Algal Proteins: Extraction, Application, and Challenges Concerning Production
Abstract
:1. Introduction
1.1. Characteristics of Seaweed
1.2. Characteristics of Microalgae
2. Protein Quality
2.1. Amino Acid Composition
2.2. Algal Protein Digestibility
3. Protein Extraction Methods
3.1. Conventional Protein Extraction Methods
3.1.1. Physical Processes
3.1.2. Enzymatic Hydrolysis
3.2. Current Protein Extraction Methods
3.2.1. Ultrasound-Assisted Extraction
3.2.2. Pulsed Electric Field
3.2.3. Other
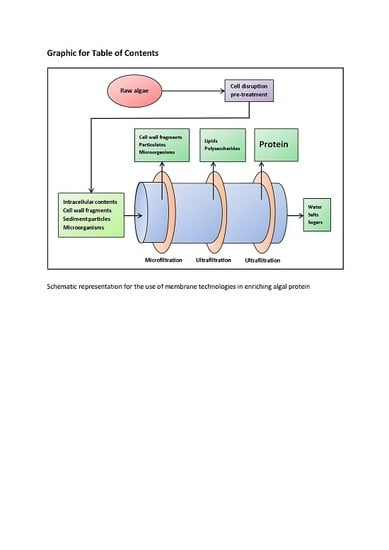
3.3. Enrichment Methods—Membrane Filtration
4. Applications
4.1. Human Nutrition
4.2. Industrial Applications
4.2.1. Lectins
4.2.2. Phycobiliproteins
4.3. Animal Feed
4.3.1. Poultry
4.3.2. Pigs
4.3.3. Ruminants
4.4. Aquaculture
4.5. Bioactive Peptides
Anti-Hypertensive Peptides
5. Challenges
5.1. Access Rights
5.2. Variability
5.3. Scalability
5.4. Digestibility
5.5. Food Safety
5.6. Price
6. Discussions
Acknowledgments
Conflicts of Interest
Abbreviations
| EAA(s) | essential amino acid(s) |
| FAO | Food and Agriculture Organisation of the United Nations |
| TNOASR | The Netherland Organisation for applied scientific research |
| TIM | TNOASR’s Intestinal Model |
| UAE | ultrasound-assisted extraction |
| PEF | pulsed electric field |
| MAE | microwave-assisted extraction |
| MF | microfiltration |
| UF | ultrafiltration |
| WHO | World Health Organisation |
| ACE-I | angiotensin-I-converting enzyme |
| RAAS | renin-angiotensin-aldosterone system |
| SHR(s) | spontaneously hypertensive rat(s) |
| SBP | systolic blood pressure |
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Gouveia, L.; Batista, A.P.; Sousa, I.; Raymundo, A.; Bandarra, N. Microalgae in Novel Food Products. In Food Chemistry Research Development; Konstantinos, N., Papadopoulos, P.P., Eds.; Nova Science Publishers: New York, NY, USA, 2008; pp. 75–112. [Google Scholar]
- Van Krimpen, M.; Bikker, P.; Van der Meer, I.; Van der Peet-Schwering, C.; Vereijken, J. Cultivation, Processing and Nutritional Aspects for Pigs and Poultry of European Protein Sources as Alternatives for Imported Soybean Products; Wageningen UR Livestock Research: Lelystad, The Netherlands, 2013; p. 48. [Google Scholar]
- Wallace, J. Increasing agricultural water use efficiency to meet future food production. Agric. Ecosyst. Environ. 2000, 82, 105–119. [Google Scholar] [CrossRef]
- Pimentel, D.; Pimentel, M. Sustainability of meat-based and plant-based diets and the environment. Am. J. Clin. Nutr. 2003, 78, 660S–663S. [Google Scholar] [PubMed]
- Sampath-Wiley, P.; Neefus, C.D.; Jahnke, L.S. Seasonal effects of sun exposure and emersion on intertidal seaweed physiology: Fluctuations in antioxidant contents, photosynthetic pigments and photosynthetic efficiency in the red alga Porphyra umbilicalis kützing (Rhodophyta, Bangiales). J. Exp. Mar. Biol. Ecol. 2008, 361, 83–91. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jonsdottir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J. An outlook on microalgal biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Cavalier-Smith, T. Evolution and relationships. In Unravelling the Algae: The Past, Present, and Future of Algal Systematics; Brodie, J., Ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 21. [Google Scholar]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. Food Balance Sheets; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Food and Agriculture Organization. The State of World Fisheries and Aquaculture: Opportunties and Challenges; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2014. [Google Scholar]
- Cock, J.M.; Sterck, L.; Rouzé, P.; Scornet, D.; Allen, A.E.; Amoutzias, G.; Anthouard, V.; Artiguenave, F.; Aury, J.-M.; Badger, J.H. The ectocarpus genome and the independent evolution of multicellularity in brown algae. Nature 2010, 465, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Hoek, C.; Mann, D.; Jahns, H.M. Algae: An Introduction to Phycology; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Foster, M.S.; Schiel, D.R. Ecology of Giant Kelp Forests in California: A Community Profile; San Jose State University, Moss Landing Marine Labs.: Moss Landing, CA, USA, 1985. [Google Scholar]
- Fitzgerald, C.; Gallagher, E.; Tasdemir, D.; Hayes, M. Heart health peptides from macroalgae and their potential use in functional foods. J. Agric. Food Chem. 2011, 59, 6829–6836. [Google Scholar] [CrossRef] [PubMed]
- Karol, K.G.; McCourt, R.M.; Cimino, M.T.; Delwiche, C.F. The closest living relatives of land plants. Science 2001, 294, 2351–2353. [Google Scholar] [CrossRef] [PubMed]
- McCourt, R.M.; Delwiche, C.F.; Karol, K.G. Charophyte algae and land plant origins. Trends Ecol. Evol. 2004, 19, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Mouritsen, O.G.; Dawczynski, C.; Duelund, L.; Jahreis, G.; Vetter, W.; Schröder, M. On the human consumption of the red seaweed dulse (Palmaria palmata (L.) weber & amp; mohr). J. Appl. Phycol. 2013, 25, 1777–1791. [Google Scholar]
- Norton, T.A.; Melkonian, M.; Andersen, R.A. Algal biodiversity. Phycologia 1996, 35, 308–326. [Google Scholar] [CrossRef]
- Chacón-Lee, T.; González-Mariño, G. Microalgae for “healthy” foods—Possibilities and challenges. Compr. Rev. Food Sci. Food Saf. 2010, 9, 655–675. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Capelli, B.; Cysewski, G.R. Potential health benefits of Spirulina microalgae. Nutrafoods 2010, 9, 19–26. [Google Scholar] [CrossRef]
- Becker, E. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, I.; Guil-Guerrero, J.L. Evaluation of the antioxidant activity of three microalgal species for use as dietary supplements and in the preservation of foods. Food Chem. 2008, 108, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Boisen, S.; Eggum, B. Critical evaluation of in vitro methods for estimating digestibility in simple-stomach animals. Nutr. Res. Rev. 1991, 4, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Young, V.R.; Pellett, P.L. Plant proteins in relation to human protein and amino acid nutrition. Am. J. Clin. Nutr. 1994, 59, 1203S–1212S. [Google Scholar] [PubMed]
- Hoffman, J.R.; Falvo, M.J. Protein-which is best. J. Sports Sci. Med. 2004, 3, 118–130. [Google Scholar] [PubMed]
- Food and Agriculture Organization; World Health Organization. Protein Quality Evaluation—Report of Joint FAO/WHO Expert Consultation; FAO: Rome, Italy, 1991. [Google Scholar]
- Mišurcová, L.; Kráčmar, S.; Klejdus, B.; Vacek, J. Nitrogen content, dietary fiber, and digestibility in algal food products. Czech J. Food Sci. 2010, 28, 27–35. [Google Scholar]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Kolb, N.; Vallorani, L.; Stocchi, V. Chemical composition and evaluation of protein quality by amino acid score method of edible brown marine algae Arame (Eisenia bicyclis) and Hijiki (Hijikia fusiforme). Acta Aliment. 1999, 28, 213–222. [Google Scholar] [CrossRef]
- Volkmann, H.; Imianovsky, U.; Oliveira, J.L.; Sant’Anna, E.S. Cultivation of arthrospira (Spirulina) platensis in desalinator wastewater and salinated synthetic medium: Protein content and amino-acid profile. Braz. J. Microbiol. 2008, 39, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Mišurcová, L.; Buňka, F.; Ambrožová, J.V.; Machů, L.; Samek, D.; Kráčmar, S. Amino acid composition of algal products and its contribution to RDI. Food Chem. 2014, 151, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, M.; Park, C.S.; Amano, H. Distribution of free l-cysteine and glutathione in seaweeds. Fish. Sci. 2001, 67, 194–196. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Etienne-Mesmin, L.; Livrelli, V.; Denis, S.; Blanquet-Diot, S.; Alric, M. Relevance and challenges in modeling human gastric and small intestinal digestion. Trends Biotechnol. 2012, 30, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Ekmekcioglu, C. A physiological approach for preparing and conducting intestinal bioavailability studies using experimental systems. Food Chem. 2002, 76, 225–230. [Google Scholar] [CrossRef]
