef]
- Bindoli, A. Lipid peroxidation in mitochondria. Free Rad. Biol. Med. 1988, 5, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Shleikin, A.G.; Medvedev, Y.V. Role of peroxidation and heme catalysis in coloration of raw meat. Acta Sci. Pol. Technol. Alim. 2014, 13, 123–127. [Google Scholar] [CrossRef]
- Tang, J.; Faustman, C.; Hoagland, T.A.; Mancini, R.A.; Seyfert, M.; Hunt, M.C. Interactions between mitochondrial lipid oxidation and oxymyoglobin oxidation and the effects of vitamin E. J. Agric. Food Chem. 2005, 53, 6073–6079. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Richards, M.P.; Undeland, I. Lipid oxidation and antioxidant delivery systems in muscle food. Comp. Rev. Food Sci. Food Saf. 2022, 21, 1275–1299. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yin, J.; Zhang, J.H.; Richards, M.P. Factors affecting lipid oxidation due to pig and turkey hemolysate. J. Agric. Food Chem. 2017, 65, 8011–8017. [Google Scholar] [CrossRef]
- Wu, H.; Ghirmai, S.; Undeland, I. Stabilization of herring (Clupea harengus) by-products against lipid oxidation by rinsing and incubation with antioxidant solutions. Food Chem. 2020, 316, 126337. [Google Scholar] [CrossRef]
- Rhee, K.S.; Dutson, T.R.; Smith, G.C. Enzymic lipid-peroxidation in microsomal fractions from beef skeletal-muscle. J. Food Sci. 1984, 49, 675–679. [Google Scholar] [CrossRef]
- Ozcan, A.; Ogun, M. Biochemistry of Reactive Oxygen and Nitrogen Species. In Basic Principles and Clinical Significance of Oxidative Stress, 1st ed.; Gowder, S.J.T., Ed.; InTech Open: Rjieka, Croatia, 2015; pp. 37–58. [Google Scholar] [CrossRef]
- Chabi, B.; Ljubicic, V.; Menzies, K.J.; Huang, J.H.; Saleem, A.; Hood, D.A. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 2008, 7, 1–118. [Google Scholar] [CrossRef]
- Wang, L.-L.; Yu, Q.-L.; Han, L.; Ma, X.-L.; Song, R.-D.; Zhao, S.-N.; Zhang, W.-H. Study on the effect of reactive oxygen species-mediated oxidative stress on the activation of mitochondrial apoptosis and the tenderness of yak meat. Food Chem. 2018, 244, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-C.; Waskell, L. The interaction of microsomal cytochrome p450 2b4 with its redox partners, cytochrome p450 reductase and cytochrome b5. Arch. Biochem. Biophys. 2011, 507, 144–153. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S.; Jaffe, R. Lipid peroxidation of muscle food as affected by sodium chloride. J. Agric. Food Chem. 1991, 39, 1017–1021. [Google Scholar] [CrossRef]
- Ramasarma, T.; Muakkassah-Kelly, S.; Hochstein, P. Inhibition of microsomal lipid peroxidation by cytosolic protein in presence of ADP and high concentration of Fe2+. Biochim. Biophys. Acta (BBA) Lipids Lipid. Met. 1984, 796, 243–250. [Google Scholar] [CrossRef]
- Markis, T.M.; Denisov, I.; Schlichting, I.; Sligar, S.G. Activation of molecular oxygen by cytochrome p450. In Cytochrome P450. Structure, Mechanism, and Biochemistry, 3rd ed.; de Montellano, P.R.O., Ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2005; pp. 149–182. [Google Scholar] [CrossRef]
- Pradhan, A.A.; Rhee, K.S.; Hernández, P. Stability of catalase and its potential role in lipid oxidation in meat. Meat Sci. 2000, 54, 385–390. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Aminzare, M.; Raeisi, M.; Afshari, A.; Alizadeh, A.M.; Rezaeigolestani, M. Comparative evaluation of edible films impregnated with sage essential oil or lactoperoxidase system: Impact on chemical and sensory quality of carp burgers. J. Food Proc. Pres. 2019, 43, e14070. [Google Scholar] [CrossRef]
- Mottley, C.; Robinson, R.E.; Mason, R.P. Free radical formation in the oxidation of malondialdehyde and acetylacetone by peroxidase enzymes. Arch. Biochem. Biophys. 1991, 289, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gunzler, W.A.; Floke, I. Glutathione peroxidase. In Handbook Methods for Oxygen Radical Research, 1st ed.; Greenwald, R.A., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1985; pp. 285–290. [Google Scholar] [CrossRef]
- Hernández, P.; Park, D.; Rhee, K.S. Chloride salt type/ionic strength, muscle site and refrigeration effects on antioxidant enzymes and lipid oxidation in pork. Meat Sci. 2002, 61, 405–410. [Google Scholar] [CrossRef]
- Sarraga, C.; Carrerras, I.; Regueiro, J.A.G. Influence of meat quality and NaCl percentage on glutathione peroxidase activity and values for acid-reactive substances of raw and dry-cured Longissimus dorsi. Meat Sci. 2002, 62, 503–507. [Google Scholar] [CrossRef]
- Carlsen, C.U.; Skovgaard, I.M.; Skibsted, L.H. Pseudoperoxidase activity of myoglobin: Kinetics and mechanism of the peroxidase cycle of myoglobin with H2O2 and 2,2-azino-bis(3-ethylbenzthiazoline-6-sulfonate) as substrates. J. Agric. Food Chem. 2003, 51, 5815–5823. [Google Scholar] [CrossRef]
- Rhee, K.S.; Seideman, S.C.; Cross, H.R. Enzymic and nonenzymic factors affecting lipid peroxidation in raw beef muscles. J. Agric. Food Chem. 1986, 34, 308–312. [Google Scholar] [CrossRef]
- Kanner, J.; Harel, S. Iniciation of membranal lipid peroxidation by activated metmyoglobin and methemoglobin. Arch. Biochem. Biophys. 1985, 237, 314–321. [Google Scholar] [CrossRef]
- Gatellier, P.; Anton, M.; Renerre, M. Lipid peroxidation induced by H2O2-activated metmyoglobin and direction of a myoglobin-derived radical. J. Agric. Food Chem. 1995, 43, 651–656. [Google Scholar] [CrossRef]
- Monahan, F.J.; Crackel, R.L.; Gray, J.I.; Buckley, D.J. Catalysis of lipid oxidation in muscle model systems by haem and inorganic iron. Meat Sci. 1993, 34, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J.; German, J.B.; Kinsella, J.E. Initiation of Lipid Peroxidation of biological systems. CRC Crit. Rev. Food Sci. Nutr. 1987, 25, 317–363. [Google Scholar] [CrossRef]
- Kubow, S. Routes of formation and toxic consequences of lipid oxidation products in foods. Free Rad. Biol. Med. 1992, 12, 63–81. [Google Scholar] [CrossRef]
- Liavonchanka, A.; Feussner, I. Lipoxygenases: Occurrence, functions and catalysis. J. Plant Physiol. 2006, 163, 348–357. [Google Scholar] [CrossRef]
- Min, B.R.; Nam, K.C.; Cordray, J.C.; Ahn, D.U. Factors Affecting Oxidative Stability of Pork, Beef, and Chicken Meat. In Iowa State University Animal Industry Report 2008, 1st ed.; Leaflet, A.S., Ed.; Iowa State University: Ames, IA, USA, 2008; pp. 1–4. [Google Scholar] [CrossRef]
- Wang, T.; Hammond, E.G. Chapter: 5 Lipoxygenase and Lipid Oxidation in Foods. In Oxidation in Foods and Beverages and Antioxidant Applications, 1st ed.; Decker, E.A., Ed.; Elsevier: Amsterdam, The Netherlands; Woodhead Publishing Ltd.: Sawston/Cambridge, UK, 2010; pp. 105–121. [Google Scholar] [CrossRef]
- Grossman, S.; Bergman, M.; Sklan, D. Lipoxygenase in chicken muscle. J. Agric. Food Chem. 1988, 36, 1268–1270. [Google Scholar] [CrossRef]
- Min, B.; Nam, K.C.; Cordray, J.; Ahn, D.U. Endogenous factors affecting oxidative stability of beef loin, pork loin, and chicken breast and thigh meats. J. Food Sci. 2008, 73, C439–C446. [Google Scholar] [CrossRef]
- Jin, G.; Zhang, J.; Yu, X.; Lei, Y.; Wang, J. Crude lipoxygenase from pig muscle: Partial characterization and interactions of temperature, NaCl and pH on its activity. Meat Sci. 2011, 87, 257–263. [Google Scholar] [CrossRef]
- Smith, W.L.; Murphy, R.C. The eicosanoids: Cyclooxygenase, lipoxygenase and epoxygenase pathways. In Biochemistry of Lipids, Lipoproteins and Membranes, 6th ed.; Ridgway, N.D., McLeod, R.S., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2015; pp. 259–296. [Google Scholar] [CrossRef]
- Decker, E.A.; Schanus, E.G. Catalysis of linoleate oxidation by soluble chicken muscle proteins. J. Am. Oil Chem. Soc. 1986, 63, 101–104. [Google Scholar] [CrossRef]
- Iwahashi, H.; Morishita, H.; Ishii, T.; Sugata, R.; Kido, R. Enhancement by catechols of hydroxyl-radical formation in the presence of ferric ions and hydrogen peroxide get access arrow. J. Biochem. 1989, 105, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Xu, S.; Wang, Z. Kinetics of lipid oxidation and off-odor formation in silver carp mince: The effect of lipoxygenase and hemoglobin. Food Res. Int. 2009, 42, 85–90. [Google Scholar] [CrossRef]
- Banerjee, S. Inhibition of mackerel (Scomber scombrus) muscle lipoxygenase by green tea polyphenols. Food Res. Int. 2006, 39, 486–491. [Google Scholar] [CrossRef]
- Mohri, S.; Cho, S.Y.; Endo, Y.; Fujimoto, K. Linoleate-1,3-(S)-lipoxygenase in sardine skin. J. Agric. Food Chem. 1992, 40, 573–576. [Google Scholar] [CrossRef]
- German, G.B. Muscle lipids. J. Muscle Foods 1990, 1, 339–361. [Google Scholar] [CrossRef]
- German, J.B.; Kinsella, J.E. Lipid oxidation in fish tissue. enzymatic initiation via lipoxygenase. J. Agric. Food Chem. 1985, 33, 680–683. [Google Scholar] [CrossRef]
- German, J.B.; Hang, H.; Berger, R. Role of lipoxygenases in lipid oxidation in foods. In ACS Symposium Series 500 Lipid Oxidation in Food, 1st ed.; St. Angelo, A.J., Ed.; American Chemical Society: Washington, DC, USA, 1992; pp. 74–92. [Google Scholar] [CrossRef]
- Hsieh, R.J.; Kinsella, J.E. Lipoxygenase-catalyzed oxidation of n-6 and n-3 polyunsaturated fatty acids: Relevance to and activity in fish tissue. J. Food Sci. 1986, 51, 940–945. [Google Scholar] [CrossRef]
- Hsieh, R.J.; Kinsella, J.E. Lipoxygenase generation of specific volatile flavor carbonyl compounds in fish tissues. J. Agric. Food Chem. 1989, 37, 1989–1994. [Google Scholar] [CrossRef]
- Harris, P.; Tall, J. Substrate specificity of mackerel flesh lipopolygenase. J. Food Sci. 1994, 59, 504–506. [Google Scholar] [CrossRef]
- Josephson, D.B.; Lindsay, R.C.; Stuiber, D.A. Variations in the occurrences of enzymically derived volatile aroma compounds in salt- and freshwater fish. J. Food Sci. 1984, 32, 1344–1347. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Miller, L.A.; Addis, P.B. Effect of heat inactivation of lipoxygenase on lipid oxidation in lake herring (Coregonus artedii). J. Am. Oil Chem. Soc. 1991, 68, 752–757. [Google Scholar] [CrossRef]
- Yılmaz, Ş.T.; Çaklı, Ş.; Yılmaz, E.B.Ş.; Kırlangıç, F.; Lee, C. Effect of fillet temperature on lipoxygenase activity in sardine mince with and without milk protein concentrate. LWT 2018, 90, 38–44. [Google Scholar] [CrossRef]
- Tall, J.; Harris, P. Rancidity in frozen fish. In Fish Oil. Technology, Nutrition and Marketing, 1st ed.; Hamilton, R.J., Rice, R.D., Eds.; PJ Barnes & Associates: High Wycombe, UK, 1995; pp. 35–47. [Google Scholar]
- Hultin, H.O. Oxidation of lipids in seafoods. In Seafoods: Chemistry, Processing Technology and Quality, 1st ed.; Shahidi, F., Botta, J.R., Eds.; Springer: Boston, MA, USA, 1994; pp. 49–74. [Google Scholar] [CrossRef]
- Apgar, M.E.; Hultin, H.O. Lipid peroxidation in fish muscles microsomes in the frozen state. Cryobiology 1982, 19, 154–162. [Google Scholar] [CrossRef]
- Eun, J.B.; Boyle, J.A.; Hearnsberger, J.M. Lipid peroxidation and chemical changes in catfish (Ictalurus punctatus) muscle microsomes during frozen storage. J. Food Sci. 1994, 59, 251–255. [Google Scholar] [CrossRef]
- McDonald, R.E.; Hultin, H.O. Some characteristics of the enzymic lipid peroxidation system in the microsomal fraction of flounder skeletal muscle. J. Food Sci. 1987, 52, 15–21, 27. [Google Scholar] [CrossRef]
- Slabyj, B.M.; Hultin, H.O. Lipid peroxidation by microsomal fractions isolated from light and dark muscles of herring (Clupea harengus). Food Res. 1982, 47, 1395–1399. [Google Scholar] [CrossRef]
- Decker, E.A.; Hultin, O.H. Factors influencing catalysis of lipid oxidation by the soluble fraction of mackerel muscle. J. Food Sci. 1990, 55, 947–950, 953. [Google Scholar] [CrossRef]
- Han, T.J.; Liston, J. Lipid peroxidation enzyme systems in rainbow trout (Salmo gairdnerii) muscle microsomes. Comp. Biochem. Physiol. 1989, 93, 485–492. [Google Scholar] [CrossRef]
- Han, T.J.; Liston, J. Lipid peroxidation protection factors in rainbow trout (Salmo gairdnerii) muscle cytosol. J. Food Sci. 1989, 54, 809–813. [Google Scholar] [CrossRef]
- Paton, S.; Keeney, M.; Kurtz, G.W. Compounds producing the Kreis color reaction with particular reference to oxidized milk fat. J Am. Oil Chem. Soc. 1951, 28, 391–393. [Google Scholar] [CrossRef]
