Chemical and Biological Sensors for Food-Quality Monitoring and Smart Packaging
Abstract
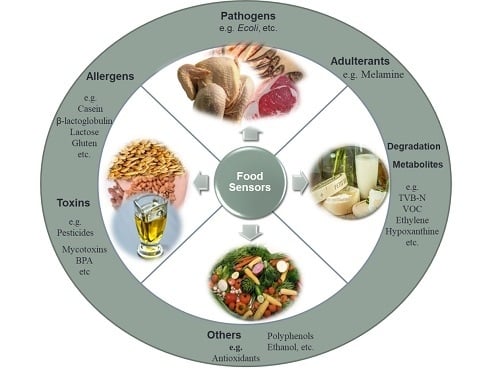
:1. Introduction
2. Current Status of Active and Functional Packaging
3. Food Freshness/Quality Monitoring
3.1. Fish, Meat, and Poultry
3.2. Cereal Grains
3.3. Fruits and Vegetables
4. Biosensors in Food Analysis
4.1. Biosensors for Food-Allergen Detection
4.2. Biosensors for Bacterial-Pathogen Detection
4.3. Biosensors for Food Adulteration, Authenticity, and Toxicicity Assessment
5. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- Neethirajan, S.; Jayas, D.S. Nanotechnology for the food and bioprocessing industries. Food Bioprocess Technol. 2011, 4, 39–47. [Google Scholar] [CrossRef]
- Kerry, J.; O’grady, M.; Hogan, S. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Karube, I.; Wilson, G.S. Biosensors: Fundamentals Applications; Oxford University Press: New York, NY, USA, 1987. [Google Scholar]
- Yang, C.; Wang, Y.; Marty, J.-L.; Yang, X. Aptamer-based colorimetric biosensing of Ochratoxin A using unmodified gold nanoparticles indicator. Biosens. Bioelectron. 2011, 26, 2724–2727. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Radecka, H.; Radecki, J. Electrochemical biosensors for food analysis. Monatsh. Chem. Chem. Mon. 2009, 140, 891. [Google Scholar] [CrossRef]
- Narsaiah, K.; Jha, S.N.; Bhardwaj, R.; Sharma, R.; Kumar, R. Optical biosensors for food quality and safety assurance—A review. J. Food Sci. Technol. 2012, 49, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, I.; Minunni, M.; Tombelli, S.; Mascini, M. Quartz crystal microbalance (QCM) affinity biosensor for genetically modified organisms (GMOs) detection. Biosens. Bioelectron. 2003, 18, 129–140. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical glucose biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef] [PubMed]
- Alves, R.C.; Barroso, M.F.; González-García, M.B.; Oliveira, M.B.P.; Delerue-Matos, C. New trends in food allergens detection: Toward biosensing strategies. Crit. Rev. Food Sci. Nutr. 2016, 56, 2304–2319. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.; Hassan, R.Y.; Andreescu, S. Multifunctional nanotechnology-enabled sensors for rapid capture and detection of pathogens. Sensors 2017, 17, 2121. [Google Scholar] [CrossRef] [PubMed]
- Kuswandi, B.; Wicaksono, Y.; Abdullah, A.; Heng, L.Y.; Ahmad, M. Smart packaging: Sensors for monitoring of food quality and safety. Sens. Instrum. Food Qual. Saf. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Applications of commercial biosensors in clinical, food, environmental, and biothreat/biowarfare analyses. Anal. Biochem. 2015, 478, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Aguilera, R.; Oliveira, J.C. Review of design engineering methods and applications of active and modified atmosphere packaging systems. Food Eng. Rev. 2009, 1, 66–83. [Google Scholar] [CrossRef]
- Otoni, C.G.; Espitia, P.J.; Avena-Bustillos, R.J.; McHugh, T.H. Trends in antimicrobial food packaging systems: Emitting sachets and absorbent pads. Food Res. Int. 2016, 83, 60–73. [Google Scholar] [CrossRef]
- Busolo, M.A.; Lagaron, J.M. Oxygen scavenging polyolefin nanocomposite films containing an iron modified kaolinite of interest in active food packaging applications. Innov. Food Sci. Emerg. Technol. 2012, 16, 211–217. [Google Scholar] [CrossRef]
- Tewari, G.; Jayas, D.S.; Jeremiah, L.E.; Holley, R.A. Absorption kinetics of oxygen scavengers. Int. J. Food Sci. Technol. 2002, 37, 209–217. [Google Scholar] [CrossRef]
- Terry, L.A.; Ilkenhans, T.; Poulston, S.; Rowsell, L.; Smith, A.W. Development of new palladium-promoted ethylene scavenger. Postharvest Biol. Technol. 2007, 45, 214–220. [Google Scholar] [CrossRef]