- Urbano, M.G.; Goñi, I. Bioavailability of nutrients in rats fed on edible seaweeds, nori (Porphyra tenera) and wakame (Undaria pinnatifida), as a source of dietary fibre. Food Chem. 2002, 76, 281–286. [Google Scholar] [CrossRef]
- Suzuki, T.; Nakai, K.; Yoshie, Y.; Shirai, T.; Hirano, T. Digestibility of dietary fiber in brown Alga, Kombu, by rats. Bull. Jpn. Soc. Sci. Fish 1993, 59, 879–884. [Google Scholar] [CrossRef]
- Wong, K.; Cheung, P.C. Influence of drying treatment on three Sargassum species. J. Appl. Phycol. 2001, 13, 43–50. [Google Scholar] [CrossRef]
- Joubert, Y.; Fleurence, J. Simultaneous extraction of proteins and DNA by an enzymatic treatment of the cell wall of Palmaria palmata (Rhodophyta). J. Appl. Phycol. 2008, 20, 55–61. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical methods for determining bioavailability and bioaccessibility of bioactive compounds from fruits and vegetables: A review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Etcheverry, P.; Grusak, M.A.; Fleige, L.E. Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D, and E. Front. Physiol. 2012, 3, 317. [Google Scholar] [CrossRef] [PubMed]
- Alegría, A.; Garcia-Llatas, G.; Cilla, A. Static digestion models: General introduction. In The Impact of Food Bioactives on Health; Springer: Heidelberg, Germany, 2015; pp. 3–12. [Google Scholar]
- Fernández-García, E.; Carvajal-Lérida, I.; Pérez-Gálvez, A. In vitro bioaccessibility assessment as a prediction tool of nutritional efficiency. Nutr. Res. 2009, 29, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.A.; Monro, J.A.; Moughan, P.J. In vitro determination of dietary protein and amino acid digestibility for humans. Br. J. Nutr. 2012, 108, S282. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J.; Lim, B.O.; Decker, E.A.; McClements, D.J. In vitro human digestion models for food applications. Food Chem. 2011, 125, 1–12. [Google Scholar] [CrossRef]
- Minekus, M.; Marteau, P.; Havenaar, R. Multicompartmental dynamic computer-controlled model simulating the stomach and small intestine. Altern. Lab. Anim. ATLA 1995, 23, 197–209. [Google Scholar]
- Minekus, M.; Smeets-Peeters, M.; Bernalier, A.; Marol-Bonnin, S.; Havenaar, R.; Marteau, P.; Alric, M.; Fonty, G. A computer-controlled system to simulate conditions of the large intestine with peristaltic mixing, water absorption and absorption of fermentation products. Appl. Microbiol. Biotechnol. 1999, 53, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Barmpalia-Davis, I.M.; Geornaras, I.; Kendall, P.A.; Sofos, J.N. Differences in survival among 13 listeria monocytogenes strains in a dynamic model of the stomach and small intestine. Appl. Environ. Microbiol. 2008, 74, 5563–5567. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Grootaert, C.; Marzorati, M.; Possemiers, S.; Verstraete, W.; Gérard, P.; Rabot, S.; Bruneau, A.; El Aidy, S.; Derrien, M. Microbial community development in a dynamic gut model is reproducible, colon region specific, and selective for Bacteroidetes and Clostridium cluster IX. Appl. Environ. Microbiol. 2010, 76, 5237–5246. [Google Scholar] [CrossRef] [PubMed]
- Vardakou, M.; Mercuri, A.; Naylor, T.; Rizzo, D.; Butler, J.; Connolly, P.; Wickham, M.; Faulks, R. Predicting the human in vivo performance of different oral capsule shell types using a novel in vitro dynamic gastric model. Int. J. Pharm. 2011, 419, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Curto, A.L.; Pitino, I.; Mandalari, G.; Dainty, J.R.; Faulks, R.M.; Wickham, M.S.J. Survival of probiotic lactobacilli in the upper gastrointestinal tract using an in vitro gastric model of digestion. Food Microbiol. 2011, 28, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Dupont, D.; Bordoni, A.; Brodkorb, A.; Capozzi, F.; Cirkovic Velickovic, T.; Corredig, M.; Cotter, P.D.; de Noni, I.; Gaudichon, C.; Golding, M. An international network for improving health properties of food by sharing our knowledge on the digestive process. Food Dig. 2011, 2, 23–25. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Egger, L.; Menard, O.; Delgado-Andrade, C.; Alvito, P.; Assunção, R.; Balance, S.; Barberá, R.; Brodkorb, A.; Cattenoz, T.; Clemente, A.; et al. The harmonized infogest in vitro digestion method: From knowledge to action. Food Res. Int. 2016, 88, 217–225. [Google Scholar] [CrossRef]
- Glahn, R.P.; Lee, O.A.; Yeung, A.; Goldman, M.I.; Miller, D.D. Caco-2 cell ferritin formation predicts nonradiolabeled food iron availability in an in vitro digestion/Caco-2 cell culture model. J. Nutr. 1998, 128, 1555–1561. [Google Scholar] [PubMed]
- Rousset, M. The human colon carcinoma cell lines HT-29 and Caco-2: Two in vitro models for the study of intestinal differentiation. Biochimie 1986, 68, 1035–1040. [Google Scholar] [CrossRef]
- Mahler, G.J.; Shuler, M.L.; Glahn, R.P. Characterization of Caco-2 and HT29-MTX cocultures in an in vitro digestion/cell culture model used to predict iron bioavailability. J. Nutr. Biochem. 2009, 20, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Cheung, P.C. Nutritional evaluation of some subtropical red and green seaweeds part II. In vitro protein digestibility and amino acid profiles of protein concentrates. Food Chem. 2001, 72, 11–17. [Google Scholar] [CrossRef]
- Bikker, P.; Krimpen, M.M.; Wikselaar, P.; Houweling-Tan, B.; Scaccia, N.; Hal, J.W.; Huijgen, W.J.; Cone, J.W.; López-Contreras, A.M. Biorefinery of the green seaweed Ulva lactuca to produce animal feed, chemicals and biofuels. J. Appl. Phycol. 2016, 28, 3511–3525. [Google Scholar] [CrossRef] [PubMed]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Nutritional quality of some wild and cultivated seaweeds: Nutrient composition, total phenolic content and in vitro digestibility. J. Appl. Phycol. 2016, 28, 3575–3585. [Google Scholar] [CrossRef]
- Barbarino, E.; Lourenço, S.O. An evaluation of methods for extraction and quantification of protein from marine macro-and microalgae. J. Appl. Phycol. 2005, 17, 447–460. [Google Scholar] [CrossRef]
- Kadam, S.U.; Álvarez, C.; Tiwari, B.K.; O’Donnell, C.P. Extraction and characterization of protein from irish brown seaweed ascophyllum nodosum. Food Res. Int. 2016. [Google Scholar] [CrossRef]
- Fleurence, J.; Le Coeur, C.; Mabeau, S.; Maurice, M.; Landrein, A. Comparison of different extractive procedures for proteins from the edible seaweeds Ulva rigida and Ulva rotundata. J. Appl. Phycol. 1995, 7, 577–582. [Google Scholar] [CrossRef]
- Jordan, P.; Vilter, H. Extraction of proteins from material rich in anionic mucilages: Partition and fractionation of vanadate-dependent bromoperoxidases from the brown algae Laminaria digitata and L. Saccharina in aqueous polymer two-phase systems. Biochim. Biophys. Acta (BBA) Gen. Subj. 1991, 1073, 98–106. [Google Scholar] [CrossRef]
- Fleurence, J. The enzymatic degradation of algal cell walls: A useful approach for improving protein accessibility? J. Appl. Phycol. 1999, 11, 313–314. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Extraction of protein from the macroalga Palmaria palmata. LWT Food Sci. Technol. 2013, 51, 375–382. [Google Scholar] [CrossRef]
- Fleurence, J.; Massiani, L.; Guyader, O.; Mabeau, S. Use of enzymatic cell wall degradation for improvement of protein extraction from chondrus crispus, gracilaria verrucosa and Palmaria palmata. J. Appl. Phycol. 1995, 7, 393–397. [Google Scholar] [CrossRef]
- Marrion, O.; Schwertz, A.; Fleurence, J.; Gueant, J.L.; Villaume, C. Improvement of the digestibility of the proteins of the red alga Palmaria palmata by physical processes and fermentation. Mol. Nutr. Food Res. 2003, 47, 339–344. [Google Scholar]
