Nutritional Profile and Carbohydrate Characterization of Spray-Dried Lentil, Pea and Chickpea Ingredients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pulse Treatments
2.2. Proximate Analyses
2.3. Carbohydrate Analysis
2.4. Microscopy
2.5. Statistical Analysis
3. Results and Discussion
3.1. Proximate Analyses
| Potato | Chickpea | Lentil | Green Pea | n 1 | |
|---|---|---|---|---|---|
| Energy (kcal/100 g) 2 | 299.8 | 282.7 | 263.7 | 245.2 | |
| Moisture (%) 3 | 7.89 ± 0.10 a | 7.03 ± 0.17 b | 5.66 ± 0.05 d | 6.30 ± 0.08 c | 3 |
| Protein (%) | 5.4 ± 0.2 d | 18.3 ± 0.3 c | 22.7 ± 0.2 a | 21.3 ± 0.5 b | 4 |
| Lipids (%) | 0.24 ± 0.06 c | 4.97 ± 0.23 a | 0.70 ± 0.08 b | 0.60 ± 0.08 b | 3 |
| Ash (%) | 3.76 ± 0.15 a | 2.79 ± 0.07 b | 2.62 ± 0.06 b | 2.74 ± 0.23 b | 3 |
| Digestible starch (%) | 68.0 ±1.3 a | 40.2 ± 1.2 bc | 42.6 ± 1.0 b | 39.2 ± 1.3 c | 6 |
| Sugars (%) | 0.99 ± 0.00 c | 2.86 ± 0.56 a | 1.71 ± 0.04 b | 3.00 ± 0.36 a | 6 |
| Total dietary fibre (%) | 7.8 ± 0.6 c | 26.2 ± 2.7 ab | 21.9 ± 1.4 b | 27.1 ± 1.6 a | 4 |
| Resistant starch (%) | 0.78 ± 0.02 d | 4.44 ± 0.16 c | 4.75 ± 0.14 b | 5.17 ± 0.07 a | 9 |
| Dietary fibre (%) | 7.0 ± 0.6 c | 19.9 ± 2.5 a | 14.6 ± 1.1 b | 18.4 ± 1.0 ab | 3 |
| Oligosaccharide (%) | ND 4 | 1.88 ± 0.09 c | 2.59 ± 0.07 b | 3.49 ± 0.59 a | 6 |
3.2. Carbohydrate Analysis

| Potato | Chickpea | Lentil | Green Pea | |
|---|---|---|---|---|
| Arabinose (%) 1 | 0.34 ± 0.02 c | 3.76 ± 0.19 a | 2.38 ± 0.16 b | 2.76 ± 0.13 b |
| Galactose (%) | 1.96 ± 0.17 b | 3.68 ± 0.20 a | 3.64 ± 0.22 a | 2.97 ± 0.31 ab |
| Glucose (%) | 72.9 ± 1.2 a | 46.9 ± 0.3 c | 50.5 ± 2.2 b | 50.2 ± 2.2 b |
| Mannose (%) | ND2 | ND | ND | ND |
| Rhamnose (%) | ND | ND | ND | 0.29 ± 0.02 |
| Uronic acids (%) | - 3 | 3.38 ± 0.37 a | 3.26 ± 0.24 a | 3.52 ± 0.16 a |
| Xylose (%) | ND | 0.50 ± 0.05 a | 0.83 ± 0.15 a | 1.10 ± 0.15 a |
| Potato | Chickpea | Lentil | Green Pea | |
|---|---|---|---|---|
| Sugars (%) 1 | 1.7 | 3.08 | 1.81 | 3.21 |
| Sucrose (%) | ND 2 | 3.04 ± 0.57 a | 1.80 ± 0.04 b | 3.17 ± 0.37 a |
| Glucose (%) | 1.69 ± 0.01 a | 0.04 ± 0.03 b | 0.01 ± 0.01 c | 0.04 ± 0.01 b |
| Oligosaccharides (%) | ND | 2.02 | 2.75 | 3.73 |
| Raffinose (%) | ND | 0.53 ± 0.03 a | 0.32 ± 0.01 a | 0.48 ± 0.07 a |
| Stachyose (%) | ND | 1.49 ± 0.07 c | 1.79 ± 0.06 b | 2.36 ± 0.39 a |
| Verbascose(%) | ND | ND | 0.64 ± 0.01 b | 0.89 ± 1.17 a |