- Smith, A.W.; Poulston, S.; Rowsell, L.; Terry, L.A.; Anderson, J.A. A new palladium-based ethylene scavenger to control ethylene-induced ripening of climacteric fruit. Platin. Met. Rev. 2009, 53, 112–122. [Google Scholar] [CrossRef]
- Wills, R.; Warton, M. Efficacy of potassium permanganate impregnated into alumina beads to reduce atmospheric ethylene. J. Am. Soc. Hortic. Sci. 2004, 129, 433–438. [Google Scholar]
- Contreras, C.B.; Charles, G.; Toselli, R.; Strumia, M.C. Antimicrobial active packaging. Biopackaging 2017, 36–54. [Google Scholar]
- Hansen, A.; Mørkøre, T.; Rudi, K.; Olsen, E.; Eie, T. Quality changes during refrigerated storage of MA-packaged pre-rigor fillets of farmed Atlantic cod (Gadus morhua L.) using traditional MAP, CO2 emitter, and vacuum. J. Food Sci. 2007, 72, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Cagri, A.; Ustunol, Z.; Ryser, E.T. Antimicrobial edible films and coatings. J. Food Prot. 2004, 67, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; López-de-Dicastillo, C.; Hernández-Muñoz, P.; Catalá, R.; Gavara, R. Advances in antioxidant active food packaging. Trends Food Sci. Technol. 2014, 35, 42–51. [Google Scholar] [CrossRef]
- Yam, K.L.; Takhistov, P.T.; Miltz, J. Intelligent packaging: Concepts and applications. J. Food Sci. 2005, 70. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Yang, M.; Zhang, Y.; Xiang, K.; Tang, R. Review of time temperature indicators as quality monitors in food packaging. Packag. Technol. Sci. 2015, 28, 839–867. [Google Scholar] [CrossRef]
- 3M™ MonitorMark™ Time Temperature Indicators. Available online: https://www.3m.com/3M/en_US/company-us/all-3m-products/~/MONMARK-3M-MonitorMark-Time-Temperature-Indicators/?N=5002385+3293785721&rt=rud (accessed on 21 August 2018).
- Time Temperature Indicators. Available online: http://freshpoint-tti.com/time-temperature-indicators/ (accessed on 21 August 2018).
- Esser, B.; Schnorr, J.M.; Swager, T.M. Selective detection of ethylene gas using carbon nanotube-based devices: Utility in determination of fruit ripeness. Angew. Chem. Int. Ed. 2012, 51, 5752–5756. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.; Clarke-Hill, C.; Hillier, D.; Comfort, D. The benefits, challenges and impacts of radio frequency identification technology (RFID) for retailers in the UK. Mark. Intell. Plan. 2005, 23, 395–402. [Google Scholar] [CrossRef]
- Biosensors. Available online: http://www2.Flex-alert.Com/flexalert/applications/biosensors (accessed on 21 August 2018).
- How Ripe Do You Like It. Available online: http://www.ripesense.co.nz/ (accessed on 21 August 2018).
- Smolander, M. Freshness indicators for food packaging. In Smart Packaging Technologies for Fast Moving Consumer Goods; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 111–127. [Google Scholar]
- Kuswandi, B.; Restyana, A.; Abdullah, A.; Heng, L.Y.; Ahmad, M. A novel colorimetric food package label for fish spoilage based on polyaniline film. Food Control 2012, 25, 184–189. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Ashie, I.; Smith, J.; Simpson, B.; Haard, N.F. Spoilage and shelf-life extension of fresh fish and shellfish. Crit. Rev. Food Sci. Nutr. 1996, 36, 87–121. [Google Scholar] [CrossRef] [PubMed]
- Karube, I.; Matsuoka, H.; Suzuki, S.; Watanabe, E.; Toyama, K. Determination of fish freshness with an enzyme sensor system. J. Agric. Food Chem. 1984, 32, 314–319. [Google Scholar] [CrossRef]