- Maehre, H.K.; Jensen, I.-J.; Eilertsen, K.-E. Enzymatic pre-treatment increases the protein bioaccessibility and extractability in Dulse (Palmaria palmata). Mar. Drugs 2016, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, V.; Galutzov, B.; Teissié, J. High yield electroextraction of proteins from yeast by a flow process. Anal. Biochem. 2003, 315, 77–84. [Google Scholar] [CrossRef]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry—A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Ashokkumar, M.; Sunartio, D.; Kentish, S.; Mawson, R.; Simons, L.; Vilkhu, K.; Versteeg, C.K. Modification of food ingredients by ultrasound to improve functionality: A preliminary study on a model system. Innov. Food Sci. Emerg. Technol. 2008, 9, 155–160. [Google Scholar] [CrossRef]
- Mason, T.; Paniwnyk, L.; Lorimer, J. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New approaches for the use of non-conventional cell disruption technologies to extract potential food additives and nutraceuticals from microalgae. Food Eng. Rev. 2015, 7, 45–62. [Google Scholar] [CrossRef]
- Parniakov, O.; Apicella, E.; Koubaa, M.; Barba, F.; Grimi, N.; Lebovka, N.; Pataro, G.; Ferrari, G.; Vorobiev, E. Ultrasound-assisted green solvent extraction of high-added value compounds from microalgae Nannochloropsis spp. Bioresour. Technol. 2015, 198, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Keris-Sen, U.D.; Sen, U.; Soydemir, G.; Gurol, M.D. An investigation of ultrasound effect on microalgal cell integrity and lipid extraction efficiency. Bioresour. Technol. 2014, 152, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Janczyk, P.; Wolf, C.; Souffrant, W.B. Evaluation of nutritional value and safety of the green microalgae Chlorella vulgaris treated with novel processing methods. Arch Zootech 2005, 8, 132–147. [Google Scholar]
- Janczyk, P.; Halle, B.; Souffrant, W. Microbial community composition of the crop and ceca contents of laying hens fed diets supplemented with Chlorella vulgaris. Poult. Sci. 2009, 88, 2324–2332. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Ma, H.; Wang, T.; Zheng, H. Alternating two-frequency countercurrent ultrasonic-assisted extraction of protein and polysaccharide from Porphyra yezoensis. Trans. Chin. Soc. Agric. Eng. 2013, 29, 285–292. [Google Scholar]
- Vanthoor-Koopmans, M.; Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H. Biorefinery of microalgae for food and fuel. Bioresour. Technol. 2013, 135, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.; Esveld, D.; Valero, A.; Luttge, R.; Mastwijk, H.; Bartels, P.; Van Den Berg, A.; Boom, R. Electroporation of cells in microfluidic devices: A review. Anal. Bioanal. Chem. 2006, 385, 474–485. [Google Scholar] [CrossRef] [PubMed]
- Grahl, T.; Märkl, H. Killing of microorganisms by pulsed electric fields. Appl. Microbiol. Biotechnol. 1996, 45, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Joannes, C.; Sipaut, C.S.; Dayou, J.; Yasir, S.M.; Mansa, R.F. The potential of using pulsed electric field (pef) technology as the cell disruption method to extract lipid from microalgae for biodiesel production. Int. J. Renew. Energy Res. 2015, 5, 598–621. [Google Scholar]
- Goettel, M.; Eing, C.; Gusbeth, C.; Straessner, R.; Frey, W. Pulsed electric field assisted extraction of intracellular valuables from microalgae. Algal Res. 2013, 2, 401–408. [Google Scholar] [CrossRef]
- Zbinden, M.D.A.; Sturm, B.S.; Nord, R.D.; Carey, W.J.; Moore, D.; Shinogle, H.; Stagg-Williams, S.M. Pulsed electric field (PEF) as an intensification pretreatment for greener solvent lipid extraction from microalgae. Biotechnol. Bioeng. 2013, 110, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Parameswaran, P.; Li, A.; Baez, M.; Rittmann, B.E. Effects of pulsed electric field treatment on enhancing lipid recovery from the microalga, scenedesmus. Bioresour. Technol. 2014, 173, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Postma, P.; Pataro, G.; Capitoli, M.; Barbosa, M.; Wijffels, R.H.; Eppink, M.; Olivieri, G.; Ferrari, G. Selective extraction of intracellular components from the microalga Chlorella vulgaris by combined pulsed electric field–temperature treatment. Bioresour. Technol. 2016, 203, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Luengo, E.; Martínez, J.M.; Coustets, M.; Álvarez, I.; Teissié, J.; Rols, M.-P.; Raso, J. A comparative study on the effects of millisecond-and microsecond-pulsed electric field treatments on the permeabilization and extraction of pigments from Chlorella vulgaris. J. Membr. Biol. 2015, 248, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted extraction of nutritionally valuable compounds from microalgae Nannochloropsis spp. Using the binary mixture of organic solvents and water. Innov. Food Sci. Emerg. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Töpfl, S. Pulsed Electric Fields (Pef) for Permeabilization of Cell Membranes in Food-and Bioprocessing–Applications, Process and Equipment Design and Cost Analysis; Berlin University of Technology: Berlin, Germany, 2006. [Google Scholar]
- Coustets, M.; Al-Karablieh, N.; Thomsen, C.; Teissié, J. Flow process for electroextraction of total proteins from microalgae. J. Membr. Biol. 2013, 246, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; Carretero, J.; Ferrer, I. Comparing pretreatment methods for improving microalgae anaerobic digestion: Thermal, hydrothermal, microwave and ultrasound. Chem. Eng. J. 2015, 279, 667–672. [Google Scholar] [CrossRef]
- Herrero, M.; Cifuentes, A.; Ibanez, E. Sub-and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-by-products, algae and microalgae: A review. Food Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, N.; Ranjan, R.; Kumar, S.; Bhat, Z.; Jeong, D.K. Perspective of membrane technology in dairy industry: A review. Asian-Australas. J. Anim. Sci. 2013, 26, 1347. [Google Scholar] [CrossRef] [PubMed]
- Yaich, H.; Garna, H.; Besbes, S.; Paquot, M.; Blecker, C.; Attia, H. Chemical composition and functional properties of Ulva lactuca seaweed collected in Tunisia. Food Chem. 2011, 128, 895–901. [Google Scholar] [CrossRef]
- Pafylias, I.; Cheryan, M.; Mehaia, M.; Saglam, N. Microfiltration of milk with ceramic membranes. Food Res. Int. 1996, 29, 141–146. [Google Scholar] [CrossRef]
- Petrusevski, B.; Bolier, G.; Van Breemen, A.; Alaerts, G. Tangential flow filtration: A method to concentrate freshwater algae. Water Res. 1995, 29, 1419–1424. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Chen, M.; Pei, Y.; Sun, P. In intro antioxidant activities of different sulfated polysaccharides from chlorophytan seaweeds Ulva fasciata. Int. J. Biol. Macromol. 2013, 59, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Denis, C.; Massé, A.; Fleurence, J.; Jaouen, P. Concentration and pre-purification with ultrafiltration of a r-phycoerythrin solution extracted from macro-algae grateloupia turuturu: Process definition and up-scaling. Sep. Purif. Technol. 2009, 69, 37–42. [Google Scholar] [CrossRef]
- Safi, C.; Liu, D.Z.; Yap, B.H.; Martin, G.J.; Vaca-Garcia, C.; Pontalier, P.-Y. A two-stage ultrafiltration process for separating multiple components of Tetraselmis suecica after cell disruption. J. Appl. Phycol. 2014, 26, 2379–2387. [Google Scholar] [CrossRef]
- American College of Sports Medicine; American Dietetic Association; Dietitians of Canada. Joint Position Statement: Nutrition and athletic performance. American College of Sports Medicine, American Dietetic Association, and Dietitians of Canada. Med. Sci. Sports Exerc. 2000, 32, 2130–2145. [Google Scholar]
- Fleurence, J.; Morançais, M.; Dumay, J.; Decottignies, P.; Turpin, V.; Munier, M.; Garcia-Bueno, N.; Jaouen, P. What are the prospects for using seaweed in human nutrition and for marine animals raised through aquaculture? Trends Food Sci. Technol. 2012, 27, 57–61. [Google Scholar] [CrossRef]
- Du, J. Research on functionality sports nutrition and health food security issues based on Circulation. Open Cybern. System. J. 2015, 9, 1945–1949. [Google Scholar] [CrossRef]
- Chakdar, H.; Jadhav, S.D.; Dhar, D.W.; Pabbi, S. Potential applications of blue green algae. J. Sci. Ind. Res. 2012, 71, 13–20. [Google Scholar]