| Total (%) | 1.07 | 5.1 | 4.56 | 6.94 |
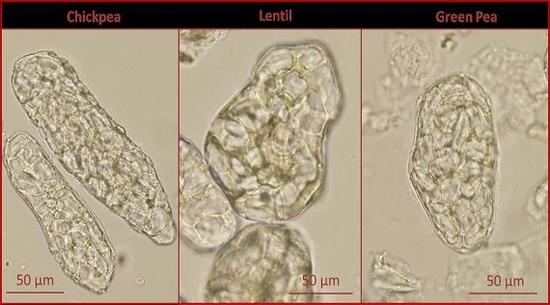
3.3. Microscopy
| Chickpea | Lentil | Green Pea | |
|---|---|---|---|
| Soluble 1 | |||
| Arabinose (%) 1 | 6.74 ± 0.18 a | 6.31 ± 0.11 a | 5.58 ± 0.11 b |
| Galactose (%) | 4.32 ± 0.07 a | 2.81 ± 0.07 b | 2.55 ± 0.04 b |
| Glucose (%) | 5.58 ± 2.62 a | 6.32 ± 1.16 a | 2.81 ± 0.37 b |
| Mannose (%) | 2.18 ± 0.24 a | 2.32 ± 0.36 a | 2.12 ± 0.40 a |
| Rhamnose (%) | 1.23 ± 0.09 a | 0.56 ± 0.02 a | 0.85 ± 0.09 a |
| Xylose (%) | 0.52 ± 0.04 a | 1.17 ± 0.09 a | 1.03 ± 0.06 a |
| Total (%) | 20.6 ± 3.2 | 19.5 ± 1.8 | 14.9 ± 1.1 |
| Insoluble 1 | |||
| Arabinose (%) | 9.02 ± 1.9 a | 8.09 ± 0.23 a | 9.08 ± 1.07 a |
| Galactose (%) | 1.17 ± 0.15 a | 1.54 ± 0.05 a | 1.65 ± 0.08 a |
| Glucose (%) | 33.5 ± 8.6 c | 36.4 ± 0.6 b | 40.7 ± 6.13 a |
| Mannose (%) | 0.69 ± 0.06 a | 0.57 ± 0.19 a | 0.76 ± 0.06 a |
| Rhamnose (%) | 0.45 ± 0.07 a | 0.48 ± 0.06 a | 0.57 ± 0.10 a. |
| Xylose (%) | 1.10 ± 0.27 b | 4.56 ± 0.37 a | 1.10 ± 0.15 b |
| Total (%) | 46.0 ± 11.1 | 51.6 ± 1.5 | 57.2 ± 8.7 |


3.4. Relation to in Vivo Data
4. Conclusions
Acknowledgements
Conflicts of Interest
References
- Leterme, P. Recommendations by health organizations for pulse consumption. Br. J. Nutr. 2002, 88, S239–S242. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Mahadevamma, S. Grain legumes—A boon to human nutrition. Trends Food Sci. Technol. 2003, 14, 507–518. [Google Scholar] [CrossRef]
- Chibbar, R.N.; Ambigaipalan, P.; Hoover, R. Molecular diversity in pulse seed starch and complex carbohydrates and its role in human nutrition and health. Cereal Chem. 2010, 87, 342–352. [Google Scholar] [CrossRef]
- Rizkalla, S.W.; Bellisle, F.; Slama, G. Health benefits of low glycaemic index foods, such as pulses, in diabetic patients and healthy individuals. Br. J. Nutr. 2002, 88, S255–S262. [Google Scholar] [CrossRef]
- Health Canada. Eating Well with Canada’s Food Guide. Available online: http://www.hc-sc.gc.ca/fn-an/food-guide-aliment/index-eng.php (accessed on 14 March 2013).
- United States Department of Agriculture (USDA). Beans and Peas Are Unique Foods. Available online: http://www.choosemyplate.gov/food-groups/vegetables-beans-peas.html (accessed on 14 March 2013).
- AOAC International. Official Methods of Analysis. Available online: http://www.eoma.aoac.org/ (accessed on 9 July 2013).