- Cunningham, S.; Keaveny, T. A two-stage enzymatic method for determination of uric acid and hypoxanthine/xanthine. Clin. Chim. Acta 1978, 86, 217–221. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, Y.; Ma, X.; Guo, L.; Qiu, B.; Chen, G.; Lin, Z. Multicolor biosensor for fish freshness assessment with the naked eye. Sens. Actuators B Chem. 2017, 252, 201–208. [Google Scholar] [CrossRef]
- Berti, G.; Fossati, P.; Tarenghi, G.; Musitelli, C.; Melzi d’Eril, G. Enzymatic colorimetric method for the determination of inorganic phosphorus in serum and urine. Clin. Chem. Lab. Med. 1988, 26, 399–404. [Google Scholar] [CrossRef]
- Lawal, A.T.; Adeloju, S.B. Progress and recent advances in fabrication and utilization of hypoxanthine biosensors for meat and fish quality assessment: A review. Talanta 2012, 100, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Sun, J.; Chen, J.; Guo, L.; Chen, Y.; Chen, G. Electrochemiluminescent biosensor for hypoxanthine based on the electrically heated carbon paste electrode modified with xanthine oxidase. Anal. Chem. 2008, 80, 2826–2831. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, H.S.; dos Santos, L.V.; Pelegrine, C.P.; Gomes, S.; Matsushita, M.; de Souza, N.E.; Visentainer, J.V. Biosensor based on xanthine oxidase for monitoring hypoxanthine in fish meat. Am. J. Biochem. Biotechnol. 2005, 1, 85–89. [Google Scholar] [CrossRef]
- Agüí, L.; Manso, J.; Yáñez-Sedeño, P.; Pingarrón, J.M. Amperometric biosensor for hypoxanthine based on immobilized xanthine oxidase on nanocrystal gold-carbon paste electrodes. Sens. Actuators B Chem. 2006, 113, 272–280. [Google Scholar] [CrossRef]
- Yan, Z.; Niu, Q.; Mou, M.; Wu, Y.; Liu, X.; Liao, S. A novel colorimetric method based on copper nanoclusters with intrinsic peroxidase-like for detecting xanthine in serum samples. J. Nanopart. Res. 2017, 19, 235. [Google Scholar] [CrossRef]
- Draisci, R.; Volpe, G.; Lucentini, L.; Cecilia, A.; Federico, R.; Palleschi, G. Determination of biogenic amines with an electrochemical biosensor and its application to salted anchovies. Food Chem. 1998, 62, 225–232. [Google Scholar] [CrossRef]
- Carelli, D.; Centonze, D.; Palermo, C.; Quinto, M.; Rotunno, T. An interference free amperometric biosensor for the detection of biogenic amines in food products. Biosens. Bioelectron. 2007, 23, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-H.; Choi, K.-J.; Bae, J.-Y.; Yoon, S.-K.; Jang, H.-I.; Lee, C.-S. Development of a detection sensor for mixed trimethylamine and ammonia gas. J. Ind. Eng. Chem. 2013, 19, 1703–1707. [Google Scholar] [CrossRef]
- Schaude, C.; Meindl, C.; Fröhlich, E.; Attard, J.; Mohr, G.J. Developing a sensor layer for the optical detection of amines during food spoilage. Talanta 2017, 170, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, Z.; Zou, X.; Shi, J.; Mao, H.; Zhao, J.; Hao, L.; Holmes, M. Detection of meat-borne trimethylamine based on nanoporous colorimetric sensor arrays. Food Chem. 2016, 197, 930–936. [Google Scholar]
- Chang, L.-Y.; Chuang, M.-Y.; Zan, H.-W.; Meng, H.-F.; Lu, C.-J.; Yeh, P.-H.; Chen, J.-N. One-minute fish freshness evaluation by testing the volatile amine gas with an ultrasensitive porous-electrode-capped organic gas sensor system. ACS Sens. 2017, 2, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Pacquit, A.; Lau, K.T.; McLaughlin, H.; Frisby, J.; Quilty, B.; Diamond, D. Development of a volatile amine sensor for the monitoring of fish spoilage. Talanta 2006, 69, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Food Sniffer. Available online: http://www.myfoodsniffer.com/foodsniffer.html (accessed on 21 August 2018).
- Distell Fish Freshness Meter. Available online: https://www.distell.com/wp-content/uploads/2014/04/Freshness-Meter-User-Manual-v2.9.pdf (accessed on 21 August 2018).