- Sinnhuber, R.O.; Yu, T.C. Characterization of the red pigment formed in the 2-thiobarbituric acid determination of oxidative rancidity. Food Res. 1958, 23, 66–71. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Biological effect of protein modifications by lipid peroxidation products. Chem. Phys. Lipids 2019, 221, 46–52. [Google Scholar] [CrossRef]
- Caslake, M.J.; Packard, C.J.; Suckling, K.E.; Holmes, S.D.; Chamberlain, P.; Macphee, C.H. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase: A potential new risk factor for coronary artery disease. Atherosclerosis 2000, 150, 413–419. [Google Scholar] [CrossRef]
- Dahle, L.K.; Hill, E.G.; Holmer, R.T. The thiobarbituric acid reaction and the autoxidation of polyunsaturated fatty acid methyl esters. Arch. Biochem. Biophys. 1962, 98, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Stanley, J.P.; Blair, E. Autoxidation of polyunsaturated fatty acids. II. A suggested mechanism for the formation of TBA-reactive materials from prostaglandin-like endoperoxides. Lipids 1976, 11, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C. An update on products and mechanisms of lipid peroxidation. Mol. Nut. Food Res. 2009, 53, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Baron, G.; Gianazza, E.; Banfi, C.; Carini, M.; Aldini, G. Lipid peroxidation derived reactive carbonyl species in free and conjugated forms as an index of lipid peroxidation: Limits and perspectives. Redox Biol. 2021, 42, 101899. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacog. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Chemistry and reactions of reactive oxygen species in foods. J. Food Sci. 2005, 70, R142–R159. [Google Scholar] [CrossRef]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A Comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef]
- Baron, C.P.; Andersen, H.J. Myoglobin-Induced lipid oxidation. A review. J. Agric. Food Chem. 2002, 50, 3887–3897. [Google Scholar] [CrossRef] [PubMed]
- Barata, C.; Varo, I.; Navarro, J.C.; Arun, S.; Porte, C. Antioxidant enzyme activities and lipid peroxidation in the freshwater cladoceran Daphnia magna exposed to redox cycling compounds. Compar. Biochem. Physiol. Part C Toxicol. Pharmocol. 2005, 140, 175–186. [Google Scholar] [CrossRef]
- Dimova, M.; Tugai, A.; Tugai, T.; Iutynska, G.; Dordevic, D.; Kushkevych, I. Molecular research of lipid peroxidation and antioxidant enzyme activity of Comamonas testosteroni bacterial cells under the hexachlorobenzene impact. Int. J. Mol. Sci. 2022, 23, 11415. [Google Scholar] [CrossRef]
- Cotton, A.F.; Willkinson, G. Basic Inorganic Chemistry, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1976; pp. 355–401. [Google Scholar]
- Kumara, A.; Prasada, A.; Sedlárová, M.; Kalec, R.; Frankelc, L.K.; Sallansd, L.; Brickerc, T.M.; Pospíšil, P. Tocopherol controls D1 amino acid oxidation by oxygen radicals in photosystem II. Proc. Natl. Acad. Sci. USA 2021, 118, e2019246118. [Google Scholar] [CrossRef]
- Panov, A.V.; Dikalov, S.I. Cardiolipin, perhydroxyl radicals, and lipid peroxidation in mitochondrial dysfunctions and aging. Oxid. Med. Cell. Long. 2020, 2020, 1323028. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Clarke, F.M.; Purslow, P.P.; Warner, R.D. Meat color is determined not only by chromatic heme pigments but also by the physical structure and achromatic light scattering properties of the muscle. Comp. Rev. Food Sci. Food Saf. 2020, 19, 44–63. [Google Scholar] [CrossRef]
- de Avila Souza, M.A.; Shimokomaki, M.; Terra, N.N.; Petracci, M. Oxidative changes in cooled and cooked pale, soft, exudative (PSE) chicken meat. Food Chem. 2022, 385, 132471. [Google Scholar] [CrossRef]
- Arai, K.; Moriai, K.; Ogawa, A.; Iwaoka, M. An amphiphilic selenide catalyst behaves like a hybrid mimic of protein disulfide isomerase and glutathione peroxidase 7. Chem. Asian J. 2014, 9, 3464–3471. [Google Scholar] [CrossRef]
- Burek, B.O.; Bormann, S.; Hollmann, F.; Bloh, J.Z.; Holtmann, D. Hydrogen peroxide driven biocatalysis. Green Chem. 2019, 21, 3232–3249. [Google Scholar] [CrossRef]
- Yaman, S.O.; Ayhanci, A. Lipid peroxidation. In Accenting Lipid Peroxidation, 1st ed.; Atukeren, P., Ed.; InTech Open: Rijeka, Croatia, 2021; pp. 3–26. [Google Scholar] [CrossRef]
- Fenton, H.J.H.; Jackson, H.T.I.-. The oxidation of polyhydric alcohols in presence of iron. J. Chem. Soc. Trans. 1899, 75, 1–11. [Google Scholar] [CrossRef]
- Haard, N.F. Enzymes from food myosystems. J. Muscle Foods 1990, 1, 293–338. [Google Scholar] [CrossRef]
- Lu, J.; Chen, R.; Liang, H.; Yan, Q. The influence of concentration of hydroxyl radical on the chemical mechanical polishing of SiC wafer based on the Fenton reaction. Precis. Eng. 2018, 52, 221–226. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, J.; Valiente, M.; Sánchez-Martín, M.-J. Tooth whitening: From the established treatments to novel approaches to prevent side effects. J. Esth. Rest. Dent. 2019, 31, 431–440. [Google Scholar] [CrossRef]
- Das, T.K.; Wati, M.R.; Fatima-Shad, K. Oxidative stress gated by Fenton and Haber Weiss reactions and its association with Alzheimer’s disease. Arch. Neurosci. 2015, 2, e60038. [Google Scholar] [CrossRef]
- de Montellano, P.R.O. Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem. Rev. 2010, 110, 932–948. [Google Scholar] [CrossRef]
- Sokolov, A.; Solovyov, K.V.; Kostevich, V.A.; Chekanov, A.V.; Pulina, M.O.; Zakharova, E.T.; Shavlovski, M.M.; Panasenko, O.M.; Vasilyev, V.B. Protection of ceruloplasmin by lactoferrin against hydroxyl radicals is pH dependent. Biochem. Cell Biol. 2012, 90, 397–404. [Google Scholar] [CrossRef]
- Gonzalez, D.H.; Diaz, D.A.; Baumann, J.P.; Ghio, A.J.; Paulson, S.E. Effects of albumin, transferrin and humic-like substances on iron-mediated OH radical formation in human lung fluids. Free Rad. Biol. Med. 2021, 165, 79–87. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kang, J.H. Generation of hydroxyl radicals in the reaction of Ferritin with hydrogen peroxide. Bull. Korean Chem. Soc. 2009, 30, 1644–1646. Available online: https://koreascience.kr/article/JAKO200902727014927.pdf (accessed on 12 October 2023).
- Ishikawa, S.-I.; Yano, Y.; Arihara, K.; Itoh, M. Egg yolk phosvitin inhibits hydroxyl radical formation from the Fenton reaction. Biosci. Biotechnol. Biochem. 2004, 68, 1324–1331. [Google Scholar] [CrossRef]
- Harel, S.; Kanner, J. Muscle membranal lipid peroxidation initiated by H2O2-activated metmyoglobin. J. Amer. Food Chem. 1985, 33, 1188–1192. [Google Scholar] [CrossRef]
- Parke, D.V.; Symons, A.M.; Parke, A.L. Oxyradicals, inflammation and drugs acting on oxyradical production. In New Developments in Antirheumatic Therapy, 1st ed.; Rainsford, K.D., Velo, G.P., Eds.; Springer, Kluwer Academic Publishers: Dordrecht, The Netherlands, 1989; Volume 3, pp. 187–206. [Google Scholar] [CrossRef]
- Kristinova, V.; Mozuraityte, R.; Aaneby, J.; Storrø, I.; Rustad, T. Iron-mediated peroxidation in marine emulsions and liposomes studied by dissolved oxygen consumption. Eur. J. Lipid Sci. Technol. 2014, 116, 207–225. [Google Scholar] [CrossRef]
- Mozuraityte, R.; Rustad, T.; Storrø, I. The role of iron in peroxidation of polyunsaturated fatty acids in liposomes. J. Agric. Food Chem. 2008, 56, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Thanonkaew, A.; Benjakul, S.; Visessanguan, W.; Decker, E.A. The effect of metal ions on lipid oxidation, colour and physicochemical properties of cuttlefish (Sepia pharaonis) subjected to multiple freeze-thaw cycles. Food Chem. 2006, 95, 591–599. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, Y.; Qian, Z.; Shen, X. The mechanism of Fe2+-initiated lipid peroxidation in liposomes: The dual function of ferrous ions, the roles of the pre-existing lipid peroxides and the lipid peroxyl radical. Biochem. J. 2000, 352, 27–36. [Google Scholar] [CrossRef]
- Stoyanovsky, D.A.; Tyurina, Y.Y.; Shrivastava, I.; Bahar, I.; Tyurin, V.A.; Protchenko, O.; Jadhav, S.; Bolevich, S.B.; Kozlov, A.V.; Vladimirov, Y.A.; et al. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Rad. Biol. Med. 2019, 133, 153–161. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry and reactions of reactive oxygen species in foods. Crit. Rev. Food Sci. Nutr. 2006, 46, 1–22. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms of antioxidants in the oxidation of foods. Comp. Rev. Food Sci. Food Saf. 2009, 8, 345–358. [Google Scholar] [CrossRef]
- Nawar, W.W. 5. Lipids. In Food Chemistry, 3rd ed.; Fennema, O.R., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 225–320. [Google Scholar]
- Nagababu, E.; Rifkind, J.M. Heme degradation by reactive oxygen species. Antioxid. Redox Signal. 2004, 6, 967–977. [Google Scholar] [CrossRef]
- Praneeth, V.K.K.; Ringenberg, M.R.; Ward, T.R. Redox-active ligands in catalysis. Angew. Chem. 2012, 62, 201204100. [Google Scholar] [CrossRef]
- Hockin, B.M.; Li, C.; Robertson, N.; Zysman-Colman, E. Photoredox catalysts based on earth-abundant metal complexes. Catal. Sci. Technol. 2019, 9, 889–915. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, L.; Wang, P.; Deng, L. High-oxidation-state 3d metal (Ti–Cu) complexes with N-heterocyclic carbene ligation. Chem. Rev. 2018, 118, 9930–9987. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper active sites in biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef]
- Kanner, J. Metals and food oxidation. In Oxidation in Foods and Beverages and Antioxidant Applications. Understanding Mechanisms of Oxidation and Antioxidant Activity, 1st ed.; Decker, E.A., Ed.; Woodhead Publishing Ltd.: Sawston, UK, 2010; pp. 36–56. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, Y. What is responsible for the initiating chemistry of iron-mediated lipid peroxidation: An update. Chem. Rev. 2007, 107, 748–766. [Google Scholar] [CrossRef]
- Gilbert, D.L. From the breath of life to reactive oxygen species. In Reactive Oxygen Species in Biological Systems: An Interdisciplinary Approach, 3rd.; Colton, C., Gilbert, D., Eds.; Springer Science+Business Media: Boston, MA, USA, 2007; pp. 3–31. [Google Scholar] [CrossRef]
- de Souza, P.A.L.; Camacho, F.G.; da Silva, I.R.A.; Gonçalves, F.F.; Benincá, C.; Zanoelo, E.F. An experimental and modeling study of the chain initiation reaction in heterogeneous fenton systems with zero valent iron. Chem. Eng. J. 2020, 393, 124665. [Google Scholar] [CrossRef]
- Oueslati, K.; de La Pomélie, D.; Santé-Lhoutellier, V.; Gatellier, P. Impact of the Fenton process in meat digestion as assessed using an in vitro gastro-intestinal model. Food Chem. 2016, 209, 43–49. [Google Scholar] [CrossRef]
- Carlsen, C.U.; Møller, J.K.S.; Skibsted, L.H. Heme-iron in lipid oxidation. Coord. Chem. Rev. 2005, 249, 485–498. [Google Scholar] [CrossRef]
- Soladoye, O.P.; Juárez, M.L.; Aalhus, J.L.; Shand, P.; Estévez, M. Protein oxidation in processed meat: Mechanisms and potential implications on human health. Crit. Rev. Food Sci. Food Saf. 2015, 14, 106–122. [Google Scholar] [CrossRef]
- Celia María, C.A.; José Manuel, P.L.; Celia, A.J.; Francisco José, P.; Eduardo, P.-L. Superoxide anion chemistry–Its role at the core of the innate immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Chen, C.C.; Pearson, A.M.; Gray, J.I.; Fooladi, M.H.; Ku, P.K. Some factors influencing the non-heme iron content of meat and its implication in oxidation. J. Food Sci. 1984, 49, 581–584. [Google Scholar] [CrossRef]
- Yin, M.C.; Faustman, C. Influence of temperature, pH, and phospholipid composition upon the stability of myoglobin and phospholipid–A liposome model. J. Agric. Food Chem. 1993, 41, 853–857. [Google Scholar] [CrossRef]
- Batifoulier, F.; Mercier, Y.; Gatellier, P.; Renerre, M. Influence of vitamin E on lipid and protein oxidation induced by H2O2-activated metMb in microsomal membranes from turkey muscle. Meat Sci. 2002, 61, 389–395. [Google Scholar] [CrossRef]
- Williams, D.E.; Carpenter, H.M.; Buhler, D.R.; Kelly, J.D.; Dutchuk, M. Alterations in lipid peroxidation, antioxidant enzymes, and carcinogen metabolism in liver microsomes of vitamin E–deficient trout and rat. Toxic. Appl. Phar. 1992, 116, 78–84. [Google Scholar] [CrossRef]
- Huang, C.H.; Hulton, H.O. Soluble and bound iron equally effect lipid oxidation of sarcoplasmic reticulum. J. Food Biochem. 1992, 16, 1–13. [Google Scholar] [CrossRef]