- Tokuşoglu, Ö.; Üunal, M. Biomass nutrient profiles of three microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Liang, S.; Liu, X.; Chen, F.; Chen, Z. Current Microalgal Health Food R & D Activities in China. In Asian Pacific Phycology in the 21st Century: Prospects and Challenges; Springer: Heidelberg, Germany, 2004; pp. 45–48. [Google Scholar]
- Marcus, J.B. Enhancing umami taste in foods. In Modifying Flavour in Food; Taylor, A., Hort, J., Eds.; CRC Press: Cambridge, UK, 2007; pp. 202–220. [Google Scholar]
- Prabhasankar, P.; Ganesan, P.; Bhaskar, N.; Hirose, A.; Stephen, N.; Gowda, L.R.; Hosokawa, M.; Miyashita, K. Edible Japanese seaweed, wakame (Undaria pinnatifida) as an ingredient in pasta: Chemical, functional and structural evaluation. Food Chem. 2009, 115, 501–508. [Google Scholar] [CrossRef]
- Hall, A.; Fairclough, A.; Mahadevan, K.; Paxman, J. Ascophyllum nodosum enriched bread reduces subsequent energy intake with no effect on post-prandial glucose and cholesterol in healthy, overweight males. A pilot study. Appetite 2012, 58, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, C.; Gallagher, E.; Doran, L.; Auty, M.; Prieto, J.; Hayes, M. Increasing the health benefits of bread: Assessment of the physical and sensory qualities of bread formulated using a renin inhibitory Palmaria palmata protein hydrolysate. LWT-Food Sci. Technol. 2014, 56, 398–405. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sajilata, M.; Singhal, R.; Kamat, M. Fractionation of lipids and purification of γ-linolenic acid (GLA) from Spirulina platensis. Food Chem. 2008, 109, 580–586. [Google Scholar] [CrossRef]
- Khan, Z.; Bhadouria, P.; Bisen, P. Nutritional and therapeutic potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K. Recent advances in microalgal bioscience in Japan, with special reference to utilization of biomass and metabolites: A review. J. Appl. Phycol. 1996, 8, 487–502. [Google Scholar] [CrossRef]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res. Thessalon. 2014, 21, 1. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M. Biological Activities of Proteins and Marine-Derived Peptides from Byproducts and Seaweeds. In Marine Proteins and Peptides: Biological Activities and Applications; Kim, S.-K., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2013; pp. 139–165. [Google Scholar]
- Wang, G.; Sun, H.; Fan, X.; Tseng, C. Large-scale isolation and purification of R-phycoerythrin from red alga Palmaria palmata using the expanded bed adsorption method. Acta Bot. Sin. 2001, 44, 541–546. [Google Scholar]
- Kawakubo, A.; Makino, H.; Ohnishi, J.-I.; Hirohara, H.; Hori, K. The marine red alga Eucheuma serra J. Agardh, a high yielding source of two isolectins. J. Appl. Phycol. 1997, 9, 331–338. [Google Scholar] [CrossRef]
- Weis, W.I.; Drickamer, K. Structural basis of lectin-carbohydrate recognition. Annu. Rev. Biochem. 1996, 65, 441–473. [Google Scholar] [CrossRef] [PubMed]
- Ziółkowska, N.E.; Wlodawer, A. Structural studies of algal lectins with anti-HIV activity. Acta Biochim. Pol. 2006, 53, 617–626. [Google Scholar] [PubMed]
- Naeem, A.; Saleemuddin, M.; Hasan Khan, R. Glycoprotein targeting and other applications of lectins in biotechnology. Curr. Protein Pept. Sci. 2007, 8, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Harnedy, P.A.; FitzGerald, R.J. Bioactive Proteins, Peptides, and Amino Acids from macroalgae. J. Phycol. 2011, 47, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Glazer, A.N. Phycobiliproteins—A family of valuable, widely used fluorophores. J. Appl. Phycol. 1994, 6, 105–112. [Google Scholar] [CrossRef]
- De Marsac, N.T. Phycobiliproteins and phycobilisomes: The early observations. Photosynth. Res. 2003, 76, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a commodity: Trends in applied research, patents and commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Kronick, M.; Grossman, P.D. Immunoassay techniques with fluorescent phycobiliprotein conjugates. Clin. Chem. 1983, 29, 1582–1586. [Google Scholar] [PubMed]
- Aneiros, A.; Garateix, A. Bioactive peptides from marine sources: Pharmacological properties and isolation procedures. J. Chromatogr. B 2004, 803, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Becker, W. Microalgae in Human and Animal Nutrition. In Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Richmond, A., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2004; p. 312. [Google Scholar]
- Holman, B.; Malau-Aduli, A. Spirulina as a livestock supplement and animal feed. J. Anim. Physiol. Anim. Nutr. 2013, 97, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Evans, F.; Critchley, A. Seaweeds for animal production use. J. Appl. Phycol. 2014, 26, 891–899. [Google Scholar] [CrossRef]
- Allen, V.; Pond, K.; Saker, K.; Fontenot, J.; Bagley, C.; Ivy, R.; Evans, R.; Brown, C.; Miller, M.; Montgomery, J. Tasco-Forage: III. Influence of a seaweed extract on performance, monocyte immune cell response, and carcass characteristics in feedlot-finished steers. J. Anim. Sci. 2001, 79, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Saker, K.; Allen, V.; Fontenot, J.; Bagley, C.; Ivy, R.; Evans, R.; Wester, D. Tasco-Forage: II. Monocyte immune cell response and performance of beef steers grazing tall fescue treated with a seaweed extract. J. Anim. Sci. 2001, 79, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.; Allen, V.; Pond, K.; Miller, M.; Wester, D.; Brown, C.; Evans, R.; Bagley, C.; Ivy, R.; Fontenot, J. Tasco-Forage: IV. Influence of a seaweed extract applied to tall fescue pastures on sensory characteristics, shelf-life, and vitamin e status in feedlot-finished steers. J. Anim. Sci. 2001, 79, 884–894. [Google Scholar] [CrossRef] [PubMed]
- Braden, K.; Blanton, J.; Allen, V.; Pond, K.; Miller, M. Ascophyllum nodosum supplementation: A preharvest intervention for reducing Escherichia Coli O157: H7 and Salmonella spp. In feedlot steers. J. Food Prot. 2004, 67, 1824–1828. [Google Scholar] [CrossRef]
- Allen, V.; Pond, K.; Saker, K.; Fontenot, J.; Bagley, C.; Ivy, R.; Evans, R.; Schmidt, R.; Fike, J.; Zhang, X. Tasco: Influence of a brown seaweed on antioxidants in forages and livestock—A review. J. Anim. Sci. 2001, 79, E21–E31. [Google Scholar] [CrossRef]
- Ginzberg, A.; Cohen, M.; Sod-Moriah, U.A.; Shany, S.; Rosenshtrauch, A.; Arad, S.M. Chickens fed with biomass of the red microalga Porphyridium sp. Have reduced blood cholesterol level and modified fatty acid composition in egg yolk. J. Appl. Phycol. 2000, 12, 325–330. [Google Scholar] [CrossRef]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Mariey, Y.; Samak, H.; Abou-Khashba, H.; Sayed, M.; Abou-Zeid, A. Effect of using Spirulina platensis algae as a feed additives for poultry diets: 2 productive performance of broiler. Egypt. Poult. Sci. 2014, 34, 245–258. [Google Scholar]
- Al-Batshan, H.A.; Al-Mufarrej, S.I.; Al-Homaidan, A.A.; Qureshi, M.A. Enhancement of chicken macrophage phagocytic function and nitrite production by dietary Spirulina platensis. Immunopharmacol. Immunotoxicol. 2001, 23, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.; Dominy, W. The nutritional value of dehydrated, blue-green algae (Spirulina plantensis) for poultry. Poult. Sci. 1990, 69, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Zahroojian, N.; Moravej, H.; Shivazad, M. Effects of dietary marine algae (Spirulina platensis) on egg quality and production performance of laying hens. J. Agric. Sci. Technol. 2013, 15, 1353–1360. [Google Scholar]
- Mariey, Y.; Samak, H.; Ibrahem, M. Effect of using Spirulina platensis algae as a feed additive for poultry diets. 1. Productive and reproductive performances of local laying hens. Egypt. Poult. Sci. 2012, 32, 201–215. [Google Scholar]
- Anderson, D.W.; Tang, C.S.; Ross, E. The xanthophylls of Spirulina and their effect on egg-yolk pigmentationthe xanthophylls of Spirulina and their effect on egg-yolk pigmentation. Poult. Sci. 1991, 70, 115–119. [Google Scholar] [CrossRef]