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakul, P. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Maier, D.; Channaiah, L.; Martinez-Kawas, A.; Lawrence, J.; Chaves, E.; Coradi, P.; Fromme, G. Monitoring carbon dioxide concentration for early detection of spoilage in stored grain. Julius-Kühn-Archiv 2010, 425, 505. [Google Scholar]
- Vidal, J.C.; Bonel, L.; Ezquerra, A.; Hernández, S.; Bertolín, J.R.; Cubel, C.; Castillo, J.R. Electrochemical affinity biosensors for detection of mycotoxins: A review. Biosens. Bioelectron. 2013, 49, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Jun, D.; Kuca, K. Mycotoxin assays using biosensor technology: A review. Drug Chem. Toxicol. 2007, 30, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Karunakaran, C.; Jayas, D.; White, N. Detection techniques for stored-product insects in grain. Food Control 2007, 18, 157–162. [Google Scholar] [CrossRef]
- Neethirajan, S.; Jayas, D.; Sadistap, S. Carbon dioxide (CO2) sensors for the agri-food industry—A review. Food Bioprocess Technol. 2009, 2, 115–121. [Google Scholar] [CrossRef]
- Puligundla, P.; Jung, J.; Ko, S. Carbon dioxide sensors for intelligent food packaging applications. Food Control 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Neethirajan, S.; Freund, M.; Jayas, D.; Shafai, C.; Thomson, D.; White, N. Development of carbon dioxide (CO2) sensor for grain quality monitoring. Biosyst. Eng. 2010, 106, 395–404. [Google Scholar] [CrossRef]
- Malvano, F.; Albanese, D.; Pilloton, R.; Di Matteo, M. A new label-free impedimetric aptasensor for gluten detection. Food Control 2017, 79, 200–206. [Google Scholar] [CrossRef]
- Nassef, H.M.; Bermudo Redondo, M.C.; Ciclitira, P.J.; Ellis, H.J.; Fragoso, A.; O’Sullivan, C.K. Electrochemical immunosensor for detection of celiac disease toxic gliadin in foodstuff. Anal. Chem. 2008, 80, 9265–9271. [Google Scholar] [CrossRef] [PubMed]
- Test Your Food Gluten: Anytime, Anywhere. Available online: https://nimasensor.com/gluten/ (accessed on 21 August 2018).
- White, S.P.; Frisbie, C.D.; Dorfman, K.D. Detection and sourcing of gluten in grain with multiple floating-gate transistor biosensors. ACS Sens. 2018, 3, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Van der Gaag, B.; Spath, S.; Dietrich, H.; Stigter, E.; Boonzaaijer, G.; van Osenbruggen, T.; Koopal, K. Biosensors and multiple mycotoxin analysis. Food Control 2003, 14, 251–254. [Google Scholar] [CrossRef]
- Chen, J.; Fang, Z.; Liu, J.; Zeng, L. A simple and rapid biosensor for ochratoxin a based on a structure-switching signaling aptamer. Food Control 2012, 25, 555–560. [Google Scholar] [CrossRef]
- Bulbul, G.; Hayat, A.; Andreescu, S. A generic amplification strategy for electrochemical aptasensors using a non-enzymatic nanoceria tag. Nanoscale 2015, 7, 13230–13238. [Google Scholar] [CrossRef] [PubMed]
- Bonel, L.; Vidal, J.C.; Duato, P.; Castillo, J.R. An electrochemical competitive biosensor for ochratoxin a based on a DNA biotinylated aptamer. Biosens. Bioelectron. 2011, 26, 3254–3259. [Google Scholar] [CrossRef] [PubMed]
- Bülbül, G.; Hayat, A.; Andreescu, S. Ssdna-functionalized nanoceria: A redox-active aptaswitch for biomolecular recognition. Adv. Healthc. Mater. 2016, 5, 822–828. [Google Scholar] [CrossRef] [PubMed]
- FAO. The Future of Food and Agriculture—Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Gunders, D. Wasted: How America is Losing up to 40 Percent of its Food from Farm to Fork to Landfill; Natural Resources Defense Council: New York, NY, USA, 2012. [Google Scholar]
- Buzby, J.C.; Wells, H.F.; Axtman, B.; Mickey, J. Supermarket loss estimates for fresh fruit, vegetables, meat, poultry, and seafood and their use in the ERS loss-adjusted food availability data. Econ. Inf. Bull.-USDA Econ. Res. Serv. 2009, 44, 26. [Google Scholar]
- Adams, D.; Yang, S. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc. Natl. Acad. Sci. USA 1979, 76, 170–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Hara, K.; Fukuoka, A. Low-temperature oxidation of ethylene over platinum nanoparticles supported on mesoporous silica. Angew. Chem. Int. Ed. 2013, 52, 6265–6268. [Google Scholar] [CrossRef] [PubMed]
- Vermeiren, L.; Devlieghere, F.; van Beest, M.; de Kruijf, N.; Debevere, J. Developments in the active packaging of foods. Trends Food Sci. Technol. 1999, 10, 77–86. [Google Scholar] [CrossRef]