- Kanner, J.; Shegalovich, I.; Harel, S.; Hazan, B. Muscle lipid peroxidation dependent on oxygen and free metal ions. J. Agric. Food Chem. 1988, 36, 409–412. [Google Scholar] [CrossRef]
- Decker, E.A.; Xu, Z. Minimizing rancidity in muscle foods. Food Technol. 1998, 52, 54–59. [Google Scholar]
- Watt, M.J.; Hoy, A.J. Lipid metabolism in skeletal muscle: Generation of adaptive and maladaptive intracellular signals for cellular function. Am. J. Physiol. Endo. Metabol. 2012, 302, E1315–E1328. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Hickner, R.C.; Cortright, R.L.; Dohm, G.L.; Houmard, J.A. Lipid oxidation is reduced in obese human skeletal muscle. Am. J. Physiol.-Endo. Metabol. 2000, 279, E1039–E1044. [Google Scholar] [CrossRef] [PubMed]
- Secci, G.; Parisi, G. From farm to fork: Lipid oxidation in fish products. a review. It. J. Anim. Sci. 2016, 15, 124–136. [Google Scholar] [CrossRef]
- Haard, N.F. Seafood enzymes: The role of adaptation and other intraspecific factors. In Seafood Enzymes. Utilization and Influence of Postharvest Seafood Quality, 1st.; Haard, N.F., Simpson, B.K., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2000; pp. 1–36. [Google Scholar] [CrossRef]
- Rhee, K.S.; Ziprin, Y.A.; Ordonez, G.; Bohae, C.E. Fatty acid profiles and lipid oxidation in beef steer muscle from different anatomical locations. Meat Sci. 1988, 23, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Hamulka, J.; Bryś, J.; Górska, A.; Janaszek-Mańkowska, M.; Górnicka, M. The quality and composition of fatty acids in adipose tissue-derived from wild animals; a pilot study. Appl. Sci. 2021, 11, 10029. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, S.; Yin, J.; Zhang, J.; Richards, M.P. Mechanisms involved in the inhibitory effects of free fatty acids on lipid peroxidation in turkey muscle. Food Chem. 2021, 342, 128333. [Google Scholar] [CrossRef] [PubMed]
- Salami, S.A.; Guinguina, A.; Agboola, J.O.; Omede, A.A.; Agbonlahor, E.M.; Tayyab, U. Review: In vivo and postmortem effects of feed antioxidants in livestock: A review of the implications on authorization of antioxidant feed additives. Animal 2016, 8, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.B.; Da Silva, M.V.; Lannes, S.C.S. Lipid oxidation in meat: Mechanisms and protective factors–A review. Food Sci. Technol. 2018, 38, 32518. [Google Scholar] [CrossRef]
- Hadidi, M.; Orellana-Palacios, J.C.; Aghababaei, F.; Gonzalez-Serrano, D.J.; Moreno, A.; Lorenzo, J.M. Plant by-product antioxidants: Control of protein-lipid oxidation in meat and meat products. LWT 2022, 169, 114003. [Google Scholar] [CrossRef]
- Bian, H.; Ma, J.; Geng, Z.; Liu, T.; Sun, C.; Wang, D.; Zhang, M.; Xu, W. Changes of hydroxyl-linoleic acids during Chinese-style sausage processing and their relationships with lipids oxidation. Food Chem. 2019, 296, 63–68. [Google Scholar] [CrossRef]
- Han, G.; Zhang, L.; Li, Q.; Wang, Y.; Chen, Q.; Kong, B. Impacts of different altitudes and natural drying times on lipolysis, lipid oxidation and flavour profile of traditional Tibetan yak jerky. Meat Sci. 2020, 162, 108030. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Abreu, V.K.G. Lipid Peroxidation in Meat and Meat Products. In Lipid Peroxidation Research, 1st ed.; Mansour, M.A., Ed.; InTech Open: Rijeka, Croatia, 2020; pp. 29–42. [Google Scholar] [CrossRef]
- Ke, Y.Y.; Liu, W.J.; Wang, Z.X.; Chen, Y.X. Effects of monochromatic light on quality properties and antioxidation of meat in broilers. Poult. Sci. 2011, 90, 2632–2637. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Bragagnolo, N. Influence of salt on lipid oxidation in meat and seafood products: A review. Food Res. Int. 2017, 94, 90–100. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, W.G.; Lee, E.J.; Ma, C.W.; Ahn, D.U. Effects of diet, packaging, and irradiation on protein oxidation, lipid oxidation, and color of raw broiler thigh meat during refrigerated storage. Poult. Sci. 2011, 90, 1348–1357. [Google Scholar] [CrossRef]
- Igene, J.O.; Pearson, A.M. Role of phospholipids and triglycerides in warmed over flavor development in meat model systems. J. Food Sci. 1979, 44, 1285–1290. [Google Scholar] [CrossRef]
- Ackman, R.G.; Gunnlaugsdottir, H. Seafoods and Fishery Byproducts. Natural and Unnatural Environments for Longer Chain Omega-3 fatty Acids. In ACS Symposium Series 500 Lipid Oxidation in Food, 1st ed.; St. Angelo, A.J., Ed.; American Chemical Society: Washington, DC, USA, 1992; pp. 208–230. [Google Scholar] [CrossRef]
- Naudí, A.; Cabré, R.; Dominguez-Gonzalez, M.; Ayala, V.; Jové, M.; Mota-Martorell, N.; Piñol-Ripoll, G.; Gil-Villar, M.P.; Rué, M.; Portero-Otín, M.; et al. Region-specific vulnerability to lipid peroxidation and evidence of neuronal mechanisms for polyunsaturated fatty acid biosynthesis in the healthy adult human central nervous system. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2017, 1862, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Jamilah, B.; Abbas, K.A.; Abdul Rahman, R. A review on lipid oxidation of meat in active and modified atmosphere packaging and usage of some stabilizers. J. Food Agric. Environ. 2008, 6, 76–81. Available online: http://psasir.upm.edu.my/id/eprint/12838 (accessed on 14 December 2023).
- Dang, T.T.; Rode, T.M.; Skipnes, D. Independent and combined effects of high pressure, microwave, soluble gas stabilization, modified atmosphere and vacuum packaging on microbiological and physicochemical shelf life of precooked chicken breast slices. J. Food Eng. 2021, 292, 110352. [Google Scholar] [CrossRef]
- Barahona, M.; Campo, M.M.; Hachemi, M.A.; González, M.M.; Olleta, J.L. Feeding, muscle and packaging effects on lipid oxidation and color of Avileña Negra-Ibérica beef. Animals 2021, 11, 2863. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, D.; Liu, X. Effects of Lactobacillus sakei C2 and Sakacin C2 individually or in combination on the growth of Listeria monocytogenes, chemical and odor changes of vacuum-packed sliced cooked ham. Food Control. 2015, 47, 27–31. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Gan, X.; Li, H. Interrelationship among ferrous myoglobin, lipid and protein oxidations in rabbit meat during refrigerated and superchilled storage. Meat Sci. 2018, 146, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, M.; Wang, T.; Wang, D.; Sun, C.; Bian, H.; Li, P.; Zou, Y.; Xu, W. Lipid oxidation induced by heating in chicken meat and the relationship with oxidants and antioxidant enzymes activities. Poult. Sci. 2020, 99, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Pikul, J.; Kummerov, F.A. Lipid oxidation in chicken muscles and skin after roasting and refrigerated storage of meat broiler parts. J. Food Sci. 1990, 55, 30–37. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.U.; Mustapha, A. Effects of plant extracts on microbial growth, color change, and lipid oxidation in cooked beef. Food Microb. 2007, 24, 7–14. [Google Scholar] [CrossRef]
- Conforti, F.D.; Giuffrida, M.L. Determination of oxidative changes in precooked chicken parts by non-heme iron content and thiobarbituric acid value. Poult. Sci. 1995, 74, 1224–1231. [Google Scholar] [CrossRef]
- Soyer, A.; Özalp, B.; Dalmış, Ü.; Bilgin, V. Effects of freezing temperature and duration of frozen storage on lipid and protein oxidation in chicken meat. Food Chem. 2010, 120, 1025–1030. [Google Scholar] [CrossRef]
- Berisha, A.; Endo, Y.; Fujimoto, K. The effect of heating temperature on the prooxidant and hydroperoxide decomposition activity of myoglobin. Food Sci. Technol. Res. 2000, 6, 257–262. [Google Scholar] [CrossRef]
- Hansen, E.; Lauridsen, L.; Skibsted, L.H.; Moawad, R.K.; Andersen, M.L. Oxidative stability of frozen pork patties: Effect of fluctuating temperature on lipid oxidation. Meat Sci. 2004, 68, 185–191. [Google Scholar] [CrossRef]
- Rahman, M.H.; Hossain, M.M.; Rahman, S.M.E.; Amin, M.R.; Oh, D.-H. Evaluation of physicochemical deterioration and lipid oxidation of beef muscle affected by freeze-thaw cycles. Korean J. Food Sci. Anim. Res. 2015, 35, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Wereńska, M.; Okruszek, A.; Haraf, G.; Wołoszyn, J.; Goluch, Z. Impact of frozen storage on oxidation changes of some components in goose meat. Poult. Sci. 2022, 101, 101517. [Google Scholar] [CrossRef] [PubMed]
- Kunsman, J.E.; Field, R.A.; Kazantzis, D. Lipid oxidation of mechanically deboned red meat. J. Food Sci. 1978, 43, 1375–1378. [Google Scholar] [CrossRef]
- Bernardi, D.M.; Bertol, T.M.; Pflanzer, S.B.; Sgarbieri, V.C.; Pollonio, M.A.R. Ω-3 in meat products: Benefits and effects on lipid oxidative stability. J. Sci. Food Agric. 2016, 96, 2620–2634. [Google Scholar] [CrossRef]
- Kilic, B.; Richards, M.P. Lipid oxidation in poultry döner kebab: Pro-oxidative and anti-oxidative factors. J. Food Sci. 2003, 68, 686–689. [Google Scholar] [CrossRef]
- Bigolin, J.; Weber, C.I.; Alfaro, A.T. Lipid oxidation in mechanically deboned chicken meat: Effect of the addition of different agents. Food Nutr. Sci. 2013, 4, 35324. [Google Scholar] [CrossRef]
- Barbut, S.; Darper, H.H.; Hadley, M. Lipid oxidation in chicken nuggets as affected by meat type, phosphate and packaging. J. Food Prot. 1989, 52, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, A.C.S.; Albergaria, F.C.; de Silva, L.M.S.F.; Fernandes, L.A.C.; de Sousa Gomes, M.E.; Pimenta, C.J. Effect of natural and synthetic antioxidants on oxidation and storage stability of mechanically separated tilapia meat. LWT 2022, 154, 112679. [Google Scholar] [CrossRef]
- Secci, G.; Borgogno, M.; Lupi, P.; Rossi, S.; Paci, G.; Mancini, S.; Bonelli, A.; Parisi, G. Effect of mechanical separation process on lipid oxidation in European aquacultured sea bass, gilthead sea bream, and rainbow trout products. Food Control 2016, 67, 75–81. [Google Scholar] [CrossRef]
- Wu, H.; Abdollahi, M.; Undeland, I. Effect of recovery technique, antioxidant addition and compositional features on lipid oxidation in protein enriched products from cod- salmon and herring backbones. Food Chem. 2021, 360, 129973. [Google Scholar] [CrossRef]
- Morris, D.M.; Dawson, L.E. Storage stability of mechanically deboned sucker (Catostomidae) flesh. J. Food Sci. 1979, 44, 1093–1096. [Google Scholar] [CrossRef]
- Gomez-Basauri, J.V.; Regenstein, J.M. Processing and frozen storage effect on the iron content of cod and mackerel. J. Food Sci. 1992, 57, 1332–1336. [Google Scholar] [CrossRef]
- Guyon, C.; Meynier, A.; de Lamballerie, M. Protein and lipid oxidation in meat: A review with emphasis on high-pressure treatments. Trends Food Sci. Technol. 2016, 50, 131–143. [Google Scholar] [CrossRef]
- Rhee, K.S.; Smith, G.C.; Rhee, K.C. Retardation by glandless cottonseed flour on lipid oxidation and discoloration in raw ground beef containing salt. J. Food Sci. 1983, 48, 351–360. [Google Scholar] [CrossRef]
- Rousset-Akrim, S.; Got, F.; Bably, M.C.; Culioli, J. Influence of CaCI2 and NaCl injections on the texture and flavour of beef. Int. J. Food Sci. Tech. 1996, 31, 333–343. [Google Scholar] [CrossRef]
- Auborng, S.P.; Ugliano, M. Effect of brine pre-treatment on lipid a stability of frozen horse mackerel (Trachurus trachurus). Eur. J. Food Sci. Technol. 2002, 215, 91–95. [Google Scholar] [CrossRef]
- Osinchak, J.E.; Hultin, H.O.; Zaijcek, O.T.; Kelleher, S.D. Effect of NaCl on catalysis of lipid oxidation by the soluble fraction of fish muscle. Free Rad. Biol. Med. 1992, 12, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Farouk, M.M.; Price, J.F.; Salih, A.M. Effect of Fe2+, salt, cooking and Shredded coffi® on thiobarbituric acid (TBA) numbers in ground beef. J. Food Sci. 1991, 56, 172–174. [Google Scholar] [CrossRef]
- Ramanathan, L.; Das, N.P. Natural products inhibit oxidative rancidity in salted cooked ground fish. J. Food Sci. 1993, 58, 318–320. [Google Scholar] [CrossRef]
- Davis, L.; Smith, G.; Hole, M. Lipid oxidation in salted-dried fish: II. The effect of photosensitisers on the rate of oxidation of a fish oil. J. Sci. Food Agric. 1995, 67, 493–499. [Google Scholar] [CrossRef]