- Sujatha, T.; Narahari, D. Effect of designer diets on egg yolk composition of ‘white leghorn’ hens. J. Food Sci. Technol. 2011, 48, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Abudabos, A.M.; Okab, A.B.; Aljumaah, R.S.; Samara, E.M.; Abdoun, K.A.; Al-Haidary, A.A. Nutritional value of green seaweed (Ulva lactuca) for broiler chickens. Ital. J. Anim. Sci. 2013, 12, e28. [Google Scholar] [CrossRef]
- El-Deek, A.; Brikaa, M.A. Nutritional and biological evaluation of marine seaweed as a feedstuff and as a pellet binder in poultry diet. Int. J. Poult. Sci. 2009, 8, 875–881. [Google Scholar] [CrossRef]
- Kulshreshtha, G.; Rathgeber, B.; Stratton, G.; Thomas, N.; Evans, F.; Critchley, A.; Hafting, J.; Prithiviraj, B. Feed supplementation with red seaweeds, Chondrus crispus and Sarcodiotheca gaudichaudii, affects performance, egg quality, and gut microbiota of layer hens. Poult. Sci. 2014, 93, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Gatrell, S.; Lum, K.; Kim, J.; Lei, X. Nonruminant Nutrition Symposium: Potential of defatted microalgae from the biofuel industry as an ingredient to replace corn and soybean meal in swine and poultry diets. J. Anim. Sci. 2014, 92, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Grinstead, G.; Tokach, M.; Dritz, S.; Goodband, R.; Nelssen, J. Effects of Spirulina platensis on growth performance of weanling pigs. Anim. Feed Sci. Technol. 2000, 83, 237–247. [Google Scholar] [CrossRef]
- Granaci, V. Achievements in the Artificial Insemination of Swine; University of Agricultural Sciences and Veterinary Medicine: Cluj-Napoca, Romania, 2007. [Google Scholar]
- He, M.; Hollwich, W.; Rambeck, W. Supplementation of algae to the diet of pigs: A new possibility to improve the iodine content in the meat. J. Anim. Physiol. Anim. Nutr. 2002, 86, 97–104. [Google Scholar] [CrossRef]
- Dierick, N.; Ovyn, A.; De Smet, S. Effect of feeding intact brown seaweed Ascophyllum nodosum on some digestive parameters and on iodine content in edible tissues in pigs. J. Sci. Food Agric. 2009, 89, 584–594. [Google Scholar] [CrossRef]
- Reilly, P.; O’doherty, J.; Pierce, K.; Callan, J.; O’sullivan, J.; Sweeney, T. The effects of seaweed extract inclusion on gut morphology, selected intestinal microbiota, nutrient digestibility, volatile fatty acid concentrations and the immune status of the weaned pig. Animal 2008, 2, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Angell, A.R.; Angell, S.F.; de Nys, R.; Paul, N.A. Seaweed as a protein source for mono-gastric livestock. Trends Food Sci. Technol. 2016, 54, 74–84. [Google Scholar] [CrossRef]
- Panjaitan, T.; Quigley, S.; McLennan, S.; Poppi, D. Effect of the concentration of Spirulina (Spirulina platensis) algae in the drinking water on water intake by cattle and the proportion of algae bypassing the rumen. Anim. Prod. Sci. 2010, 50, 405–409. [Google Scholar] [CrossRef]
- Kulpys, J.; Paulauskas, E.; Pilipavicius, V.; Stankevicius, R. Influence of cyanobacteria arthrospira (Spirulina) platensis biomass additive towards the body condition of lactation cows and biochemical milk indexes. Agron. Res 2009, 7, 823–835. [Google Scholar]
- Christaki, E.; Karatzia, M.; Bonos, E.; Florou-Paneri, P.; Karatzias, C. Effect of dietary Spirulina Platensis on milk fatty acid profile of dairy cows. Asian J. Anim. Vet. Adv. 2012, 7, 597–604. [Google Scholar] [CrossRef]
- Šimkus, A.; Oberauskas, V.; Laugalis, J.; Želvytė, R.; Monkevičienė, I.; Sederevičius, A.; Šimkienė, A.; Pauliukas, K. The effect of weed Spirulina platensis on the milk production in cows. Vet. Zootech. 2007, 38, 74–77. [Google Scholar]
- Boeckaert, C.; Vlaeminck, B.; Dijkstra, J.; Issa-Zacharia, A.; Van Nespen, T.; Van Straalen, W.; Fievez, V. Effect of dietary starch or micro algae supplementation on rumen fermentation and milk fatty acid composition of dairy cows. J. Dairy Sci. 2008, 91, 4714–4727. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, L.; Silva, A.; Azevedo, S.; Mendes, R.; Mangueira, J.; Gomes, A. Performance of santa inês lambs submitted to the use of artificial milk enriched with Spirulina Platensis. Ciênc. Anim. Bras. 2010, 11, 258–263. [Google Scholar]
- Peiretti, P.; Meineri, G. Effects of diets with increasing levels of Spirulina Platensis on the performance and apparent digestibility in growing rabbits. Livest. Sci. 2008, 118, 173–177. [Google Scholar] [CrossRef]
- Arieli, A.; Sklan, D.; Kissil, G. A note on the nutritive value of Ulva lactuca for ruminants. Anim. Sci. 1993, 57, 329–331. [Google Scholar] [CrossRef]
- Ventura, M.; Castañón, J. The nutritive value of seaweed (Ulva lactuca) for goats. Small Rumin. Res. 1998, 29, 325–327. [Google Scholar] [CrossRef]
- Novoa-Garrido, M.; Aanensen, L.; Lind, V.; Larsen, H.J.S.; Jensen, S.K.; Govasmark, E.; Steinshamn, H. Immunological effects of feeding macroalgae and various vitamin e supplements in Norwegian white sheep-ewes and their offspring. Livest. Sci. 2014, 167, 126–136. [Google Scholar] [CrossRef]
- Lio-Po, G.D.; Leaño, E.M.; Peñaranda, M.M.D.; Villa-Franco, A.U.; Sombito, C.D.; Guanzon, N.G. Anti-luminous vibrio factors associated with the ‘green water’grow-out culture of the tiger shrimp Penaeus monodon. Aquaculture 2005, 250, 1–7. [Google Scholar] [CrossRef]
- Chuntapa, B.; Powtongsook, S.; Menasveta, P. Water quality control using Spirulina Platensis in shrimp culture tanks. Aquaculture 2003, 220, 355–366. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Microalgae for aquaculture: Opportunities and constraints. J. Appl. Phycol. 1997, 9, 393–401. [Google Scholar] [CrossRef]
- Müller-Fuega, A. Microalgae for Aquaculture: The current global situation and future trends. In Handbook of Microalgal C Ulture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; John Wiley Sons, Ltd.: Oxford, UK, 2013; pp. 352–364. [Google Scholar]
- Robinson, C.; Samocha, T.; Fox, J.; Gandy, R.; McKee, D. The use of inert artificial commercial food sources as replacements of traditional live food items in the culture of larval shrimp, Farfantepenaeus aztecus. Aquaculture 2005, 245, 135–147. [Google Scholar] [CrossRef]
- Muller-Feuga, A. The role of microalgae in aquaculture: Situation and trends. J. Appl. Phycol. 2000, 12, 527–534. [Google Scholar] [CrossRef]
- Lozano, I.; Wacyk, J.M.; Carrasco, J.; Cortez-San Martín, M.A. Red macroalgae Pyropia columbina and Gracilaria chilensis: Sustainable feed additive in the Salmo salar diet and the evaluation of potential antiviral activity against infectious salmon anemia virus. J. Appl. Phycol. 2016, 28, 1343–1351. [Google Scholar] [CrossRef]
- Wan, A.H.; Soler-Vila, A.; O’Keeffe, D.; Casburn, P.; Fitzgerald, R.; Johnson, M.P. The inclusion of Palmaria palmata macroalgae in Atlantic salmon (Ulva lactuca) diets: Effects on growth, haematology, immunity and liver function. J. Appl. Phycol. 2016, 28, 3091–3100. [Google Scholar] [CrossRef]
- Bansemer, M.S.; Qin, J.G.; Harris, J.O.; Howarth, G.S.; Stone, D.A. Nutritional requirements and use of macroalgae as ingredients in abalone feed. Rev. Aquac. 2016, 8, 121–135. [Google Scholar] [CrossRef]
- Viera, M.; de Vicose, G.C.; Gómez-Pinchetti, J.; Bilbao, A.; Fernandez-Palacios, H.; Izquierdo, M. Comparative performances of juvenile abalone (Haliotis tuberculata coccinea Reeve) fed enriched vs non-enriched macroalgae: Effect on growth and body composition. Aquaculture 2011, 319, 423–429. [Google Scholar] [CrossRef]
- Xia, S.; Yang, H.; Li, Y.; Liu, S.; Zhou, Y.; Zhang, L. Effects of different seaweed diets on growth, digestibility, and ammonia-nitrogen production of the sea cucumber Apostichopus japonicus (selenka). Aquaculture 2012, 338, 304–308. [Google Scholar] [CrossRef]