- Weber, W.; Luzi, S.; Karlsson, M.; Fussenegger, M. A novel hybrid dual-channel catalytic-biological sensor system for assessment of fruit quality. J. Biotechnol. 2009, 139, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, P.M. Non-ethylene, non-respiratory volatiles in harvested fruits and vegetables: Their occurrence, biological activity and control. Postharvest Biol. Technol. 1997, 12, 109–125. [Google Scholar] [CrossRef]
- Kuswandi, B.; Maryska, C.; Abdullah, A.; Heng, L.Y. Real time on-package freshness indicator for guavas packaging. J. Food Meas. Charact. 2013, 7, 29–39. [Google Scholar] [CrossRef]
- IUPAC. Compendium of Chemical Terminology, 2nd ed.; IUPAC: Zurich, Switzerland, 1997. [Google Scholar]
- Silva, N.F.; Magalhães, J.M.; Freire, C.; Delerue-Matos, C. Electrochemical biosensors for salmonella: State of the art and challenges in food safety assessment. Biosens. Bioelectron. 2018, 99, 667–682. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Weng, X.; Tah, A.; Cordero, J.; Ragavan, K. Nano-biosensor platforms for detecting food allergens-new trends. Sens. Bio-Sens. Res. 2018, 18, 13–30. [Google Scholar] [CrossRef]
- Nikoleli, G.-P.; Nikolelis, D.P.; Siontorou, C.G.; Karapetis, S.; Varzakas, T. Novel biosensors for the rapid detection of toxicants in foods. Adv. Food Nutr. Res. 2018, 84, 57–102. [Google Scholar] [PubMed]
- Prescott, S.L.; Pawankar, R.; Allen, K.J.; Campbell, D.E.; Sinn, J.K.; Fiocchi, A.; Ebisawa, M.; Sampson, H.A.; Beyer, K.; Lee, B.-W. A global survey of changing patterns of food allergy burden in children. World Allergy Organ. J. 2013, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Yman, I.M.; Eriksson, A.; Johansson, M.A.; Hellens, K.-E. Food allergen detection with biosensor immunoassays. J. AOAC Int. 2006, 89, 856–861. [Google Scholar] [PubMed]
- Test Your Food for Peanuts: anytime, Anywhere. Available online: https://nimasensor.Com/peanut/ (accessed on 21 August 2018).
- Pollet, J.; Delport, F.; Janssen, K.; Tran, D.; Wouters, J.; Verbiest, T.; Lammertyn, J. Fast and accurate peanut allergen detection with nanobead enhanced optical fiber SPR biosensor. Talanta 2011, 83, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Han, J.; Wu, Y.; Yuan, F.; Chen, Y.; Ge, Y. Simultaneous detection of eight food allergens using optical thin-film biosensor chips. J. Agric. Food Chem. 2011, 59, 6889–6894. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.M.; Hill, I.D. Celiac disease and autoimmunity: Review and controversies. Curr. Allergy Asthma Rep. 2013, 13, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Tlili, C.; L’Hocine, L.; Zourob, M. Electrochemical immunosensor for the milk allergen β-lactoglobulin based on electrografting of organic film on graphene modified screen-printed carbon electrodes. Biosens. Bioelectron. 2012, 38, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Zourob, M. In vitro selection of DNA aptamers targeting β-lactoglobulin and their integration in graphene-based biosensor for the detection of milk allergen. Biosens. Bioelectron. 2017, 91, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Hiep, H.M.; Endo, T.; Kerman, K.; Chikae, M.; Kim, D.-K.; Yamamura, S.; Takamura, Y.; Tamiya, E. A localized surface plasmon resonance based immunosensor for the detection of casein in milk. Sci. Technol. Adv. Mater. 2007, 8, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Swagerty, D.L.; Walling, A.D.; Klein, R.M. Lactose intolerance. Am. Fam. Physician 2002, 65, 1845–1860. [Google Scholar] [PubMed]
- Paige, D.M.; Bayless, T.M.; Huang, S.-S.; Wexler, R. Lactose intolerance and lactose hydrolyzed milk. ACS Symp. Ser. Am. Chem. Soc. 1975, 15, 191–206. [Google Scholar]
- Ammam, M.; Fransaer, J. Two-enzyme lactose biosensor based on β-galactosidase and glucose oxidase deposited by ac-electrophoresis: Characteristics and performance for lactose determination in milk. Sens. Actuators B Chem. 2010, 148, 583–589. [Google Scholar] [CrossRef]
- Watanabe, E.; Takagi, M.; Takei, S.; Hoshi, M.; Cao, S. Development of biosensors for the simultaneous determination of sucrose and glucose, lactose and glucose, and starch and glucose. Biotechnol. Bioeng. 1991, 38, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.L.; López-Munguia, A.; Galindo, E. Modeling the non-steady-state response of an enzyme electrode for lactose. Enzym. Microb. Technol. 1991, 13, 672–675. [Google Scholar] [CrossRef]
- Marrakchi, M.; Dzyadevych, S.V.; Lagarde, F.; Martelet, C.; Jaffrezic-Renault, N. Conductometric biosensor based on glucose oxidase and beta-galactosidase for specific lactose determination in milk. Mater. Sci. Eng. C 2008, 28, 872–875. [Google Scholar] [CrossRef]
- Lactose Biosensor Test System. Available online: http://www.directsens.com/lactosens/ (accessed on 21 August 2018).