- Sickler, M.L.; Claus, J.R.; Marriott, N.G.; Eigel, W.N.; Wang, H. Reduction in lipid oxidation by incorporation of encapsulated sodium tripolyphosphate in ground turkey. Meat Sci. 2013, 95, 376–380. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Primožič, J.; Poljšak, B.; Jamnik, P.; Kovač, V.; Jurešić, G.Č.; Spalj, S. Risk assessment of oxidative stress induced by metal ions released from fixed orthodontic appliances during treatment and indications for supportive antioxidant therapy: A narrative review. Antioxidants 2021, 10, 1359. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Inter. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential metals in health and disease. Chem. Biol. Inter. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Kostova, I. The role of complexes of biogenic metals in living organisms. Inorganics 2023, 11, 56. [Google Scholar] [CrossRef]
- Decker, E.A.; Hultin, O.H. Lipid oxidation in muscle foods via redox iron. In Lipid Oxidation in Food. ACS Symposium Series, 1st ed.; St. Angelo, A.J., Ed.; American Chemical Society: Washington, DC, USA, 1992; Volume 500, pp. 33–53. [Google Scholar] [CrossRef]
- Sabow, A.B.; Sazili, A.Q.; Zulkifli, I.; Goh, Y.M.; Ab Kadir, M.Z.A.; Abdulla, N.R.; Nakyinsige, K.; Kaka, U.; Adeyemi, K.D. A comparison of bleeding efficiency, microbiological quality and lipid oxidation in goats subjected to conscious halal slaughter and slaughter following minimal anesthesia. Meat Sci. 2015, 104, 78–84. [Google Scholar] [CrossRef]
- Sohn, J.-H.; Ushio, H.; Ishida, N.; Yamashita, M.; Terayama, M.; Ohshima, T. Effect of bleeding treatment and perfusion of yellowtail on lipid oxidation in post-mortem muscle. Food Chem. 2007, 104, 962–970. [Google Scholar] [CrossRef]
- Carvajal, A.K.; Rustad, T.; Mozuraityte, R.; Storrø, I. Kinetic studies of lipid oxidation induced by hemoglobin measured by consumption of dissolved oxygen in a liposome model system. J. Agric. Food Chem. 2009, 57, 7826–7833. [Google Scholar] [CrossRef] [PubMed]
- Sajib, M.; Wu, H.; Fristedt, R.; Undeland, I. Hemoglobin-mediated lipid oxidation of herring filleting co-products during ensilaging and its inhibition by pre-incubation in antioxidant solutions. Sci. Rep. 2021, 11, 19492. [Google Scholar] [CrossRef]
- Repetto, M.G.; Ferrarotti, N.F.; Boveris, A. The involvement of transition metal ions on iron-dependent lipid peroxidation. Arch. Toxicol. 2010, 84, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Jegasothy, H.; Weerakkody, R.; Selby-Pham, S.; Bennett, L.E. In vitro heme and non-heme iron capture from hemoglobin, myoglobin and ferritin by bovine lactoferrin and implications for suppression of reactive oxygen species in vivo. BioMetals 2014, 27, 1371–1382. [Google Scholar] [CrossRef]
- Lombardi-Boccia, G.; Martinez-Dominguez, B.; Aguzzi, A. Total heme and non-heme iron in raw and cooked meats. J. Food Sci. 2002, 67, 1738–1741. [Google Scholar] [CrossRef]
- Bou, R.; Guardiola, F.; Codony, R.; Faustman, C.; Elias, R.J.; Decker, E.A. Effect of heating oxymyoglobin and metmyoglobin on the oxidation of muscle microsomes. J. Agric. Food Chem. 2008, 56, 9612–9620. [Google Scholar] [CrossRef]
- Vaudagna, S.R.; Sánchez, G.; Neira, M.S.; Insani, E.M.; Picallo, A.B.; Gallinger, M.M.; Lasta, J.A. Sous vide cooked beef muscles: Effects of low temperature-long time (LT–LT) treatments on their quality characteristics and storage stability. Int. J. Food Sci. Technol. 2002, 37, 425–441. [Google Scholar] [CrossRef]
- Richards, M.P.; Modra, A.M.; Li, R. Role of deoxyhemiglobin in lipid oxidation of washed cod muscle mediated by trout, poultry and beef hemoglobines. Meat Sci. 2002, 62, 157–163. [Google Scholar] [CrossRef]
- Kongkachuichai, R.; Napatthalung, P.; Charoensiri, R. Heme and nonheme iron content of animal products commodity consumed in Thailand. J. Food Compos. Anal. 2002, 15, 389–398. [Google Scholar] [CrossRef]
- Poli, G.; Schaur, R.J.; Siems, W.G.; Leonarduzzi, G. 4-Hydroxynonenal: A membrane lipid oxidation product of medicinal interest. Med. Res. Rev. 2008, 28, 569–631. [Google Scholar] [CrossRef] [PubMed]
- King, J.G.; Fukumoto, R.; Gorbunov, N.V. Lipid Peroxidation after Ionizing Irradiation Leeds to Apoptosis and Autophagy. In Lipid Peroxidation, 1st ed.; Catala, A., Ed.; InTech Open: Rijeka, Croatia, 2012; pp. 261–278. [Google Scholar] [CrossRef]
- Bakalivanova, T.; Grigorova, S.; Kaloyanov, N. Effect of irradiation and packaging on lipid fraction of Bulgarian salami during storage. Rad. Phys. Chem. 2009, 78, 273–276. [Google Scholar] [CrossRef]
- Bakalivanova, T.; Tsvetkova, E.; Grigorova, S.; Kaloyanov, N. Gamma treatment effect on the lipid peroxidation of poultry meat cooked-smoked delicacies. Bulg. J. Agric. Sci. 2005, 11, 331–339. Available online: https://www.agrojournal.org/11/03-09.htm (accessed on 9 March 2005).
- Dokmeci, D.; Akpolat, M.; Aydogdu, N.; Uzal, C.; Doganay, L.; Turan, F.N. The protective effect of l-carnitine on ionizing radiation-induced free oxygen radicals. Scan. J. Lab. Anim. Sci. 2006, 33, 75–83. [Google Scholar] [CrossRef]
- Kaloyanov, N.; Brachkova, S. Study of the effect of gamma rays treatment on lipid peroxidation of non-perishable raw-dried salami. J. Univ. Chem. Technol. Metal. 2004, 39, 187–192. [Google Scholar]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef]
- Supruniuk, E.; Górski, J.; Chabowski, A. Endogenous and exogenous antioxidants in skeletal muscle fatigue development during exercise. Antioxidants 2023, 12, 501. [Google Scholar] [CrossRef]
- Voljč, M.; Frankič, T.; Levart, A.; Nemec, M.; Salobir, J. Evaluation of different vitamin E recommendations and bioactivity of α-tocopherol isomers in broiler nutrition by measuring oxidative stress in vivo and the oxidative stability of meat. Poult. Sci. 2011, 90, 1478–1488. [Google Scholar] [CrossRef]
- Bjelaković, L.; Kocic, G.; Radovanovic, D.; Antic, V.; Bjelakovic, B.; Antic, Z. Antioxidants and their importance during muscular exercise: A review. Facta Univer. Ser. Med. Biol. 2016, 18, 48–56. [Google Scholar] [CrossRef]
- Ribeiro, D.; Freitas, M.; Silva, A.M.S.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef]
- Dewanjee, S.; Bhattacharjee, N.; Chakraborty, P.; Bhattacharjee, S. Carotenoids as Antioxidants. In Carotenoids: Structure and Function in the Human Body, 1st ed.; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer: Cham, Switzerland, 2021; Volume 1, pp. 447–473. [Google Scholar] [CrossRef]
- Sandmann, G. Antioxidant protection from UV- and light-stress related to carotenoid structures. Antioxidants 2019, 8, 219. [Google Scholar] [CrossRef] [PubMed]
- Cenedella, R.J.; Neely, A.R.; Sexton, P. Concentration and distribution of ubiquinone (coenzyme Q), the endogenous lipid antioxidant, in the rat lens: Effect of treatment with simvastatin. Mol. Vis. 2005, 11, 594–602. Available online: http://www.molvis.org/molvis/v11/a70/v11a70-cenedella.pdf (accessed on 10 August 2005). [PubMed]
- Rezaharsamto, B.; Subroto, E. A review on bioactive peptides derived from various sources of meat and meat by-products. Int. J. Sci. Technol. Res. 2019, 8, 3151–3156. Available online: https://www.ijstr.org/final-print/dec2019/-A-Review-On-Bioactive-Peptides-Derived-From-Various-Sources-Of-Meat-And-Meat-By-products.pdf (accessed on 10 October 2023).
- Martini, S.; Conte, A.; Tagliazucchi, D. Comparative peptidomic profile and bioactivities of cooked beef, pork, chicken and turkey meat after in vitro gastro-intestinal digestion. J. Proteom. 2019, 208, 103500. [Google Scholar] [CrossRef] [PubMed]
- Promeyrat, A.; Sayd, T.; Laville, E.; Chambon, C.; Lebret, B.; Gatellier, P. Early post-mortem sarcoplasmic proteome of porcine muscle related to protein oxidation. Food Chem. 2011, 127, 1097–1104. [Google Scholar] [CrossRef]
- Wu, H.-C.; Shiau, C.-Y.; Chen, H.-M.; Chiou, T.-K. Antioxidant activities of carnosine, anserine, some free amino acids and their combination. J. Food Drug Anal. 2003, 11, 13. [Google Scholar] [CrossRef]
- Kim, H.C.; Ko, Y.-J.; Jo, C. Potential of 2D qNMR spectroscopy for distinguishing chicken breeds based on the metabolic differences. Food Chem. 2021, 342, 128316. [Google Scholar] [CrossRef]
- Kwon, J.A.; Yim, D.-G.; Kim, H.-J.; Ismail, A.; Kim, S.-S.; Lee, H.J.; Jo, C. Effect of temperature abuse on quality and metabolites of frozen/thawed beef loins. Food Sci. Anim. Res. 2022, 42, 341–349. [Google Scholar] [CrossRef]
- Vraneš, M.; Panić, J.; Tot, A.; Papović, S.; Gadžurić, S.; Podlipnik, Č.; Bešter-Rogač, M. From amino acids to dipeptide: The changes in thermal stability and hydration properties of β-alanine, L-histidine and L-carnosine. J. Mol. Liq. 2021, 328, 115250. [Google Scholar] [CrossRef]
- Kopec, W.; Jamroz, D.; Wiliczkiewicz, A.; Biazik, E.; Pudlo, A.; Korzeniowska, M.; Hikawczuk, T.; Skiba, T. Antioxidative characteristics of chicken breast meat and blood after diet supplementation with carnosine, L-histidine, and β-alanine. Antioxidants 2020, 9, 1093. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, J.; Cui, D.; Liu, L.; Zhang, S.; Shen, B.; Wu, Y.; Zhang, Q. Protective effect of carnosine on hydrogen peroxide-induced oxidative stress in human kidney tubular epithelial cells. Biochem. Biophys. Res. Com. 2021, 534, 576–582. [Google Scholar] [CrossRef]
- Rai, S.R.; Bhattacharyya, C.; Sarkar, A.; Chakraborty, S.; Sircar, E.; Dutta, S.; Sengupta, R. Glutathione: Role in oxidative/nitrosative stress, antioxidant defence, and treatments. ChemistrySelect 2021, 6, 4566–4590. [Google Scholar] [CrossRef]
- Iannitti, T.; Rottigni, V.; Palmieri, B. Role of free radicals and antioxidant defences in oral cavity-related pathologies. J. Oral Pathol. Med. 2012, 41, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.N.; Dunn, R.J.; Jeong, S.Y.; Zhu, Q.; Julien, J.-P.; David, S. Ceruloplasmin regulates iron levels in the CNS and prevents free radical injury. J. Neurosci. 2002, 22, 6578–6586. [Google Scholar] [CrossRef]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent developments in effective antioxidants: The structure and antioxidant properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and anti-inflammatory activity of ascorbic acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef] [PubMed]
- Lahučký, R.; Bahelka, I.; Novotná, K.; Vašíčková., K. Effects of dietary vitamin E and vitamin C supplementation on the level of α-tocopherol and L-ascorbic acid in muscle and on the antioxidative status and meat quality of pigs. Czech J. Anim. Sci. 2005, 50, 175–184. [Google Scholar] [CrossRef]
- Padayatty, S.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef]
- Kalač, P. Biologically active polyamines in beef, pork and meat products: A review. Meat Sci. 2006, 73, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Li, F.; Zhuang, H.; Chen, X.; Li, L.; Qiao, W.; Zhang, J. Effects of plant polyphenols and α-tocopherol on lipid oxidation, residual nitrites, biogenic amines, and N-nitrosamines formation during ripening and storage of dry-cured bacon. LWT Food Sci. Technol. 2015, 60, 199–206. [Google Scholar] [CrossRef]
- Qi, Q.; Hu, C.; Zhang, H.; Sun, R.; Liu, Q.; Ouyang, K.; Xie, Y.; Li, X.; Wu, W.; Liu, Y.; et al. Dietary supplementation with putrescine improves growth performance and meat quality of wenchang chickens. Animals 2023, 13, 1564. [Google Scholar] [CrossRef]
- Gherghina, M.-E.; Peride, I.; Tiglis, M.; Neagu, T.P.; Niculae, A.; Checherita, I.A. Uric acid and oxidative stress-relationship with cardiovascular, metabolic, and renal impairment. Int. J. Mol. Sci. 2022, 23, 3188. [Google Scholar] [CrossRef]
- Terevinto, A.; Ramos, A.; Castroman, G.; Cabrera, M.C.; Saadoun, A. Oxidative status, in vitro iron-induced lipid oxidation and superoxide dismutase, catalase and glutathione peroxidase activities in rhea meat. Meat Sci. 2010, 84, 706–710. [Google Scholar] [CrossRef]
- Gheisari, H.R.; Motamedi, H. Chloride salt type/ionic strength and refrigeration effects on antioxidant enzymes and lipid oxidation in cattle, camel and chicken meat. Meat Sci. 2010, 86, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Gheisar, H.R. Correlation between acid, TBA, peroxide and iodine values, catalase and glutathione peroxidase activities of chicken, cattle and camel meat during refrigerated storage. Veter. World 2011, 4, 153–157. [Google Scholar] [CrossRef]