- Saito, T. Antihypertensive peptides derived from Bovine Casein and Whey Proteins. In Bioactive Components of Milk; Bösze, Z., Ed.; Springer: New York, NY, USA, 2008; pp. 295–317. [Google Scholar]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Vercruysse, L.; Van Camp, J.; Smagghe, G. ACE inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein: A review. J. Agric. Food Chem. 2005, 53, 8106–8115. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Aleixandre, A. Antihypertensive peptides derived from egg proteins. J. Nutr. 2006, 136, 1457–1460. [Google Scholar] [PubMed]
- Jung, W.K.; Mendis, E.; Je, J.Y.; Park, P.J.; Son, B.W.; Kim, H.C.; Kim, S.K. Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive ratsangiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem 2006, 94, 26. [Google Scholar]
- Yu, Y.; Hu, J.; Miyaguchi, Y.; Bai, X.; Du, Y.; Lin, B. Isolation and characterization of angiotensin I-converting enzyme inhibitory peptides derived from porcine hemoglobin. Peptides 2006, 27, 2950–2956. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Qu, M.R.; Wan, J.Z.; You, J.M. Antihypertensive effect of rice protein hydrolysate with in vitro angiotensin I-converting enzyme inhibitory activity in spontaneously hypertensive rats. Asia. Pac. J. Clin. Nutr. 2007, 16, 275–280. [Google Scholar] [PubMed]
- Rho, S.J.; Lee, J.S.; Chung, Y.I.; Kim, Y.W.; Lee, H.G. Purification and identification of an angiotensin I-converting enzyme inhibitory peptide from fermented soybean extract. Process Biochem. 2009, 44, 490. [Google Scholar] [CrossRef]
- Motoi, H.; Kodama, T. Isolation and characterization of angiotensin I-converting enzyme inhibitory peptides from wheat gliadin hydrolysate. Nahrung 2003, 47, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Aluko, R.E. Determination of nutritional and bioactive properties of peptides in enzymatic pea, chickpea, and mung bean protein hydrolysates. J. AOAC Int. 2008, 91, 947–956. [Google Scholar] [PubMed]
- Lee, J.-E.; Bae, I.Y.; Lee, H.G.; Yang, C.-B. Tyr-Pro-Lys, an angiotensin I-converting enzyme inhibitory peptide derived from broccoli (Brassica oleracea Italica). Food Chem. 2006, 99, 143–148. [Google Scholar] [CrossRef]
- Suetsuna, K. Isolation and characterization of angiotensin I-converting enzyme inhibitor dipeptides derived from Allium sativum L. (garlic) Isolation and characterization of angiotensin I-converting enzyme inhibitor dipeptides derived from. J. Nutr. Biochem. 1998, 9, 415. [Google Scholar] [CrossRef]
- Fan, X.; Bai, L.; Zhu, L.; Yang, L.; Zhang, X. Marine algae-derived bioactive peptides for human nutrition and health. J. Agric. Food Chem. 2014, 62, 9211–9222. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, L.; Bondu, S.; Doiron, K.; Rioux, L.-E.; Turgeon, S.L. Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J. Funct. Foods 2015, 17, 685–697. [Google Scholar] [CrossRef]
- Ennamany, R.; Saboureau, D.; Mekideche, N.; Creppy, E. Secma 1®, a mitogenic hexapeptide from Ulva algeae modulates the production of proteoglycans and glycosaminoglycans in human foreskin fibroblast. Hum. Exp. Toxicol. 1998, 17, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Nakano, T. Identification of an antihypertensive peptide from peptic digest of wakame (Undaria pinnatifida). J. Nutr. Biochem. 2000, 11, 450–454. [Google Scholar] [CrossRef]
- Suetsuna, K.; Maekawa, K.; Chen, J.-R. Antihypertensive effects of Undaria pinnatifida (wakame) peptide on blood pressure in spontaneously hypertensive rats. J. Nutr. Biochem. 2004, 15, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-converting enzyme inhibitory peptides derived from wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-H.; Ahn, G.-N.; Heo, S.-J.; Kim, K.-N.; Lee, K.-W.; Song, C.-B.; Jeon, Y.-J. Screening of extracts from marine green and brown algae in Jeju for potential marine angiotensin-I converting enzyme (ACE) inhibitory activity. J. Korean Soc. Food Sci. Nutr. 2006, 35, 307–314. [Google Scholar]
- Suetsuna, K. Purification and identification of angiotensin I-converting enzyme inhibitors from the red alga Porphyra yezoensis. J. Mar. Biotechnol. 1998, 6, 163–167. [Google Scholar] [PubMed]
- Suetsuna, K. Separation and identification of angiotensin I-converting enzyme inhibitory peptides from peptic digest of Hizikia fusiformis protein. Nippon. Suisan. Gakkaishi. 1998, 64, 862–866. [Google Scholar] [CrossRef]
- Furuta, T.; Miyabe, Y.; Yasui, H.; Kinoshita, Y.; Kishimura, H. Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Phycobiliproteins of Dulse Palmaria palmata. Mar. Drugs 2016, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Chen, J.-R. Identification of antihypertensive peptides from peptic digest of two microalgae, Chlorella vulgaris and Spirulina Platensis. Mar. Biotechnol. 2001, 3, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-J.; Heo, S.-J.; Oh, C.H.; Kang, D.-H.; Jeong, S.H.; Park, W.S.; Choi, I.-W.; Jeon, Y.-J.; Jung, W.-K. Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptide Isolated from Biodiesel Byproducts of Marine Microalgae, Nannochloropsis oculata. J. Biobased Mater. Bioenergy 2013, 7, 135–142. [Google Scholar] [CrossRef]
- Samarakoon, K.W.; Kwon, O.-N.; Ko, J.-Y.; Lee, J.-H.; Kang, M.-C.; Kim, D.; Lee, J.B.; Lee, J.-S.; Jeon, Y.-J. Purification and identification of novel angiotensin-I converting enzyme (ACE) inhibitory peptides from cultured marine microalgae (Nannochloropsis oculata) protein hydrolysate. J. Appl. Phycol. 2013, 25, 1595–1606. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kang, N.; Kim, E.-A.; Kang, M.C.; Lee, S.-H.; Kang, S.-M.; Lee, J.-B.; Jeon, B.-T.; Kim, S.-K.; Park, S.-J. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem. 2012, 47, 2005–2011. [Google Scholar] [CrossRef]
- Sheih, I.-C.; Fang, T.J.; Wu, T.-K. Isolation and characterisation of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem. 2009, 115, 279–284. [Google Scholar] [CrossRef]
- Tierney, M.S.; Croft, A.K.; Hayes, M. A review of antihypertensive and antioxidant activities in macroalgae. Bot. Mar. 2010, 53, 387–408. [Google Scholar] [CrossRef]
- Sheih, I.C.; Fang, T.J.; Wu, T.K.; Lin, P.H. Anticancer and antioxidant activities of the peptide fraction from algae protein waste. J. Agric. Food Chem. 2010, 58, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Sheih, I.C.; Wu, T.K.; Fang, T.J. Antioxidant properties of a new antioxidative peptide from algae protein waste hydrolysate in different oxidation systems. Bioresour. Technol. 2009, 100, 3419–3425. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Qian, Z.J.; Ryu, B.; Karadeniz, F.; Kim, D.; Kim, S.K. Antioxidant peptides from protein hydrolysate of microalgae Navicula incerta and their protective effects in HepG2/CYP2E1 cells induced by ethanol. Phytother. Res. 2012, 26, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-C.; Kim, D.; Jeon, Y.-J. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, A.; O’Callaghan, Y.; O’Grady, M.; Queguineur, B.; Hanniffy, D.; Troy, D.; Kerry, J.; O’Brien, N. In vitro and cellular antioxidant activities of seaweed extracts prepared from five brown seaweeds harvested in spring from the west coast of Ireland. Food Chem. 2011, 126, 1064–1070. [Google Scholar] [CrossRef]
- Heo, S.J.; Lee, G.W.; Song, C.B.; Jeon, Y.J. Antioxidant activity of enzymatic extracts from brown seaweeds. Algae 2003, 18, 71–81. [Google Scholar] [CrossRef]
- Heo, S.-J.; Park, E.-J.; Lee, K.-W.; Jeon, Y.-J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Lakmal, H.C.; Samarakoon, K.W.; Lee, W.; Lee, J.-H.; Abeytunga, D.; Lee, H.-S.; Jeon, Y.-J. Anticancer and antioxidant effects of selected Sri Lankan marine algae. J. Natl. Sci. Found. Sri Lanka 2014, 42, 315–323. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X. Separation, antitumor activities, and encapsulation of polypeptide from Chlorella pyrenoidosa. Biotechnol. Progress 2013, 29, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, X. Separation and nanoencapsulation of antitumor polypeptide from Spirulina Platensis. Biotechnol. Progress 2013, 29, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Martínez-Augustin, O.; Drago, S.R. Bioactive properties of peptides obtained by enzymatic hydrolysis from protein byproducts of Porphyra columbina. Food Res. Int. 2012, 49, 364–372. [Google Scholar] [CrossRef]