- Centers for Disease Control and Prevention (CDC). Foodborne Illness: Frequently Asked Questions; CDC: Atlanta, GA, USA, 2018. [Google Scholar]
- WHO. Global Burden of Foodborne Diseases; WHO Press: Geneva, Switzerland, 2015. [Google Scholar]
- Centers for Disease Control and Prevention. Surveillance for foodborne disease outbreaks-united states, 2009–2010. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 41. [Google Scholar]
- De Boer, E.; Beumer, R.R. Methodology for detection and typing of foodborne microorganisms. Int. J. Food Microbiol. 1999, 50, 119–130. [Google Scholar] [CrossRef]
- Beumer, R.R.; Brinkman, E. Detection of Listeria spp. With a monoclonal antibody-based enzyme-linked immunosorbent assay (ELISA). Food Microbiol. 1989, 6, 171–177. [Google Scholar] [CrossRef]
- Palumbo, J.D.; Borucki, M.K.; Mandrell, R.E.; Gorski, L. Serotyping of listeria monocytogenes by enzyme-linked immunosorbent assay and identification of mixed-serotype cultures by colony immunoblotting. J. Clin. Microbiol. 2003, 41, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.Y.; Caswell, J.L.; Prescott, J.F. Nonculture molecular techniques for diagnosis of bacterial disease in animals: A diagnostic laboratory perspective. Vet. Pathol. Online 2014, 51, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Marrakchi, M.; Xu, D.; Dong, H.; Andreescu, S. Biosensors based on modularly designed synthetic peptides for recognition, detection and live/dead differentiation of pathogenic bacteria. Biosens. Bioelectron. 2016, 80, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.; Scott, M.G.; Karunaratne, N.; Yan, H.; Hancock, R.E.W. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob. Agents Chemother. 1999, 43, 1542–1548. [Google Scholar] [CrossRef] [PubMed]
- Rydlo, T.; Rotem, S.; Mor, A. Antibacterial properties of dermaseptin S4 derivatives under extreme incubation conditions. Antimicrob. Agents Chemother. 2006, 50, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Alhogail, S.; Suaifan, G.A.; Zourob, M. Rapid colorimetric sensing platform for the detection of Listeria monocytogenes foodborne pathogen. Biosens. Bioelectron. 2016, 86, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Ali, M.M.; Su, H.-M.; Filipe, C.D.; Didar, T.F. Sentinel wraps: Real-time monitoring of food contamination by printing dnazyme probes on food packaging. ACS Nano 2018, 12, 3287–3294. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.V. Applications of nanotechnology in food packaging and food safety: Barrier materials, antimicrobials and sensors. J. Colloid Interface Sci. 2011, 363, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-F.; Rhim, J.-W. Preparation and application of agar/alginate/collagen ternary blend functional food packaging films. Int. J. Biol. Macromol. 2015, 80, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Gallocchio, F.; Cibin, V.; Biancotto, G.; Roccato, A.; Muzzolon, O.; Carmen, L.; Simone, B.; Manodori, L.; Fabrizi, A.; Patuzzi, I. Testing nano-silver food packaging to evaluate silver migration and food spoilage bacteria on chicken meat. Food Addit. Contam. Part A 2016, 33, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- De Moura, M.R.; Mattoso, L.H.; Zucolotto, V. Development of cellulose-based bactericidal nanocomposites containing silver nanoparticles and their use as active food packaging. J. Food Eng. 2012, 109, 520–524. [Google Scholar] [CrossRef]
- Tankhiwale, R.; Bajpai, S. Graft copolymerization onto cellulose-based filter paper and its further development as silver nanoparticles loaded antibacterial food-packaging material. Colloids Surf. B Biointerfaces 2009, 69, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Kuorwel, K.K.; Cran, M.J.; Orbell, J.D.; Buddhadasa, S.; Bigger, S.W. Review of mechanical properties, migration, and potential applications in active food packaging systems containing nanoclays and nanosilver. Compr. Rev. Food Sci. Food Saf. 2015, 14, 411–430. [Google Scholar] [CrossRef]