- Daun, C.; Johansson, M.; Önning, G.; Åkesson, B. Glutathione peroxidase activity, tissue and soluble selenium content in beef and pork in relation to meat ageing and pig RN phenotype. Food Chem. 2001, 73, 313–319. [Google Scholar] [CrossRef]
- Daun, C.; Åkesson, B. Glutathione peroxidase activity, and content of total and soluble selenium in five bovine and porcine organs used in meat production. Meat Sci. 2004, 66, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R. Tissue-specific functions of individual glutathione peroxidases. Free Radic. Biol. Med. 1999, 27, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, E.N.; Hesketh, J.E.; Sinclair, B.R.; Koolaard, J.P.; Roy, N.C. Selenium-enriched foods are more effective at increasing glutathione peroxidase (gpx) activity compared with selenomethionine: A meta-analysis. Nutrients 2014, 6, 4002–4031. [Google Scholar] [CrossRef] [PubMed]
- Bayram, I.; Decker, E.A. Underlying mechanisms of synergistic antioxidant interactions during lipid oxidation. Trends Food Sci. Technol. 2023, 133, 219–230. [Google Scholar] [CrossRef]
- Vlahova-Vangelova, D.; Balev, D.; Kolev, N.; Popova, T.; Dragoev, S. Quality changes of Longissimus dorsi and Semimembranosus muscles and perirenal adipose tissue during frozen storage of lambs fed dihydroquercetin or dry distilled rose petals supplemented diet. Food Sci. Appl. Biotechnol. 2022, 5, 240–255. [Google Scholar] [CrossRef]
- McCarthy, T.L.; Kerry, J.P.; Kerry, J.F.; Lynch, P.B.; Buckley, D.J. Evaluation of the antioxidant potential of natural food/plant extract as compared with synthetic antioxidants and vitamin E in raw and cooked pork patties. Meat Sci. 2001, 57, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.; Zhang, H.; Xiong, Y.L. Antioxidant activity of spice extracts in a liposome system and in cooked pork patties and the possible mode of action. Meat Sci. 2010, 85, 772–778. [Google Scholar] [CrossRef]
- Yoo, K.M.; Lee, C.H.; Lee, H.; Moon, B.; Lee, C.Y. Relative antioxidant and cytoprotective activities of common herbs. Food Chem. 2008, 106, 929–936. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Balev, D.; Vulkova, T.; Dragoev, S.; Zlatanov, M.; Bahtchevanska, S. A comparative study on the effect of some antioxidants on the lipid and pigment oxidation of dry fermented sausages. Int. J. Food Sci. Technol. 2005, 40, 977–983. [Google Scholar] [CrossRef]
- Dragoev, S. Inhibition of lipid peroxidation of frozen mackerel by pre-storage antioxidant superficial treatment. Bulg. J. Agric. Sci. 2008, 14, 283–289. [Google Scholar]
- Dragoev, S.G.; Balev, D.K.; Nenov, N.S.; Vassilev, K.P.; Vlahova-Vangelova, D.B. Antioxidant capacity of essential oil spice extracts versus ground spices and addition of antioxidants in Bulgarian type dry-fermented sausages. Eur. J. Lipid Sci. Technol. 2016, 118, 1450–1462. [Google Scholar] [CrossRef]
- Balev, D.K.; Nenov, N.S.; Dragoev, S.G.; Vassilev, K.P.; Kirisheva, G.D.; Vlahova-Vangelova, D.B. Comparison of the effect of new spice freon extracts towards ground spices and antioxidants for improving the quality of Bulgarian-type dry-cured sausage. Pol. J. Food Nutr. Sci. 2017, 67, 59–66. [Google Scholar] [CrossRef]
- Pokorni, J.; Schmidt, S. Natural antioxidants functionality during food processing. In Antioxidants in Food. Practical Applications, 1st ed.; Pokorny, J., Yanishlieva, N., Gordon, M., Eds.; Woodhead Publishing Ltd.: Washington, DC, USA; CRC Press: Boca Raton, FL, USA, 2001; Chapter 14; pp. 331–354. [Google Scholar]
- Estévez, M. Critical overview of the use of plant antioxidants in the meat industry: Opportunities, innovative applications and future perspectives. Meat Sci. 2021, 181, 108610. [Google Scholar] [CrossRef]
- Timoshnikov, V.A.; Selyutina, O.Y.; Polyakov, N.E.; Didichenko, V.; Kontoghiorghes, G.J. Mechanistic insights of chelator complexes with essential transition metals: Antioxidant/pro-oxidant activity and applications in medicine. Int. J. Mol. Sci. 2022, 23, 1247. [Google Scholar] [CrossRef]
- Gulcin, I.; Alwasel, S.H. Metal ions, metal chelators and metal chelating assay as antioxidant method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- Sun, M.; He, Z.; Jaisi, D.P. Role of metal complexation on the solubility and enzymatic hydrolysis of phytate. PLoS ONE 2021, 16, e0255787. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M. Synergistic, antagonistic and additive antioxidant effects in the binary mixtures. Phytochem. Rev. 2020, 19, 63–103. [Google Scholar] [CrossRef]
- Sáyago-Ayerdi, S.G.; Brenes, A.; Viveros, A.; Goñi, I. Antioxidative effect of dietary grape pomace concentrate on lipid oxidation of chilled and long-term frozen stored chicken patties. Meat Sci. 2009, 83, 528–533. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E.; Siebenhandl, S.; Bauer, F. The antioxidative properties of Holy basil and Galangal in cooked ground pork. Meat Sci. 2006, 72, 446–456. [Google Scholar] [CrossRef] [PubMed]
- El-Alim, S.S.L.A.; Lugasi, A.; Hóvári, J.; Dworschák, E. Culinary herbs inhibit lipid oxidation in raw and cooked minced meat patties during storage. J. Sci. Food Agric. 1999, 79, 277–285. [Google Scholar] [CrossRef]
- Tanabe, H.; Yoshida, M.; Tomita, N. Comparison of the antioxidant activities of 22 commonly used culinary herbs and spices on the lipid oxidation of pork meat. Anim. Sci. J. 2002, 73, 389–393. [Google Scholar] [CrossRef]
- Dimitrov, N.; Dyankova, S.; Solak, A.; Miteva, D.; Ivanova, S. Study of the effect of treatment with aqueous extracts by oregano and wild basil on raw poultry meat. BIO Web Conf. 2023, 58, 01009. [Google Scholar] [CrossRef]
- Bak, K.H.; Bauer, S.; Bauer, F. Effect of different genotypes and harvest times of sage (Salvia spp. labiatae) on lipid oxidation of cooked meat. Antioxidants 2023, 12, 616. [Google Scholar] [CrossRef]
- Panda, A.K.; Cherian, G. Tissue tocopherol status, meat lipid stability, and serum lipids in broiler chickens fed Artemisia annua. Eur. J. Lipid Sci. Technol. 2017, 119, 1500438. [Google Scholar] [CrossRef]
- Mattos, G.N.; Tonon, R.V.; Furtado, A.A.L.; Cabral, L.M.C. Grape by-product extracts against microbial proliferation and lipid oxidation: A review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef]
- Bennato, F.; Di Luca, A.; Martino, C.; Ianni, A.; Marone, E.; Grotta, L.; Ramazzotti, S.; Cichelli, A.; Martino, G. Influence of grape pomace intake on nutritional value, lipid oxidation and volatile profile of poultry meat. Foods 2020, 9, 508. [Google Scholar] [CrossRef]
- Untea, A.E.; Varzaru, I.; Vlaicu, P.A.; Turcu, R.P.; Panaite, T.D. Studies on antioxidant activities of grape pomace using in vitro, ex vivo, and in vivo models. Food Meas. 2023, 17, 121–128. [Google Scholar] [CrossRef]
- Goñi, I.; Brenes, A.; Centeno, C.; Viveros, A.; Saura-Calixto, F.; Rebolé, A.; Arija, I.; Estevez, R. Effect of dietary grape pomace and vitamin e on growth performance, nutrient digestibility, and susceptibility to meat lipid oxidation in chickens. Poult. Sci. 2007, 86, 508–516. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Rebolé, A.; Rica, B.D.; Arija, I.; Brenes, A. Influence of dietary enzyme addition on polyphenol utilization and meat lipid oxidation of chicks fed grape pomace. Food Res. Int. 2015, 73, 197–203. [Google Scholar] [CrossRef]
- Guerra-Rivas, C.; Vieira, C.; Rubio, B.; Martínez, B.; Gallardo, B.; Mantecón, A.R.; Lavín, P.; Manso, T. Effects of grape pomace in growing lamb diets compared with vitamin E and grape seed extract on meat shelf life. Meat Sci. 2016, 116, 221–229. [Google Scholar] [CrossRef]
- Zhao, J.X.; Li, Q.; Zhang, R.X.; Liu, W.Z.; Ren, Y.S.; Zhang, C.X.; Zhang, J.X. Effect of dietary grape pomace on growth performance, meat quality and antioxidant activity in ram lambs. Anim. Feed Sci. Technol. 2018, 236, 76–85. [Google Scholar] [CrossRef]
- Garrido, M.D.; Auqui, M.; Martí, N.; Linares, M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT Food Sci. Technol. 2011, 44, 2238–2243. [Google Scholar] [CrossRef]
- Carpes, S.T.; Pereira, D.; de Moura, C.; dos Reis, A.S.; da Silva, L.D.; Oldoni, T.L.C.; Almeida, J.F.; Plata-Oviedo, M.V.S. Lyophilized and microencapsulated extracts of grape pomace from winemaking industry to prevent lipid oxidation in chicken pâté. Braz. J. Food Technol. 2020, 23, e2019112. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Saldanha, T.; Soares, R.A.M.; Torres, E.A.F.S. Effect of natural antioxidant combinations on lipid oxidation in cooked chicken meat during refrigerated storage. Food Chem. 2012, 135, 1383–1390. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E.; Siebenhandl, S.; Bauer, F. Antioxidative effect of added dried Holy basil and its ethanolic extracts on susceptibility of cooked ground pork to lipid oxidation. Food Chem. 2007, 100, 129–135. [Google Scholar] [CrossRef]
- Falowo, A.B.; Mukumbo, F.E.; Idamokoro, E.M.; Afolayan, A.J.; Muchenje, V. Phytochemical constituents and antioxidant activity of sweet basil (Ocimum basilicum L.) essential oil on ground beef from boran and nguni cattle. Int. J. Food Sci. 2019, 2019, 2628747. [Google Scholar] [CrossRef] [PubMed]
- Sharafati-Chaleshtori, R.; Rokni, N.; Rafieian-Kopaei, M.; Drees, F.; Saleh, E. Antioxidant and antibacterial activity of basil (Ocimum basilicum L.) essential oil in beef burger. J. Agr. Sci. Tech. 2015, 17, 817–826. Available online: https://jast.modares.ac.ir/article-23-11118-en.pdf (accessed on 14 December 2023).
- Cichoski, A.J.; Cansian, R.L.; Oliveira, D.; Gaio, I.; Saggirato, A.G. Lipid and protein oxidation in the internal part of Italian type salami containing basil essential oil (Ocimum basilicum L.). Food Sci. Technol. 2011, 31, 200024. [Google Scholar] [CrossRef]
- Wong, J.W.; Hashimoto, K.; Shibamoto, T. Antioxidant activities of rosemary and sage extracts and vitamin E in a model meat system. J. Agric. Food Chem. 1995, 43, 2707–2712. [Google Scholar] [CrossRef]
- Lopez-Bote, C.J.; Gray, J.I.; Gomaa, E.A.; Flegal, C.J. Effect of dietary administration of oil extracts from rosemary and sage on lipid oxidation in broiler meat. Br. Poult. Sci. 1998, 39, 235–240. [Google Scholar] [CrossRef]
- Gantner, M.; Brodowska, M.; Górska-Horczyczak, E.; Wojtasik-Kalinowska, I.; Najda, A.; Pogorzelska, E.; Godziszewska, J. Antioxidant effect of sage (Salvia officinalis L.) extract on turkey meatballs packed in cold modified atmosphere. CyTA J. Food 2018, 16, 628–636. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant activity in meat treated with oregano and sage essential oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Ünal, K.; Babaoglu, A.S.; Karakaya, M. Effect of oregano, sage and rosemary essential oils on lipid oxidation and color properties of minced beef during refrigerated storage. J. Ess. Oil Bear. Plants 2014, 17, 797–805. [Google Scholar] [CrossRef]
- Mariutti, L.R.B.; Nogueira, G.C.; Bragagnolo, N. Lipid and cholesterol oxidation in chicken meat are inhibited by sage but not by garlic. J. Food Sci. 2011, 76, C909–C915. [Google Scholar] [CrossRef] [PubMed]
- Bianchin, M.M.; Pereira, D.; de Florio Almeida, J.; de Moura, C.; Pinheiro, R.S.; Heldt, L.F.S.; Haminiuk, C.W.I.; Carpes, S.T. Antioxidant properties of lyophilized rosemary and sage extracts and its effect to prevent lipid oxidation in poultry pátê. Molecules 2020, 25, 5160. [Google Scholar] [CrossRef]
- Forte, C.; Branciari, R.; Pacetti, D.; Miraglia, D.; Ranucci, D.; Acuti, G.; Balzano, M.; Frega, N.G.; Trabalza-Marinucci, M. Dietary oregano (Origanum vulgare L.) aqueous extract improves oxidative stability and consumer acceptance of meat enriched with CLA and n-3 PUFA in broilers. Poult. Sci. 2018, 97, 1774–1785. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Christaki, E.; Fletouris, D.J.; Florou-Paneri, P.; Spais, A.B. The effect of dietary oregano essential oil on lipid oxidation in raw and cooked chicken during refrigerated storage. Meat Sci. 2002, 62, 259–265. [Google Scholar] [CrossRef]
- Marcincak, S.; Cabadaj, R.; Popelka, P.; Šoltýsová, L. Antioxidative effect of oregano supplemented to broilers on oxidative stability of poultry meat. Sloven. Vet. Res. 2008, 45, 61–66. Available online: https://www.slovetres.si/index.php/SVR/issue/view/31/34 (accessed on 28 April 2008).