- Shih, M.F.; Chen, L.C.; Cherng, J.Y. Chlorella 11-peptide inhibits the production of macrophage-induced adhesion molecules and reduces endothelin-1 expression and endothelial permeability. Mar. Drugs 2013, 11, 3861–3874. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Kim, S.-K. Down-regulation of histamine-induced endothelial cell activation as potential anti-atherosclerotic activity of peptides from Spirulina maxima. Eur. J. Pharm. Sci. 2013, 50, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Ryu, B.; Kim, S.-K. Purification of novel anti-inflammatory peptides from enzymatic hydrolysate of the edible microalgal Spirulina maxima. J. Funct. Foods 2013, 5, 1336–1346. [Google Scholar] [CrossRef]
- Fitzgerald, C.; Gallagher, E.; O’Connor, P.; Prieto, J.; Mora-Soler, L.; Grealy, M.; Hayes, M. Development of a seaweed derived platelet activating factor acetylhydrolase (PAF-AH) inhibitory hydrolysate, synthesis of inhibitory peptides and assessment of their toxicity using the zebrafish larvae assay. Peptides 2013, 50, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-H.; Qian, Z.-J.; Ryu, B.; Karadeniz, F.; Kim, D.; Kim, S.-K. Hepatic fibrosis inhibitory effect of peptides isolated from navicula incerta on TGF-β1 Induced activation of LX-2 human hepatic stellate cells. Prev. Nutr. Food Sci. 2013, 18, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.; Hwang, I.; Park, E.; Kim, J.; Jeon, Y.-J.; Lee, J.; Park, J.W.; Jee, Y. Immunomodulatory effects of an enzymatic extract from Ecklonia cava on murine splenocytes. Mar. Biotechnol. 2008, 10, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Liou, S.-F.; Chen, S.-J.; Shih, M.-F. Protective effects of Chlorella-derived peptide on uvb-induced production of MMP-1 and degradation of procollagen genes in human skin fibroblasts. Regul. Toxicol. Pharm. 2011, 60, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.-F.; Cherng, J.-Y. Potential protective effect of fresh grown unicellular green algae component (resilient factor) against PMA-and UVB-induced MMP1 expression in skin fibroblasts. Eur. J. Dermatol. 2008, 18, 303–307. [Google Scholar] [PubMed]
- Nguyen, M.H.T.; Qian, Z.-J.; Nguyen, V.-T.; Choi, I.-W.; Heo, S.-J.; Oh, C.H.; Kang, D.-H.; Kim, G.H.; Jung, W.-K. Tetrameric peptide purified from hydrolysates of biodiesel byproducts of Nannochloropsis oculata induces osteoblastic differentiation through MAPK and SMAD pathway on MG-63 and D1 cells. Process Biochem. 2013, 48, 1387–1394. [Google Scholar] [CrossRef]
- Athukorala, Y.; Lee, K.-W.; Kim, S.-K.; Jeon, Y.-J. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Technol. 2007, 98, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Cian, R.E.; Garzón, A.G.; Ancona, D.B.; Guerrero, L.C.; Drago, S.R. Hydrolyzates from Pyropia columbina seaweed have antiplatelet aggregation, antioxidant and ACE I inhibitory peptides which maintain bioactivity after simulated gastrointestinal digestion. LWT-Food Sci. Technol. 2015, 64, 881–888. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Van Camp, J.; Verstraete, W. Bioavailability of angiotensin I converting enzyme inhibitory peptides. Br. J. Nutr. 2004, 92, 357–366. [Google Scholar] [CrossRef] [PubMed]
- World Heath Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Turk, B. Targeting proteases: Successes, failures and future prospects. Nature Rev. Drug Discov. 2006, 5, 785–799. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Peng, C.; Jiao, R.; Wong, Y.M.; Yang, N.; Huang, Y. Anti-hypertensive nutraceuticals and functional foods. J. Agric. Food Chem. 2009, 57, 4485–4499. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cai, Q.-F.; Yoshida, A.; Sun, L.-C.; Liu, Y.-X.; Liu, G.-M.; Su, W.-J.; Cao, M.-J. Purification and characterization of two novel angiotensin I-converting enzyme inhibitory peptides derived from r-phycoerythrin of red algae (Bangia fusco-purpurea). Eur. Food Res. Technol. 2017, 243, 779–789. [Google Scholar] [CrossRef]
- Fitzgerald, C.N.; Mora-Soler, L.; Gallagher, E.; O’Connor, P.; Prieto, J.; Soler-Vila, A.; Hayes, M. Isolation and characterization of bioactive pro-peptides with in vitro renin inhibitory activities from the macroalga Palmaria palmata. J. Agric. Food Chem. 2012, 60, 7421–7427. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Angeli, F.; Mazzotta, G.; Gentile, G.; Reboldi, G. The renin angiotensin system in the development of cardiovascular disease: Role of aliskiren in risk reduction. Vasc. Health Risk Manag. 2008, 4, 971–981. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Kraan, S. Review of the Potential Mechanisation of Kelp Harvesting in Ireland; Marine Institute: Dublin, Ireland, 2004; p. 52. [Google Scholar]
- Werner, A.; Clarke, D.; Kraan, S. Strategic Review and the Feasibility of Seaweed Aquaculture in Ireland; Irish Seaweed Centre, Martin Ryan Institute, National University of Ireland: Galway, Ireland, 2004; p. 120. [Google Scholar]
- De Grave, S.; Fazakerley, H.; Kelly, L.; Guiry, M.; Ryan, M.; Walshe, J. A Study of Selected Mmaërl Beds in Irish Waters and Their Potential for Sustainable Extraction; Marine Institute: Dublin, Ireland, 2000. [Google Scholar]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Galland-Irmouli, A.-V.; Fleurence, J.; Lamghari, R.; Luçon, M.; Rouxel, C.; Barbaroux, O.; Bronowicki, J.-P.; Villaume, C.; Guéant, J.-L. Nutritional value of proteins from edible seaweed Palmaria palmata (dulse). J. Nutr. Biochem. 1999, 10, 353–359. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Fonseca, P.; Carneiro, M.; Moreira, W. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.Y.; Robertson, J.; Hamid, N.; Ma, Q.; Lu, J. Changes in total nitrogen and amino acid composition of New Zealand Undaria pinnatifida with growth, location and plant parts. Food Chem. 2015, 186, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Vannela, R.; Rittmann, B. Disruption of Synechocystis PCC 6803 for lipid extraction. Water Sci. Technol. 2012, 65, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Marrion, O.; Fleurence, J.; Schwertz, A.; Guéant, J.-L.; Mamelouk, L.; Ksouri, J.; Villaume, C. Evaluation of protein in vitro digestibility of Palmaria palmata and Gracilaria verrucosa. J. Appl. Phycol. 2005, 17, 99–102. [Google Scholar] [CrossRef]
- Meade, S.J.; Reid, E.A.; Gerrard, J.A. The impact of processing on the nutritional quality of food proteins. J. AOAC Int. 2005, 88, 904–922. [Google Scholar] [PubMed]
- Maehre, H.K.; Edvinsen, G.K.; Eilertsen, K.-E.; Elvevoll, E.O. Heat treatment increases the protein bioaccessibility in the red seaweed dulse (Palmaria palmata), but not in the brown seaweed winged kelp (Alaria esculenta). J. Appl. Phycol. 2016, 28, 581–590. [Google Scholar] [CrossRef]
- Setting Maximum Levels for Certain Contaminants in Foodstuffs. 2006. Available online: https://www.fsai.ie/uploadedFiles/Consol_Reg1881_2006.pdf (accessed on 20 April 2017).
- Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission Related to Mercury and Methylmercury in Food. Available online: http://www.efsa.europa.eu/sites/default/files/scientific_output/files/main_documents/34.pdf (accessed on 20 April 2017).
- European Food Safety Authority. Scientific Opinion on Arsenic in Food. EFSA Panel on Contaminants in the Food Chain. Available online: http://www.iss.it/binary/meta/cont/AsSummary2009en.pdf (accessed on 20 April 2017).
- European Food Safety Authority. Scientific Opinion on Lead in Food. EFSA Panel on Contaminants in the Food Chain. Available online: http://www.iss.it/binary/meta/cont/Pb_Opinion2010.pdf (accessed on 20 April 2017).
- European Food Safety Authority. Statement on Tolerable Weekly Intake for Cadmium. EFSA Panel on Contaminants in the Food Chain. Available online: http://www.megapesca.com/megashop/FH201102_tgf/EFSA_Scientific_Opinion_Cadmium.pdf (accessed on 20 April 2017).