- Bainy, E.M.; Tosh, S.M.; Corredig, M.; Poysa, V.; Woodrow, L. Varietal differences of carbohydrates in defatted soybean flour and soy protein isolate by-products. Carbohydr. Polym. 2008, 72, 664–672. [Google Scholar] [CrossRef]
- Brummer, Y.; Jones, S.; Tosh, S.M.; Wood, P.J. Extraction and physicochemical characterisation of rye β-glucan and effects of barium on polysaccharide molecular weight. Cereal Chem. 2008, 85, 174–181. [Google Scholar] [CrossRef]
- Schols, H.A.; Voragen, A.G.J. Cell wall polysaccharides from soybean (Glycine max.) meal. Isolation and characterisation. Carbohydr. Polym. 1998, 37, 87–95. [Google Scholar] [CrossRef]
- Neter, J; Wasserman, W; Kutner, M.H. Applied Linear Statistical Models, 3rd ed.; Irwin: Homewood, IL, USA, 1990; pp. 741–771. [Google Scholar]
- Dalgetty, D.D.; Baik, B.-K. Isolation and characterization of cotyledon fibers from peas, lentils, and chickpeas. Cereal Chem. 2003, 80, 310–315. [Google Scholar] [CrossRef]
- Han, I.H.; Baik, B.K. Oligosaccharide content and composition of legumes and their reduction by soaking, cooking, ultrasound, and high hydrostatic pressure. Cereal Chem. 2006, 83, 428–433. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Simpson, B.K.; Shiv, O.; Prasher, S.O.; Monpetit, D.; Malcolmson, L. Thermal processing effects on the functional properties and microstructure of lentil, chickpea, and pea flours. Food Res. Int. 2011, 44, 2534–2544. [Google Scholar] [CrossRef]
- Guillon, F.; Champ, M.M.-J. Carbohydrate fractions of legumes: Uses in human nutrition and potential for health. Br. J. Nutr. 2002, 88, S293–S306. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Raynoso-Camacho, R.; Pedraza-Aboytes, G.; Acosta-Gallegos, J.A.; Guzman-Maldonado, S.H.; Paredes-Lopez, O.; Oomah, B.D.; Loarca-Pina, G. Chemical composition and in vitro polysaccharide fermentation of different beans (Phaseolus vulgaris L.). J. Food Sci. 2009, 74, T59–T65. [Google Scholar] [CrossRef]
- Veenstra, J.M.; Cryne, C.N.; Deschambault, B.R.; Boye, J.I.; Benali, M.; Marcotte, M.; Tosh, S.M.; Farnworth, E.R.; Duncan, A.M.; Wright, A.J. Effect of pulse consumption on perceived flatulence and gastrointestinal function in healthy males. Food Res. Int. 2010, 43, 553–559. [Google Scholar] [CrossRef]
- Farnworth, E.R.; Duncan, A.M.; Wright, A.J.; Boye, J.; Tosh, S.M.; Marcotte, M.; Benali, M.; Cryne, C.N.; Deschambault, B.R.; Veenstra, J.M.; et al. The effects of pulse consumption on fecal composition, fecal bacteria and fecal enzyme activities in healthy young men. 2011; unpublished work. [Google Scholar]
- Abeysekara, S.; Chilibeck, P.D.; Vatanparast, H.; Zello, G.A. A pulse-based diet is effective for reducing total and LDL-cholesterol in older adults. Br. J. Nutr. 2012, 108, S103–S110. [Google Scholar] [CrossRef]
- Cryne, C.N.; Veenstra, J.M.; Deschambault, B.R.; Benali, M.; Marcotte, M.; Boye, J.I.; Tosh, S.M.; Farnworth, E.R.; Wright, A.J.; Duncan, A.M. Spray-dried pulse consumption does not affect cardiovascular disease risk or glycemic control in healthy males. Food Res. Int. 2012, 48, 131–139. [Google Scholar] [CrossRef]
- Winham, D.M.; Hutchins, A.M.; Melde, C.L. Pinto bean, navy bean, and black-eye pea consumption do not significantly lower the glycemic response to a high glycemic index treatment in normoglycemic adults. Nutr. Res. 2007, 27, 535–541. [Google Scholar] [CrossRef]
- Thompson, S.V.; Winham, D.M.; Hutchins, A.M. Black bean and chickpea consumption reduce glycemic response as part of a rice meal. FASEB J. 2009, 23, 540–542. [Google Scholar]
- Thompson, S.V.; Winham, D.M.; Hutchins, A.M. Bean and rice meals reduce postprandial glycemic response in adults with type 2 diabetes: A cross-over study. Nutr. J. 2012, 11, 23. [Google Scholar] [CrossRef]
- Hutchins, A.M.; Winham, D.M.; Thompson, S.V. Phaseolus beans: Impact on glycaemic response and chronic disease risk in human subjects. Br. J. Nutr. 2012, 108, S52–S65. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tosh, S.M.; Farnworth, E.R.; Brummer, Y.; Duncan, A.M.; Wright, A.J.; Boye, J.I.; Marcotte, M.; Benali, M. Nutritional Profile and Carbohydrate Characterization of Spray-Dried Lentil, Pea and Chickpea Ingredients. Foods 2013, 2, 338-349. https://doi.org/10.3390/foods2030338
Tosh SM, Farnworth ER, Brummer Y, Duncan AM, Wright AJ, Boye JI, Marcotte M, Benali M. Nutritional Profile and Carbohydrate Characterization of Spray-Dried Lentil, Pea and Chickpea Ingredients. Foods. 2013; 2(3):338-349. https://doi.org/10.3390/foods2030338
Chicago/Turabian StyleTosh, Susan M., Edward R. Farnworth, Yolanda Brummer, Alison M. Duncan, Amanda J. Wright, Joyce I. Boye, Michèle Marcotte, and Marzouk Benali. 2013. "Nutritional Profile and Carbohydrate Characterization of Spray-Dried Lentil, Pea and Chickpea Ingredients" Foods 2, no. 3: 338-349. https://doi.org/10.3390/foods2030338
APA StyleTosh, S. M., Farnworth, E. R., Brummer, Y., Duncan, A. M., Wright, A. J., Boye, J. I., Marcotte, M., & Benali, M. (2013). Nutritional Profile and Carbohydrate Characterization of Spray-Dried Lentil, Pea and Chickpea Ingredients. Foods, 2(3), 338-349. https://doi.org/10.3390/foods2030338