- Park, J.H.; Kang, S.N.; Shin, D.; Shim, K.S. Antioxidant enzyme activity and meat quality of meat type ducks fed with dried oregano (Origanum vulgare L.) powder. Asian Australas J. Anim. Sci. 2015, 28, 79–85. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Deligeorgis, S.G.; Bizelis, J.A.; Dardamani, A.; Theodosiou, I.; Fegeros, K. Effect of dietary oregano oil supplementation on lamb meat characteristics. Meat Sci. 2008, 79, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Botsoglou, N.A.; Fletouris, D.J.; Florou-Paneri, P.; Christaki, E.; Spais, A.B. Inhibition of lipid oxidation in long-term frozen stored chicken meat by dietary oregano essential oil and α-tocopheryl acetate supplementation. Food Res. Int. 2003, 36, 207–213. [Google Scholar] [CrossRef]
- Govaris, A.; Botsoglou, N.; Papageorgiou, G.; Botsoglou, E.; Ambrosiadis, I. Dietary versus post-mortem use of oregano oil and/or α-tocopherol in turkeys to inhibit development of lipid oxidation in meat during refrigerated storage. Int. J. Food Sci. Nutr. 2004, 55, 115–123. [Google Scholar] [CrossRef]
- Oleynikov, V. Antioxidant and antimicrobial properties of oregano extract (Origani vulgaris herba L.). Foods Raw Mater. 2020, 8, 84–90. [Google Scholar] [CrossRef]
- Manhani, M.R.; Nicoletti, M.A.; Barretto, A.C.D.S.; De Jesus, G.R.; Munhoz, C.C.; De Abreu, G.R.; Zaccarelli-Magalhães, J.; Fukushima, A.R. Antioxidant action of rosemary and oregano extract in pre-cooked meat hamburger. Food Nutr. Sci. 2018, 9, 806–817. [Google Scholar] [CrossRef]
- Pirmohamammadi, A.; Daneshyar, M.; Farhoomand, P.; Aliakbarlu, J.; Hamian, F. Effects of Thymus vulgaris and Mentha pulegium on colour, nutrients and peroxidation of meat in heat-stressed broilers. South Afr. J. Anim. Sci. 2016, 46, 7. [Google Scholar] [CrossRef]
- Mehdipour, Z.; Afsharmanesh, M.; Sami, M. Effects of supplemental thyme extract (Thymus vulgaris L.) on growth performance, intestinal microbial populations, and meat quality in Japanese quails. Comp. Clin. Pathol. 2014, 23, 1503–1508. [Google Scholar] [CrossRef]
- Dalle Zotte, A.; Cullere, M.; Sartori, A.; Szendrő, Z.; Kovàcs, M.; Giaccone, V.; Dal Bosco, A. Dietary spirulina (Arthrospira platensis) and thyme (Thymus vulgaris) supplementation to growing rabbits: Effects on raw and cooked meat quality, nutrient true retention and oxidative stability. Meat Sci. 2014, 98, 94–103. [Google Scholar] [CrossRef]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef]
- Hailemariam, G.A.; Emire, S.A. Antioxidant activity and preservative effect of thyme (Thymus schimperi R.). Br. J. Appl. Sci. Technol. 2013, 3, 1311–1326. [Google Scholar] [CrossRef]
- Boskovic, M.; Glisic, M.; Djordjevic, J.; Starcevic, M.; Glamoclija, N.; Djordjevic, V.; Baltic, M.Z. Antioxidative activity of thyme (Thymus vulgaris) and oregano (Origanum vulgare) essential oils and their effect on oxidative stability of minced pork packaged under vacuum and modified atmosphere. J. Food Sci. 2019, 84, 2467–2474. [Google Scholar] [CrossRef]
- Hęś, M.; Gramza-Michałowska, A. Effect of plant extracts on lipid oxidation and changes in nutritive value of protein in frozen-stored meat products. J. Food Proc. Preserv. 2017, 41, e12989. [Google Scholar] [CrossRef]
- Gahruie, H.H.; Hosseini, S.M.H.; Taghavifard, M.H.; Eskandari, M.H.; Golmakani, M.-T.; Shad, E. Lipid oxidation, color changes, and microbiological quality of frozen beef burgers incorporated with shirazi thyme, cinnamon, and rosemary extracts. J. Food Qual. 2017, 2017, 6350156. [Google Scholar] [CrossRef]
- Kostadinović, L.; Lević, J.; Popović, S.; Čabarkapa, I.; Puvača, N.; Đuragić, O.; Kormanjoš, Š. Dietary inclusion of Artemisia absinthium for management of growth performance, antioxidative status and quality of chicken meat. Europ. Poult. Sci. 2015, 79, 75. [Google Scholar] [CrossRef]
- Wan, X.L.; Song, Z.H.; Niu, Y.; Cheng, K.; Zhang, J.F.; Ahmad, H.; Zhang, L.L.; Wang, T. Evaluation of enzymatically treated Artemisia annua L. on growth performance, meat quality, and oxidative stability of breast and thigh muscles in broilers. Poult. Sci. 2017, 96, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Rahiminiat, F.; Ghazanfari, S.; Mohammadi, Z.; Sharifi, S.D. Feeding artemisia sieberi, coriander and clove essential oils alters muscle lipid oxidation in broiler chicken. Bulg. J. Agric. Sci. 2017, 23, 625–631. Available online: https://www.agrojournal.org/23/04-17.pdf (accessed on 14 December 2023).
- Kim, C.H.; Kim, G.-B.; Chang, M.B.; Bae, G.S.; Paik, I.K.; Kil, D.Y. Effect of dietary supplementation of Lactobacillus-fermented Artemisia princeps on growth performance, meat lipid peroxidation, and intestinal microflora in Hy-line Brown male chickens. Poult. Sci. 2012, 91, 2845–2851. [Google Scholar] [CrossRef] [PubMed]
- Cherian, G.; Orr, A.; Burke, I.C.; Pan, W. Feeding Artemisia annua alters digesta pH and muscle lipid oxidation products in broiler chickens. Poultry Sci. 2013, 92, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Aouadi, D.; Luciano, G.; Vasta, V.; Nasri, S.; Brogna, D.M.R.; Abidi, S.; Priolo, A.; Salem, H.B. The antioxidant status and oxidative stability of muscle from lambs receiving oral administration of Artemisia herba alba and Rosmarinus officinalis essential oils. Meat Sci. 2014, 97, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.E.; Kim, H.W.; Choi, Y.S.; Lee, S.Y.; Yeo, E.J.; Ham, Y.K.; Choi, S.M.; Lee, M.A.; Kim, C.J. Evaluation of the antioxidant effect of Ganghwayakssuk (Artemisia princeps Pamp.) extract alone and in combination with ascorbic acid in raw chicken patties. Poult. Sci. 2013, 92, 3244–3250. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-S.; Choi, J.-H.; Kim, H.-Y.; Kim, H.-W.; Lee, M.-A.; Chung, H.-K.; Kim, C.-J. The antioxidative properties of Ganghwayakssuk (Artemisia princeps Pamp.) extracts added to refrigerated raw chicken nugget batter against lipid oxidation. Food Sci. Anim. Res. 2011, 31, 166–175. [Google Scholar] [CrossRef]
- Kolev, N. Natural antioxidants–An alternative for reduction of nitrites in cooked meat products. Food Sci. Appl. Biotechnol. 2022, 5, 64–76. [Google Scholar] [CrossRef]
- Balev, D.; Ivanov, G.; Nikolov, H.; Dragoev, S. Effect of natural antioxidant pretreatment on the properties of colour surface of chilled stored salmon discs. Bulg. J. Agric. Sci. 2009, 15, 379–385. Available online: http://www.agrojournal.org/15/05-01-09.pdf (accessed on 10 October 2023).
- Shah, R.; Margison, K.; Pratt, D.A. The potency of diarylamine radical-trapping antioxidants as inhibitors of ferroptosis underscores the role of autoxidation in the mechanism of cell death. ACS Chem. Biol. 2017, 12, 2538–2545. [Google Scholar] [CrossRef]
- Cores, Á.; Carmona-Zafra, N.; Clerigué, J.; Villacampa, M.; Menéndez, J.C. Quinones as neuroprotective agents. Antioxidants 2023, 12, 1464. [Google Scholar] [CrossRef] [PubMed]
- Ingold, K.U.; Pratt, D.A. Advances in radical-trapping antioxidant chemistry in the 21st century: A kinetics and mechanisms perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef]
- Staykov, A.S.; Dragoev, S.G.; Balev, D.K.; Vlahova-Vangelova, D.B.; Pavlova, M.R. Preserving the quality and prolongation the shelf-life of beef packed under vacuum or modified atmosphere using ternary antioxidant blend. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 617–622. [Google Scholar] [CrossRef]
- Kolev, N.; Vlahova-Vangelova, D.; Balev, D.; Dragoev, S.G. Effect of three-component antioxidant blend on oxidative stability and nitrite reduction of cooked sausages. ACTA Scien. Polon. Technol. Alim. 2022, 21, 205–212. [Google Scholar] [CrossRef]
- Vlahova-Vangelova, D.B.; Dragoev, S.; Balev, D.; Ivanova, S.; Nikolova, T.; Nakev, J.; Gerrard, D. Improving the oxidative stability of pork by antioxidant type phytonutrients. Biointer. Res. Appl. Chem. 2020, 10, 5624–5633. [Google Scholar] [CrossRef]
- Abilmazhinova, N.; Vlahova-Vangelova, D.; Dragoev, S.; Abzhanova, S.; Balev, D. Optimization of the oxidative stability of horse minced meat enriched with dihydroquercetin and vitamin C as a new functional food. Comptes Rendus De L’academie Bulg. Des Sci. 2020, 73, 1033–1040. [Google Scholar] [CrossRef]
- Balev, D.; Vlahova-Vangelova, D.; Dragoev, S.; Baleva, L.; Dimitrova, M.; Kolev, N. Antioxidative effect of dry distilled rose petals extract in traditional Bulgarian dry fermented sausages with reduced nitrate content. Food Sci. Appl. Biotechnol. 2022, 5, 173–180. [Google Scholar] [CrossRef]
- Malomo, A.; Odubanjo, O.; Olawoye, B.; Olaniyi, O.; Lawal, M.; Fasogbon, B. Effect of turmeric on the quality of canned African catfish in tomato sauce during storage at 25 °C and 45 °C. Food Sci. Appl. Biotechnol. 2022, 5, 12–21. [Google Scholar] [CrossRef]
- Dragoev, S. Influence of the vacuum packaging, glazing and superficial treatment with solution of sodium erythrobate on lipid peroxidation of deep-frozen mackerel (Scomber scombrus). Sci. Work. UFT 2008, 55, 41–46. (In Bulgarian) [Google Scholar]
- Ivanov, G.; Balev, D.; Nikolov, H.; Dragoev, S. Improvement of the chilled salmon sensory quality by pulverisation with natural dihydroquercetin solutions. Bulg. J. Agric. Sci. 2009, 15, 154–162. Available online: http://www.agrojournal.org/15/02-08-09.pdf (accessed on 10 October 2023).
- Ivanov, G.Y.; Staykov, A.S.; Balev, D.K.; Dragoev, S.G.; Filizov, E.H.; Vassilev, K.P.; Grozdeva, T.G. Effect of treatment with natural antioxidant on the chilled beef lipid oxidation. Adv. J. Food Sci. Technol. 2010, 2, 213–217. Available online: https://maxwellsci.com/print/ajfst/v2-213-218.pdf (accessed on 30 July 2010).
- Dragoev, S.G.; Balev, D.K.; Ivanov, G.Y.; Nikolova-Damyanova, B.M.; Grozdeva, T.G.; Filizov, E.H.; Vassilev, K.P. Effect of superficial treatment with new natural antioxidant on salmon (Salmo salar) lipid oxidation. Acta Alim. 2014, 43, 1–8. [Google Scholar] [CrossRef]
- Staykov, A.S.; Vassilev, K.P.; Dragoev, S.G.; Balev, D.K.; Vlahova-Vangelova, D.B.; Vuleva, A.I.; Rustemova, F.Y. Inhibition of lipid oxidation in different types packaged beef by a composition of natural antioxidants. Oxid. Com. 2015, 38, 666–676. [Google Scholar]
- Dragoev, S.G. Inhibition of lipid oxidation of frozen chicken legs by treatment with sodium lactate, natural antioxidants and vacuum-packaging. EC Nutr. 2015, 1, 203–216. Available online: https://ecronicon.net/assets/ecnu/pdf/ECNU-01-000026.pdf (accessed on 9 May 2015).