- Kapetanakou, A.; Agathaggelou, E.; Skandamis, P. Storage of pork meat under modified atmospheres containing vapors from commercial alcoholic beverages. Int. J. Food Microbiol. 2014, 178, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Seydim, A.; Sarikus, G. Antimicrobial activity of whey protein based edible films incorporated with oregano, rosemary and garlic essential oils. Food Res. Int. 2006, 39, 639–644. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Y.; Jiang, X.; Sun, B.; Zhu, Y.; Wang, H.; Su, Y.; He, Y. Simultaneous capture, cetection, and inactivation of bacteria as enabled by a surface-enhanced raman scattering multifunctional chip. Angew. Chem. Int. Ed. 2015, 54, 5132–5136. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Zhang, D. A novel multifunctional electrochemical platform for simultaneous detection, elimination, and inactivation of pathogenic bacteria based on the Vancomycin-functionalised AgNPs/3d-ZnO nanorod arrays. Biosens. Bioelectron. 2017, 98, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Grundy, H.; Kelly, S.; Charlton, A.; Donarski, J.; Hird, S.; Collins, M. Food authenticity and food fraud research: Achievements and emerging issues. J. Assoc. Public Analy. (Online) 2012, 40, 65–68. [Google Scholar]
- Poiana, M.-A.; Alexa, E.; Munteanu, M.-F.; Gligor, R.; Moigradean, D.; Mateescu, C. Use of ATR-FTIR spectroscopy to detect the changes in extra virgin olive oil by adulteration with soybean oil and high temperature heat treatment. Open Chem. 2015, 13, 1. [Google Scholar] [CrossRef]
- Gossner, C.M.-E.; Schlundt, J.; Embarek, P.B.; Hird, S.; Lo-Fo-Wong, D.; Beltran, J.J.O.; Teoh, K.N.; Tritscher, A. The melamine incident: Implications for international food and feed safety. Environ. Health Perspect. 2009, 117, 1803. [Google Scholar] [CrossRef] [PubMed]
- Fodey, T.L.; Thompson, C.S.; Traynor, I.M.; Haughey, S.A.; Kennedy, D.G.; Crooks, S.R. Development of an optical biosensor based immunoassay to screen infant formula milk samples for adulteration with melamine. Anal. Chem. 2011, 83, 5012–5016. [Google Scholar] [CrossRef] [PubMed]
- Ping, H.; Zhang, M.; Li, H.; Li, S.; Chen, Q.; Sun, C.; Zhang, T. Visual detection of melamine in raw milk by label-free silver nanoparticles. Food Control 2012, 23, 191–197. [Google Scholar] [CrossRef]
- Ni, P.; Dai, H.; Wang, Y.; Sun, Y.; Shi, Y.; Hu, J.; Li, Z. Visual detection of melamine based on the peroxidase-like activity enhancement of bare gold nanoparticles. Biosens. Bioelectron. 2014, 60, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Boujtita, M.; Hart, J.P.; Pittson, R. Development of a disposable ethanol biosensor based on a chemically modified screen-printed electrode coated with alcohol oxidase for the analysis of beer. Biosens. Bioelectron. 2000, 15, 257–263. [Google Scholar] [CrossRef]
- Santos, A.S.; Pereira, A.C.; Durán, N.; Kubota, L.T. Amperometric biosensor for ethanol based on co-immobilization of alcohol dehydrogenase and meldola’s blue on multi-wall carbon nanotube. Electrochim. Acta 2006, 52, 215–220. [Google Scholar] [CrossRef]
- Palmisano, F.; Rizzi, R.; Centonze, D.; Zambonin, P. Simultaneous monitoring of glucose and lactate by an interference and cross-talk free dual electrode amperometric biosensor based on electropolymerized thin films. Biosens. Bioelectron. 2000, 15, 531–539. [Google Scholar] [CrossRef]
- Antiochia, R.; Gorton, L. Development of a carbon nanotube paste electrode osmium polymer-mediated biosensor for determination of glucose in alcoholic beverages. Biosens. Bioelectron. 2007, 22, 2611–2617. [Google Scholar] [CrossRef] [PubMed]
- Mello, L.D.; Sotomayor, M.D.P.T.; Kubota, L.T. HRP-based amperometric biosensor for the polyphenols determination in vegetables extract. Sens. Actuators B Chem. 2003, 96, 636–645. [Google Scholar] [CrossRef]
- Apetrei, C.; Rodriguez-Mendez, M.; De Saja, J. Modified carbon paste electrodes for discrimination of vegetable oils. Sens. Actuators B Chem. 2005, 111, 403–409. [Google Scholar] [CrossRef]
- Della Pelle, F.; Compagnone, D. Nanomaterial-based sensing and biosensing of phenolic compounds and related antioxidant capacity in food. Sensors 2018, 18, 462. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.; Frasco, T.; Andreescu, D.; Andreescu, S. Portable ceria nanoparticle-based assay for rapid detection of food antioxidants (NanoCerac). Analyst 2013, 138, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Teerasong, S.; Jinnarak, A.; Chaneam, S.; Wilairat, P.; Nacapricha, D. Poly (vinyl alcohol) capped silver nanoparticles for antioxidant assay based on seed-mediated nanoparticle growth. Talanta 2017, 170, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Jinap, S.; Hajeb, P. Glutamate. Its applications in food and contribution to health. Appetite 2010, 55, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1988, 1, 623–634. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. Amperometric biosensor for glutamate using prussian blue-based “artificial peroxidase” as a transducer for hydrogen peroxide. Anal. Chem. 2000, 72, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Arnold, M.A. Selectivity enhancement for glutamate with a Nafion/glutamate oxidase biosensor. Talanta 1996, 43, 1157–1162. [Google Scholar] [CrossRef]