- Almela, C.; Algora, S.; Benito, V.; Clemente, M.; Devesa, V.; Suner, M.; Velez, D.; Montoro, R. Heavy metal, total arsenic, and inorganic arsenic contents of algae food products. J. Agric. Food Chem. 2002, 50, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Besada, V.; Andrade, J.M.; Schultze, F.; González, J.J. Heavy metals in edible seaweeds commercialised for human consumption. J. Mar. Syst. 2009, 75, 305–313. [Google Scholar] [CrossRef]
- Van der Spiegel, M.; Noordam, M.; Fels-Klerx, H. Safety of novel protein sources (insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- Grobbelaar, J.U. Quality control and assurance: Crucial for the sustainability of the applied phycology industry. J. Appl. Phycol. 2003, 15, 209–215. [Google Scholar] [CrossRef]
- Görs, M.; Schumann, R.; Hepperle, D.; Karsten, U. Quality analysis of commercial Chlorella products used as dietary supplement in human nutrition. J. Appl. Phycol. 2010, 22, 265–276. [Google Scholar] [CrossRef]
- Harnedy, P.A.; FitzGerald, R.J. Extraction and Enrichment of Protein from red and green Macroalgae. Nat. Prod. Mar. Algae Methods Protoc. 2015, 1308, 103–108. [Google Scholar]
- Machmudah, S.; Shotipruk, A.; Goto, M.; Sasaki, M.; Hirose, T. Extraction of Astaxanthin from Haematococcus pluvialis Using Supercritical CO2 and Ethanol as Entrainer. Ind. Eng. Chem. Res. 2006, 45, 3652–3657. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and purification of high-value metabolites from microalgae: Essential lipids, astaxanthin and phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Marella, C.; Muthukumarappan, K.; Metzger, L. Application of membrane separation technology for developing novel dairy food ingredients. J. Food Process. Technol. 2013, 4. [Google Scholar] [CrossRef]
- Van Hal, J.W.; Huijgen, W.; López-Contreras, A. Opportunities and challenges for seaweed in the biobased economy. Trends Biotechnol. 2014, 32, 231–233. [Google Scholar] [CrossRef] [PubMed]


| Extraction Method | Species | Extraction Name | Reagents | Protein Yield | Reference |
|---|---|---|---|---|---|
| Enzymatic hydrolysis | Palmaria palmata | Polysaccharidase degradation | Cellulase (Cellucast®) and xylanase (Shearzyme®) | Factor 3.3 compared to control | [46] |
| Chondrus crispus, Gracilaria verrucosa, and Palmaria palmata | Polysaccharidase degradation | κ-carrageenase, β-agarase, xylanase, cellulase | - | [74] | |
| Palmaria palmata | Polysaccharidase degradation | Cellulase (Cellucast®), xylanase (Shearzyme®) and Ultraflo® (β-glucanase) | 11.57 ± 0.08 g/100 g dw (67% yield) | [73] | |
| Physical Process | Porphyra acanthophora var. acanthophora, Sargassum vulgare and Ulva fasciata | Aqueous treatment and Potter homogenisation | Ultra-pure water | 8.9 g/100 g dw, 6.9 g /100 g dw, 7.3 g /100 g dw | [68] |
| Palmaria palmata | Osmotic stress | - | 6.77 ± 0.22 g/100 g dw (39% yield) | [73] | |
| High shear force | - | 6.92 ± 0.12 g/100 g dw (40% yield) | |||
| Chemical extraction | Ascophyylum nodosum | Acid-alkaline treatment | 0.4 M HCl and 0.4 M NaOH | 59.76% yield | [69] |
| Ulva rigida | Two-phase system | NaOH and 2-mercaptoethanol | - | [70] | |
| Ulva rotunda | |||||
| Laminaria digitata | Two-phase system | Polyethylene glycol (PEG) and potassium carbonate | - | [71] | |
| Palmaria palmata | Alkaline and aqueous | NaOH and N-acetyl- l-cysteine (NAC) | 4.16 g/100 g dw (24% yield) | [73] |
| Source | Hydrolytic Method | Peptide Sequence | IC50 | Reference |
|---|---|---|---|---|
| Undaria pinnatifida (wakame) | Pepsin | Ala-Ile-Tyr-Lys | 213 μM | [201] |
| Tyr-Lys-Tyr-Tyr | 64.2 μM | |||
| Lys-Phe-Tyr-Gly | 90.5 μM | |||
| Tyr-Asn-Lys-Leu | 90.5 μM | |||
| Undaria pinnatifida (wakame) | Hot water extraction | Tyr-His | 5.1 μM | [202] |
| Lys-Trp | 10.8 μM | |||
| Lys-Tyr | 7.7 μM | |||
| Lys-Phe | 28.3 μM | |||
| Phe-Tyr | 3.7 μM | |||
| Val-Trp | 10.8 μM | |||
| Val-Phe | 43.7 μM | |||
| Ile-Tyr | 2.7 μM | |||
| Ile-Trp | 12.4 μM | |||
| Val-Tyr | 11.3 μM | |||
| Undaria pinnatifida (wakame) | Protease S “Amano” | Val-Tyr | 35.2 μM | [203] |
| Ile-Tyr | 6.1 μM | |||
| Ala-Trp | 18.8 μM | |||
| Phe-Tyr | 42.3 μM | |||
| Val-Trp | 3.3 μM | |||
| Ile-Trp | 1.5 μM | |||
| Leu-Trp | 23.6 μM | |||
| Ecklonia cava | Alcalase | Enzymatic digest | 2.79 μg/mL | [204] |
| Flavourzyme | Enzymatic digest | 3.56 μg/mL | ||
| Kojizyme | Enzymatic digest | 2.33 μg/mL | ||
| Neutrase | Enzymatic digest | 3.10 μg/mL | ||
| Protamex | Enzymatic digest | 3.28 μg/mL | ||
| Porphyra yezoensis | Ile-Tyr | 2.69 μM | [205] | |
| Met-Lys-Tyr | 7.26 μM | |||
| Ala-Lys-Tyr-Ser-Tyr | 1.52 μM | |||
| Leu-Arg-Tyr | 5.06 μM | |||
| Hizikia fusiformis | Gly-Lys-Tyr | 3.92 μM | [206] | |
| Ser-Val-Tyr | 8.12 μM | |||
| Ser-Lys-Thr-Tyr | 11.07 μM | |||
| Palmaria palmata (dulse) | Thermolysin | Val-Tyr-Arg-Thr | 0.14 μM | [207] |
| Leu-Asp-Tyr | 6.1 μM | |||
| Leu-Arg-Tyr | 0.044 μM | |||
| Phe-Glu-Gln-Trp-Ala-Ser | 2.8 μM | |||
| Chlorella vulgaris | Pepsin | Ile-Val-Val-Glu | 315.3 μM | [208] |
| Ala-Phe-Leu | 63.8 μM | |||
| Phe-Ala-Leu | 26.3 μM | |||
| Ala-Glu-Leu | 57.1 μM | |||
| Val-Val-Pro-Pro-Ala | 79.5 μM | |||
| Arthrospira platensis | Ile-Ala-Glu | 34.7 μM | ||
| Phe-Ala-Leu | 11.4 μM | |||
| Ala-Glu-Leu | 11.4 μM | |||
| Ile-Ala-Pro-Gly | 11.4 μM | |||
| Val-Ala-Phe | 35.8 μM | |||
| Nannochloropsis oculata | Alcalase | Leu-Val-Thr-Val-Met | 18.0 μM | [209] |
| Nannochloropsis oculata | Pepsin | Gly-Met-Asn-Asn-Leu-Thr-Pro | 123 μM | [210] |
| Leu-Glu-Gln | 173 μM | |||
| Chlorella ellipsoidea | Protamex, Kojizyme, Neutrase, Flavourzyme, Alcalase, trypsin, α-chymotrypsin, pepsin, and papain | Val-Glu-Gly-Tyr | 128.4 μM | [211] |
| Chlorella vulgaris | Flavourzyme, alcalase, papain, and pepsin | Val-Glu-Cys-Tyr-Gly-Pro-Asn-Arg-Pro-Gln-Phe | 29.6 μM | [212] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. https://doi.org/10.3390/foods6050033
Bleakley S, Hayes M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods. 2017; 6(5):33. https://doi.org/10.3390/foods6050033
Chicago/Turabian StyleBleakley, Stephen, and Maria Hayes. 2017. "Algal Proteins: Extraction, Application, and Challenges Concerning Production" Foods 6, no. 5: 33. https://doi.org/10.3390/foods6050033
APA StyleBleakley, S., & Hayes, M. (2017). Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods, 6(5), 33. https://doi.org/10.3390/foods6050033