- Dragoev, S.G.; Staykov, A.S.; Vassilev, K.P.; Balev, D.K.; Vlahova-Vangelova, D.B. Improvement the quality and the shelf life of the high oxygen modified atmosphere packaged veal by superficial spraying with dihydroquercetin solution. Int. J. Food Sci. 2014, 2014, 629062. [Google Scholar] [CrossRef]
- Shakil, M.H.; Trisha, A.T.; Rahman, M.; Talukdar, S.; Kobun, R.; Huda, N.; Zzaman, W. Nitrites in cured meats, health risk issues, alternatives to nitrites: A review. Foods 2022, 11, 3355. [Google Scholar] [CrossRef]
- Andrade, B.F.; Guimarães, A.S.; do Carmo, L.R.; Tanaka, M.S.; Fontes, P.R.; Ramos, A.L.S.; Ramos, E.M. S-nitrosothiols as nitrite alternatives: Effects on residual nitrite, lipid oxidation, volatile profile, and cured color of restructured cooked ham. Meat Sci. 2024, 209, 109397. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Manini, P.; Panzella, L. Secondary targets of nitrite-derived reactive nitrogen species: Nitrosation/nitration pathways, antioxidant defence mechanisms and toxicological implications. Chem. Res. Toxicol. 2011, 24, 2071–2092. [Google Scholar] [CrossRef]
- Perez-Alvarez, J.A.; Fernandez-Lopez, J. Chapter 4. Chemical and biochemical aspects of color in muscle foods. In Handbook of Meat, Poultry and Seafood Quality; Nollet, L.M.L., Ed.; Blackwell Publishing Professional: Ames, IA, USA, 2007; pp. 25–44. [Google Scholar]
- Kanner, J.; Harel, S.; Granit, R. Nitric oxide, an inhibitor of lipid oxidation by lipoxygenase, cyclooxygenase and hemoglobin. Lipids 1992, 27, 46–49. [Google Scholar] [CrossRef]
- Wood, I.; Trostchansky, A.; Rubbo, H. Structural considerations on lipoxygenase function, inhibition and crosstalk with nitric oxide pathways. Biochimie 2020, 178, 170–180. [Google Scholar] [CrossRef]
- Møller, J.K.S.; Skibsted, L.H. Nitric oxide and myoglobins. Chem. Rev. 2002, 102, 1167–1178. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, X.; Jiao, Y.; Liu, Q.; Li, R.; Wang, W. An out of box thinking: The changes of iron-porphyrin during meat processing and gastrointestinal tract and some methods for reducing its potential health hazard. Cr. Rev. Food Sci. Nutr. 2023, 63, 1390–1405. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ahn, D.U. Lipid oxidation and its implications to meat quality and human health. Food Sci. Biotechnol. 2019, 28, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Karwowska, M.; Kononiuk, A.; Wójciak, K.M. Impact of sodium nitrite reduction on lipid oxidation and antioxidant properties of cooked meat products. Antioxidants 2020, 9, 9. [Google Scholar] [CrossRef]
- Spiteller, G.; Afzal, M. The action of peroxyl radicals, powerful deleterious reagents, explains why neither cholesterol nor saturated fatty acids cause atherogenesis and age-related diseases. Chem. Europ. J. 2014, 20, 14928–14945. [Google Scholar] [CrossRef]
- De Los Santos, E.R.; Vera, V.V.; Del Aguila Moyano, S.; Rosas, J.V.; Ordoñez, A.M.; Galdos, M.A. Physicochemical and sensory changes of vacuum-packed, salt-ripened anchovy fillets (Engraulis ringens) stored at 8 and 20 °C. Cogent Food Agric. 2018, 4, 1549194. [Google Scholar] [CrossRef]
- Duran, A.; Kahve, H.I. The effect of chitosan coating and vacuum packaging on the microbiological and chemical properties of beef. Meat Sci. 2020, 162, 107961. [Google Scholar] [CrossRef]
- Milijasevic, J.B.; Milijasevic, M.; Djordjevic, V. Modified atmosphere packaging of fish–An impact on shelf life. The 60th International Meat Industry Conference MEATCON 2019, 22–25 September 2019, Kopaonik, Serbia. IOP Conf. Ser. Earth Environ. Sci. 2019, 333, 012028. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Q.; Xu, J.; Sun, F.; Liu, H.; Kong, B. Effects of modified atmosphere packaging with various co2 concentrations on the bacterial community and shelf-life of smoked chicken legs. Foods 2022, 11, 559. [Google Scholar] [CrossRef]
- Balev, D.K.; Staykov, A.S.; Ivanov, G.Y.; Dragoev, S.G.; Filizov, E.H.; Vassilev, K.P.; Grozdeva, T.G. Effect of natural antioxidant treatment and modified atmosphere packaging on the quality and shelf-life of chilled beef. Agric. Biol. J. North Am. 2010, 1, 451–457. Available online: https://scihub.org/ABJNA/PDF/2010/4/1-4-451-457.pdf (accessed on 12 October 2023). [CrossRef]
- Balev, D.K.; Staykov, A.S.; Ivanov, G.Y.; Dragoev, S.G.; Filizov, E.H. Color stability improvement of chilled beef by natural antioxidant treatment and modified atmosphere packaging. Am. J. Food Technol. 2011, 6, 117–128. [Google Scholar] [CrossRef]
- Kandeepan, G.; Tahseen, A. Modified atmosphere packaging (map) of meat and meat products: A review. J. Package Technol. Res. 2022, 6, 137–148. [Google Scholar] [CrossRef]
- Martínez, L.; Cilla, I.; Beltrán, J.A.; Roncalés, P. Combined effect of modified atmosphere packaging and addition of rosemary (Rosmarinus officinalis), ascorbic acid, red beet root (Beta vulgaris), and sodium lactate and their mixtures on the stability of fresh pork sausages. J. Agric. Food Chem. 2006, 54, 4674–4680. [Google Scholar] [CrossRef]
- Maciel, V.B.V.; Contini, L.R.F.; Yoshida, C.M.P.; Venturini, A.C. Application of edible biopolymer coatings on meats, poultry, and seafood. In Biopolymer Membranes and Films. Health, Food, Environment, and Energy Applications, 1st ed.; de Moraes, M.A., da Silva, C.F., Vieira, R.S., Eds.; Elsevier Inc.: Maryland Heights, MO, USA, 2020; Volume 1, pp. 515–533. [Google Scholar] [CrossRef]
- Song, D.-H.; Hoa, V.B.; Kim, H.W.; Khang, S.M.; Cho, S.-H.; Ham, J.-S.; Seol, K.-H. Edible films on meat and meat products. Coatings 2021, 11, 1344. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and characterization of grass carp collagen-chitosan-lemon essential oil composite films for application as food packaging. Int. J. Biol. Macro. 2020, 160, 340–351. [Google Scholar] [CrossRef]
- Ahmed, M.; Verma, A.K.; Patel, R. Physiochemical, antioxidant, and food simulant release properties of collagen-carboxymethyl cellulose films enriched with Berberis lyceum root extract for biodegradable active food packaging. J. Food Proc. Pres. 2022, 46, e16485. [Google Scholar] [CrossRef]
- Qu, W.; Xiong, T.; Wang, B.; Li, Y.; Zhang, X. The modification of pomegranate polyphenol with ultrasound improves mechanical, antioxidant, and antibacterial properties of tuna skin collagen-chitosan film. Ultras. Sonochem. 2022, 85, 105992. [Google Scholar] [CrossRef]
- Shokraneh, N.; Ariaii, P.; Ghahroudi, F.R.; Hasannia, F.; Sabaghpour, S. The effect of coating with green tea extract and collagen fiber on quality attributes of vacuum packaged sausage. J. Food Biosci. Technol. (Islam. Azad Univ. Sci. Res. Branch) 2017, 7, 23–30. Available online: https://journals.srbiau.ac.ir/article_9609.html?lang=en (accessed on 14 December 2023).
- Ucak, I.; Khalily, R.; Carrillo, C.; Tomasevic, I.; Barba, F.J. Potential of propolis extract as a natural antioxidant and antimicrobial in gelatin films applied to rainbow trout (Oncorhynchus mykiss) fillets. Foods 2020, 9, 1584. [Google Scholar] [CrossRef]
- Liu, J.; Shibata, M.; Ma, Q.; Liu, F.; Lu, Q.; Shan, Q.; Hagiwara, T.; Bao, J. Characterization of fish collagen from blue shark skin and its application for chitosan- collagen composite coating to preserve red porgy (Pagrus major) meat. J. Food Biochem. 2020, 44, e13265. [Google Scholar] [CrossRef]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of salmon bone gelatine with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Vlahova-Vangelova, D.; Balev, D.; Kolev, N.; Nikolova, L.; Dragoev, S. Preservation of fish freshness by edible alginate coating and surface treatment with dry distilled rose petals extract or L-ascorbic acid. Food Sci. Appl. Biotechnol. 2022, 5, 181–189. [Google Scholar] [CrossRef]
- Gedif, H.D.; Tkaczewska, J.; Jamróz, E.; Zając, M.; Kasprzak, M.; Pająk, P.; Grzebieniarz, W.; Nowak, N. Developing technology for the production of innovative coatings with antioxidant properties for packaging fish products. Foods 2023, 12, 26. [Google Scholar] [CrossRef]
- Mirzaei, A.; Nezafat, Z.; Mirzaei, G.; Javanshir, S.; Karimkhani, M.M.; Jamshidi, A. Preparation of biodegradable antibacterial, antioxidant, and pH-sensitive hydrogel films based on carboxymethyl cellulose and collagen for fish freshness monitoring. Res. Sq. 2023, 1, 9. [Google Scholar] [CrossRef]
- Tanha, L.D.; Khoshkhoo, Z.; Azizi, M.H. Application of edible coating made of sturgeon gelatin and Portulaca oleracea extract for improving the shelf life of fish sausages. Food Meas. 2021, 15, 4306–4313. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lin, C.-S. Enhancement of the storage quality of frozen bonito fillets by glazing with tea extract. Food Control 2005, 16, 169–175. [Google Scholar] [CrossRef]
- Fernandes, M.G.; Cervi, C.B.; de Carvalho, R.A.; Lapa-Guimarães, J. Evaluation of turmeric extract as an antioxidant for frozen streaked prochilod (Prochilodus lineatus) fillets. J. Aquat. Food Prod. Technol. 2017, 26, 1057–1069. [Google Scholar] [CrossRef]
- Méndez, L.; Zhang, B.; Aubourg, S.P. Enhancement of lipid stability of frozen fish by octopus-waste glazing. Foods 2023, 12, 2298. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.; Li, P.; Yu, W.; Wang, J.; Xie, J. Effects of glazing with preservatives on the quality changes of squid during frozen storage. Appl. Sci. 2019, 9, 3847. [Google Scholar] [CrossRef]
- Trigo, M.; Rodríguez, A.; Dovale, G.; Pastén, A.; Vega-Gálvez, A.; Aubourg, S.P. The effect of glazing based on saponin-free quinoa (Chenopodium quinoa) extract on the lipid quality of frozen fatty fish. LWT 2018, 98, 231–236. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.; Xie, J. Effect of glazing with different materials on the quality of tuna during frozen storage. Foods 2020, 9, 231. [Google Scholar] [CrossRef]
- Wang, W.; Dong, H.; Zhang, Y.; Zhao, Y.; Peng, J.; He, Q. Frozen kinetics for the effects of a functional glazing on physical, chemical, and microbial properties of tilapia during storage. J. Food Proc. Pres. 2022, 46, e16671. [Google Scholar] [CrossRef]
- Sharefiabadi, E.; Serdaroglu, M. Application of vacuum impregnation in muscle foods. Food Sci. Appl. Biotechnol. 2023, 6, 200–214. [Google Scholar] [CrossRef]
- Demir, H.; Çelik, S.; Sezer, Y.Ç. Effect of ultrasonication and vacuum impregnation pre-treatments on the quality of beef marinated in onion juice a natural meat tenderizer. Food Sci. Technol. Int. 2022, 28, 340–352. [Google Scholar] [CrossRef]
- Martinez, O.; Salmeron, J.; Guillen, M.; Casas, C. Sensorial and physicochemical characteristics of salmon (Salmo salar) treated by different smoking processes during storage. Food Sci. Technol. Int. 2007, 13, 477–484. [Google Scholar] [CrossRef]
- Shen, S.K.; Chen, Y.W.; Dong, X.P.; Liu, F.J.; Cai, W.Q.; Wei, J.L.; Jin, D.L.; Lin, M.M. The effect of different salt concentration and time combinations in physicochemical properties and microstructure of russian sturgeon (Acipenser gueldenstaedtii) fillets under vacuum impregnation. J. Food Proc. Pres. 2020, 44, e14967. [Google Scholar] [CrossRef]
- Zhao, W.; Yu, D.; Xia, W. Vacuum impregnation of chitosan coating combined with water-soluble polyphenol extracts on sensory, physical state, microbiota composition and quality of refrigerated grass carp slices. Int. J. Biol. Macromol. 2021, 193, 847–855. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.; Zhao, L.; Chen, L.; He, Y.; Yang, H. Vacuum impregnation of fish gelatin combined with grape seed extract inhibits protein oxidation and degradation of chilled tilapia fillets. Food Chem. 2019, 294, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Berdague, J.L.; Bonnaud, N.; Rousset, S.; Touraille, C. Influence of pig crossbreed on the composition, volatile compounds in dry sausages. Meat Sci. 1993, 34, 119–129. [Google Scholar] [CrossRef]
- Radovčić, N.M.; Poljanec, I.; Vidinski, P.; Novina, K.; Medić, H. Influence of different pig genotype on aroma, colour and fatty acid composition of smoked dry-cured ham. MESO: Prvi Hrvat. Časopis O Mesu 2019, 21, 548–561. [Google Scholar] [CrossRef]
- Deng, S.Y.; Liu, F.; Huang, J.; Liu, D.; Han, C.; Zhang, C.; Blecker, C. Evaluation of volatile flavor compounds in bacon made by different pig breeds during storage time. Food Chem. 2021, 357, 129765. [Google Scholar] [CrossRef]
- Radovčić, N.M.; Vidaček, S.; Janči, T.; Medić, H. Characterization of volatile compounds, physico-chemical and sensory characteristics of smoked dry-cured ham. J. Food Sci. Technol. 2016, 53, 4093–4105. [Google Scholar] [CrossRef]
- Raza, A.; Song, H.; Raza, J.; Li, P.; Li, K.; Yaoc, J. Formation of beef-like odorants from glutathione-enriched yeast extract via Maillard reaction. Food Func. 2020, 11, 8583–8601. [Google Scholar] [CrossRef] [PubMed]
- Amaya-Farfan, J.; Rodriguez-Amaya, D.B. (Eds.) The Maillard reactions. In Chemical Changes during Processing and Storage of Foods. Implications for Food Quality and Human Health, 1st ed.; Elsevier Inc.: Maryland Heights, MO, USA, 2021; Volume 1, pp. 215–263. [Google Scholar] [CrossRef]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2022, 371, 131139. [Google Scholar] [CrossRef]
- Du, H.; Ma, X.; Jiang, M.; Yan, P.; Zhang, Z.C. Highly efficient Cu/SiO2 catalyst derived from ethanolamine modification for furfural hydrogenation. Appl. Catal. A Gen. 2020, 598, 117598. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, R.; Yang, F.; Xie, Y.; Guo, Y.; Yao, W.; Zhou, W. Control strategies of pyrazines generation from Maillard reaction. Trends Food Sci. Technol. 2021, 112, 795–807. [Google Scholar] [CrossRef]
- St. Angelo, A.J.; Spainer, A.M.; Bett, K.L. Chemical and Sensory Evaluation of Flavor in Untreated and Antioxidant-Treated Meat. In Lipid Oxidation in Food, 1st ed.; St. Angelo, A.J., Ed.; ACS Symposium Series: Washington, DC, USA, 1992; Series 500; pp. 140–160. [Google Scholar] [CrossRef]