- Skládal, P.; Nunes, G.S.; Yamanaka, H.; Ribeiro, M.L. Detection of carbamate pesticides in vegetable samples using cholinesterase-based biosensors. Electroanalysis 1997, 9, 1083–1087. [Google Scholar] [CrossRef]
- Cesarino, I.; Moraes, F.C.; Lanza, M.R.; Machado, S.A. Electrochemical detection of carbamate pesticides in fruit and vegetables with a biosensor based on acetylcholinesterase immobilised on a composite of polyaniline-carbon nanotubes. Food Chem. 2012, 135, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, A.; Bragos, R.; Lacorte, S.; Marty, J. Performance of a portable biosensor for the analysis of organophosphorus and carbamate insecticides in water and food. Sens. Actuators B Chem. 2008, 133, 195–201. [Google Scholar] [CrossRef]
- Del Carlo, M.; Mascini, M.; Pepe, A.; Diletti, G.; Compagnone, D. Screening of food samples for carbamate and organophosphate pesticides using an electrochemical bioassay. Food Chem. 2004, 84, 651–656. [Google Scholar] [CrossRef]
- Andreescu, S.; Barthelmebs, L.; Marty, J.-L. Immobilization of acetylcholinesterase on screen-printed electrodes: Comparative study between three immobilization methods and applications to the detection of organophosphorus insecticides. Anal. Chim. Acta 2002, 464, 171–180. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.Y.; Min, K.; Cha, H.J.; Choi, S.S.; Yoo, Y.J. A novel organophosphorus hydrolase-based biosensor using mesoporous carbons and carbon black for the detection of organophosphate nerve agents. Biosens. Bioelectron. 2010, 25, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.S.; Kwack, S.J.; Kim, K.-B.; Kim, H.S.; Lee, B.M. Potential risk of bisphenol a migration from polycarbonate containers after heating, boiling, and microwaving. J. Toxicol. Environ. Health Part A 2009, 72, 1285–1291. [Google Scholar] [CrossRef] [PubMed]
- Goodson, A.; Robin, H.; Summerfield, W.; Cooper, I. Migration of bisphenol a from can coatings—Effects of damage, storage conditions and heating. Food Addit. Contam. 2004, 21, 1015–1026. [Google Scholar] [CrossRef] [PubMed]
- Erler, C.; Novak, J. Bisphenol A exposure: Human risk and health policy. J. Pediatr. Nurs. 2010, 25, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Indirect Food Additives: Polymers. Available online: https://www.federalregister.gov/documents/2012/07/17/2012-17366/indirect-food-additives-polymers (accessed on 24 August 2018).
- Indirect Food Additives: Adhesives and Components of Coatings. Available online: https://www.federalregister.gov/documents/2013/07/12/2013-16684/indirect-food-additives-adhesives-and-components-of-coatings (accessed on 24 August 2018).
- Alkasir, R.S.; Ornatska, M.; Andreescu, S. Colorimetric paper bioassay for the detection of phenolic compounds. Anal. Chem. 2012, 84, 9729–9737. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Xu, Z.; Kuang, Y. Electrochemical determination of bisphenol a in plastic bottled drinking water and canned beverages using a molecularly imprinted chitosan-graphene composite film modified electrode. Food Chem. 2014, 157, 490–497. [Google Scholar] [CrossRef] [PubMed]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, F.; Andreescu, S. Chemical and Biological Sensors for Food-Quality Monitoring and Smart Packaging. Foods 2018, 7, 168. https://doi.org/10.3390/foods7100168
Mustafa F, Andreescu S. Chemical and Biological Sensors for Food-Quality Monitoring and Smart Packaging. Foods. 2018; 7(10):168. https://doi.org/10.3390/foods7100168
Chicago/Turabian StyleMustafa, Fatima, and Silvana Andreescu. 2018. "Chemical and Biological Sensors for Food-Quality Monitoring and Smart Packaging" Foods 7, no. 10: 168. https://doi.org/10.3390/foods7100168
APA StyleMustafa, F., & Andreescu, S. (2018). Chemical and Biological Sensors for Food-Quality Monitoring and Smart Packaging. Foods, 7(10), 168. https://doi.org/10.3390/foods7100168