- Shahidi, F. Lipid-Derived Flavors in Meat Products. In Meat Processing: Improving Quality, 1st ed.; Kerry, J., Kerry, J., Ledward, D., Eds.; CRC Press:: Boca Raton, FL, USA, 2002; Volume 1, pp. 105–121. [Google Scholar] [CrossRef]
- Hes, M. Protein-lipid interactions in different meat systems in the presence of natural antioxidants–A review. Pol. J. Food Nutr. Sci. 2017, 67, 5–17. [Google Scholar] [CrossRef]
- Shahidi, F.; Oh, W.Y. Lipid-derived flavor and off-flavor of traditional and functional foods: An overview. J. Food Bioact. 2020, 10, 20–31. [Google Scholar] [CrossRef]
- Demasi, M.; Augusto, O.; Bechara, E.J.H.; Bicev, R.N.; Cerqueira, F.M.; da Cunha, F.M.; Denicola, A.; Gomes, F.; Miyamoto, S.; Netto, L.E.S.; et al. Oxidative modification of proteins: From damage to catalysis, signalling, and beyond. Antioxid. Redox Signal. 2021, 35, 1016–1080. [Google Scholar] [CrossRef]
- Zhang, K.; Li, D.; Zang, M.; Zhang, Z.; Li, X.; Wang, S.; Zhang, S.; Zhao, B. Comparative characterization of fatty acids, reheating volatile compounds, and warmed-over flavor (WOF) of Chinese indigenous pork and hybrid pork. LWT 2022, 155, 112981. [Google Scholar] [CrossRef]
- Farmer, E. Meat Flavour. In The Chemistry of The Muscle Based Foods, 1st ed.; Johanson, D.E., Knight, M.K., Ledwards, D.A., Eds.; Royal Society of Chemistry: Cambridge, UK, 1992; Volume 1, pp. 169–188. [Google Scholar]
- Hematyar, N.; Rustad, T.; Sampels, S.; Dalsgaard, T.K. Relationship between lipid and protein oxidation in fish. Aquacul. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Viedma-Poyatos, Á.; González-Jiménez, P.; Langlois, O.; Company-Marín, I.; Spickett, C.M.; Pérez-Sala, D. Protein lipoxidation: Basic concepts and emerging roles. Antioxidants 2021, 10, 295. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Characterisation of fluorescent Schiff bases formed during oxidation of pig myofibrils. Meat Sci. 2007, 76, 210–215. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, X.; Zhou, G. V Covalent chemical modification of myofibrillar proteins to improve their gelation properties: A systematic review. Comp. Rev. Food Sci. Food Saf. 2021, 20, 924–959. [Google Scholar] [CrossRef] [PubMed]
- Ho Ng, C.; Yao, X.Q.; Huang, Y.; Chen, Z.-Y. Oxidised cholesterol is more hypercholesterolaemic and atherogenic than non-oxidised cholesterol in hamsters. Br. J. Nutr. 2008, 99, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Broncano, J.; Petr´on, M.; Parra, V.; Tim´on, M. Effect of different cooking methods on lipid oxidation and formation of free cholesterol oxidation products (COPs) in Latissimus dorsi muscle of Iberian pigs. Meat Sci. 2009, 83, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Dantas, N.M.; Sampaio, G.R.; Ferreira, F.S.; da Silva Labre, T.; da Silva Torres, E.A.F.; Saldanha, T. Cholesterol oxidation in fish and fish products. J. Food Sci. 2015, 80, R2627–R2639. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.A. A perspective on free radical autoxidation: The physical organic chemistry of polyunsaturated fatty acid and sterol peroxidation. J. Org. Chem. 2013, 78, 3511–3524. [Google Scholar] [CrossRef] [PubMed]
- Orczewska-Dudek, S.; Bederska-Łojewska, D.; Pieszka, M.; Pietras, M. Cholesterol and lipid peroxides in animal products and health implications–A review. Ann. Anim. Sci. 2012, 12, 25–52. [Google Scholar] [CrossRef]
- Ferioli, F.; Dutta, P.C.; Caboni, M.F. Cholesterol and lipid oxidation in raw and pan-fried minced beef stored under aerobic packaging. J. Sci. Food Agric. 2010, 90, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Bragagnolo, N. Cholesterol and Cholesterol Oxides in Meat and Meat Products. In Handbook of Muscle Foods Analysis, 1st ed.; Nollet, L.M.L., Toldra, F., Eds.; CRC Press (Chapman & Hall), Taylor & Francis Group: Boca Raton, FL, USA, 2008; Series 500; pp. 197–231. [Google Scholar] [CrossRef]
- Gomes, A.R.; Varela, C.L.; Tavares-da-Silva, E.J.; Roleira, F.M.F. Epoxide containing molecules: A good or a bad drug design approach. Eur. J. Med. Chem. 2020, 201, 112327. [Google Scholar] [CrossRef] [PubMed]
- DeBose-Boyd, R. Feedback regulation of cholesterol synthesis: Sterol-accelerated ubiquitination and degradation of HMG CoA reductase. Cell. Res. 2008, 18, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Llatas, G.; Mercatante, D.; López-García, G.; Rodriguez-Estrada, M.T. Oxysterols–how much do we know about food occurrence, dietary intake and absorption? Cur. Opin. Food Sci. 2021, 41, 231–239. [Google Scholar] [CrossRef]
- Vine, D.F.; Croft, K.D.; Beilin, L.J.; Mamo, J.C.L. Effect of dietary cholesterol oxidation products on the plasma clearance of chylomicrons in the rat. Lipids 2002, 37, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Becerra, J.A.; Ochoa-Flores, A.A.; Soto-Rodriguez, I.; Rodriguez-Estrada, M.T.; García, H.S. Effect of cooking conditions on cholesterol oxidation and astaxanthin in dried salted shrimp. Eur. J. Lipid Sci. Technol. 2014, 116, 872–884. [Google Scholar] [CrossRef]
- Hur, S.J.; Park, G.B.; Joo, S.T. Formation of cholesterol oxidation products (COPs) in animal products. Food Control 2007, 18, 939–947. [Google Scholar] [CrossRef]
- Bahorun, T.; Soobrarree, M.A.; Luximon-Ramma, V.; Aruoma, O.I. Free radicals and antioxidants in cardiovascular health and disease. Internet J. Med. Update. 2006, 1, 1–17. Available online: https://akspublication.com/Paper05_Jul-Dec2006_.pdf (accessed on 14 December 2023). [CrossRef]
- National Center for Health Statistics. Multiple Cause of Death 2018-2021 on CDC WONDER Database. Available online: https://wonder.cdc.gov/mcd.html (accessed on 2 February 2023).
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Commodore-Mensah, Y.; Elkind, M.S.V.; Evenson, K.R.; et al. Heart disease and stroke statistics–2023 update: A report from the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar] [CrossRef]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Frank, A.; Bonney, M.; Bonney, S.; Weitzel, L.; Koeppen, M.; Eckle, T. Myocardial ischemia reperfusion injury: From basic science to clinical bedside. Semin. Cardiothorac. Vasc. Anesth. 2012, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion–From mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K. Aldehyde adducts generated during lipid peroxidation modification of proteins. Free Rad. Res. 2015, 49, 896–904. [Google Scholar] [CrossRef]
- Yi, X.; Luo, H.; Zhou, J.; Yu, M.; Chen, X.; Tan, L.; Wei, W.; Li, J. Prevalence of stroke and stroke related risk factors: A population based cross sectional survey in southwestern China. BMC Neurol. 2020, 20, 1592-z. [Google Scholar] [CrossRef]
- Fukui, S.; Imazeki, R.; Amano, Y.; Kudo, Y.; Amari, K.; Yamamoto, M.; Todoroki, K.; Ikeya, Y.; Okazaki, T.; Yanagimachi, N.; et al. Common and specific risk factors for ischemic stroke in elderly: Differences based on type of ischemic stroke and aging. J. Neurolog. Sci. 2017, 380, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Owolabi, M.O.; Thrift, A.G.; Mahal, A.; Ishida, M.; Martins, S.; Johnson, W.D.; Pandian, J.; Abd-Allah, F.; Yaria, J.; Phan, H.T.; et al. Primary stroke prevention worldwide: Translating evidence into action. Health Policy 2022, 7, E74–E85. [Google Scholar] [CrossRef]
- Polidori, M.C.; Griffiths, H.R.; Mariani, E.; Mecocci, P. Hallmarks of protein oxidative damage in neurodegenerative diseases: Focus on Alzheimer’s disease. Amino Acids 2007, 32, 553–559. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Ibrahim, A.A. Pathological roles of reactive oxygen species and their defence mechanism. Saudi Pharm J. 2004, 12, 1–18. Available online: https://ksascholar.dri.sa/en/publications/pathological-roles-of-reactive-oxygen-species-and-their-defence-m-2 (accessed on 12 January 2004).
- Chen, K.K.; Minakuchi, M.; Wuputra, K.; Ku, C.-C.; Pan, J.-B.; Kuo, K.-K.; Lin, Y.-C.; Saito, S.; Lin, C.-S.; Yokoyama, K.K. Redox control in the pathophysiology of influenza virus infection. BMC Microbiol. 2020, 20, 01890–01899. [Google Scholar] [CrossRef]
- Darenskaya, M.; Kolesnikova, L.; Kolesnikov, S. The association of respiratory viruses with oxidative stress and antioxidants. Implications for the COVID-19 pandemic. Curr. Pharm. Des. 2021, 27, 1618–1627. [Google Scholar] [CrossRef]
- Wang, L.; Cao, Z.; Wang, Z.; Guo, J.; Wen, J. Reactive oxygen species associated immunoregulation post influenza virus infection. Front Immunol. 2022, 13, 927593. [Google Scholar] [CrossRef]
- Farzi, R.; Aghbash, P.S.; Eslami, N.; Azadi, A.; Shamekh, A.; Hemmat, N.; Entezari-Maleki, T.; Baghi, H.B. The role of antigen-presenting cells in the pathogenesis of COVID-19. Pathol. Res. Pract. 2022, 233, 153848. [Google Scholar] [CrossRef]
- Sutipornpalangkul, W.; Morales, N.P.; Harnroongroj, T. Free radicals in primary knee osteoarthritis. J. Med. Assoc. Thai. 2009, 92 (Suppl. 6), S268–S274. Available online: https://repository.li.mahidol.ac.th/handle/123456789/27824 (accessed on 10 December 2009).
- López-Novoa, J.M.; Rodríguez-Peña, A.B.; Ortiz, A.; Martínez-Salgado, C.; Hernández, F.J.L. Etiopathology of chronic tubular, glomerular and renovascular nephropathies: Clinical implications. J. Transl. Med. 2011, 9, 13. [Google Scholar] [CrossRef]
- Jalal, D.I.; Chonchol, M.; Targher, G. Disorders of hemostasis associated with chronic kidney disease. Semin. Thromb. Hemost. 2010, 36, 34–40. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Webb, C.; Twedt, D. Oxidative stress and liver disease. Vet. Clin. North Am. Small Anim. Pract. 2008, 38, 125–135. [Google Scholar] [CrossRef]
- Lee, D.T.; Plesa, M.L. Anemia. In Family Medicine, 1st ed.; Paulman, P.M., Taylor, R.B., Paulman, A.A., Nasir, L.S., Eds.; Springer: Cham, Switzerland, 2022; Volume 1, pp. 1815–1829. [Google Scholar] [CrossRef]
- Rác, M.; Křupka, M.; Binder, S.; Sedlářová, M.; Matušková, Z.; Raška, M.; Pospíšil, P. Oxidative damage of U937 human leukemic cells caused by hydroxyl radical results in singlet oxygen formation. PLoS ONE 2015, 10, e0116958. [Google Scholar] [CrossRef]
- Musallam, K.M.; Lombard, L.; Kistler, K.D.; Arregui, M.; Gilroy, K.S.; Chamberlain, C.; Zagadailov, E.; Ruiz, K.; Taher, A.T. Epidemiology of clinically significant forms of alpha- and beta-thalassemia: A global map of evidence and gaps. Hematology 2023, 98, 1436–1451. [Google Scholar] [CrossRef]
- Shafique, F.; Ali, S.; Almansouri, T.; Van Eeden, F.; Shafi, N.; Khalid, M.; Khawaja, S.; Andleeb, S.; ul Hassan, M. Thalassemia, a human blood disorder. Braz. J. Biol. 2023, 83, 246062. [Google Scholar] [CrossRef]
- Angastiniotis, M.; Lobitz, S. Thalassemias: An overview. Int. J. Neonatal Screen. 2019, 5, 16. [Google Scholar] [CrossRef]
- Verhovsek, M.M.; Chui, D.H. The Thalassemia Syndromes. In Anemia Pathophysilogy, Diagnosis, and Management, 1st ed.; Benz, E.J., Jr., Berliner, N., Schiffman, F.J., Eds.; Cambridge University Press: Cambridge, UK, 2018; Volume 1, pp. 48–87. [Google Scholar]
- Devasagayam, T.P.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–803. Available online: https://www.japi.org/w2648494/free-radicals-and-antioxidants-in-human-health-current-status-and-future-prospects (accessed on 15 August 2004).
- Zaorsky, N.G.; Churilla, T.M.; Egleston, B.L.; Fisher, S.G.; Ridge, J.A.; Horwitz, E.M.; Meyer, J.E. Causes of death among cancer patients. Ann. Oncol. 2017, 28, 400–407. [Google Scholar] [CrossRef]
- Crandall, C.J.; Hovey, K.M.; Andrews, C.A.; Chlebowski, R.T.; Stefanick, M.L.; Lane, D.S.; Shifren, J.; Chen, C.; Kaunitz, A.M.; Cauley, J.A.; et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the women’s health initiative observational study. Menopause 2018, 25, 11–20. [Google Scholar] [CrossRef]
- Dierge, E.; Larondelle, Y.; Feron, O. Cancer diets for cancer patients: Lessons from mouse studies and new insights from the study of fatty acid metabolism in tumors. Biochimie 2020, 178, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Bojková, B.; Winklewski, P.J.; Wszedybyl-Winklewska, M. Dietary fat and cancer–which is good, which is bad, and the body of evidence. Int. J. Mol. Sci. 2020, 21, 4114. [Google Scholar] [CrossRef]
- Calder, P.C. Functional roles of fatty acids and their effects on human health. J. Parent. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Liput, K.P.; Lepczyński, A.; Ogłuszka, M.; Nawrocka, A.; Poławska, E.; Grzesiak, A.; Ślaska, B.; Pareek, C.S.; Czarnik, U.; Pierzchała, M. Effects of dietary n-3 and n-6 polyunsaturated fatty acids in inflammation and cancerogenesis. Int. J. Mol. Sci. 2021, 22, 6965. [Google Scholar] [CrossRef]
- Lee, J.Y.; Sim, T.-B.; Lee, J.-e.; Na, H.-K. Chemopreventive and chemotherapeutic effects of fish oil derived omega-3 polyunsaturated fatty acids on colon carcinogenesis. Clin. Nutr. Res. 2017, 6, 147–160. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of colorectal cancer in terms of epidemiology, risk factors, development, symptoms and diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dragoev, S.G. Lipid Peroxidation in Muscle Foods: Impact on Quality, Safety and Human Health. Foods 2024, 13, 797. https://doi.org/10.3390/foods13050797
Dragoev SG. Lipid Peroxidation in Muscle Foods: Impact on Quality, Safety and Human Health. Foods. 2024; 13(5):797. https://doi.org/10.3390/foods13050797
Chicago/Turabian StyleDragoev, Stefan G. 2024. "Lipid Peroxidation in Muscle Foods: Impact on Quality, Safety and Human Health" Foods 13, no. 5: 797. https://doi.org/10.3390/foods13050797
APA StyleDragoev, S. G. (2024). Lipid Peroxidation in Muscle Foods: Impact on Quality, Safety and Human Health. Foods, 13(5), 797. https://doi.org/10.3390/foods13050797