Characterization of Maltase and Sucrase Inhibitory Constituents from Rhodiola crenulata
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of B2-3’-O-gallate, ECG, and EC from R. crenulata
2.2. Maltase Activity Assay
2.3. Maltase Inhibitory Activity of B2-3’-O-gallate, ECG, EC, and Quercetin
2.4. Sucrase Activity Assay
2.5. Sucrase Inhibitory Activity of B2-3’-O-gallate, ECG, EC, and Quercetin
2.6. Lineweaver–Burk Plots and Dixon Plots
2.7. Statistical Analysis
3. Results and Discussion
3.1. Maltase and Sucrase Inhibitory Activity of B2-3’-O-gallate, ECG, EC, and Quercetin
3.2. Mode of Inhibition of B2-3’-O-gallate, ECG, EC, and Quercetin Toward Maltase
3.3. Mode of Inhibition of B2-3’-O-gallate, ECG, EC, and Quercetin Toward Sucrase
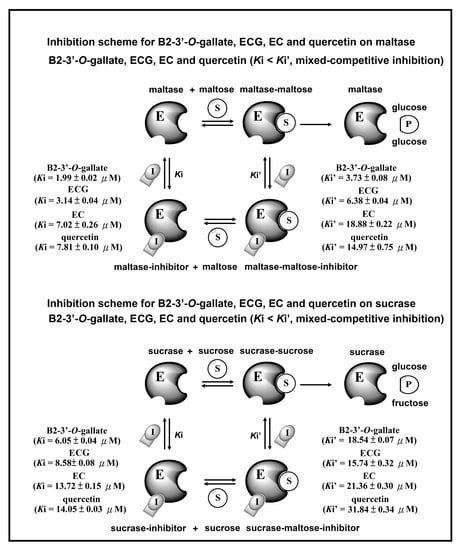
3.4. Inhibition Scheme of B2-3’-O-gallate, ECG, EC, and Quercetin for Maltase and Sucrase
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sancho, R.A.S.; Pastore, G.M. Evaluation of the effects of anthocyanins in type 2 diabetes. Food Res. Int. 2012, 46, 378–386. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Zanoveli, J.M.; Morais, H.D.; Dias, I.C.; Schreiber, A.K.; Souza, C.P.; Cunha, J.M. Depression associated with diabetes: From pathophysiology to treatment. Curr. Diabetes Rev. 2016, 12, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Girish, T.K.; Pratape, V.M.; Prasada Rao, U.J.S. Nutrient distribution, phenolic acid composition, antioxidant and α-glucosidase inhibitory potentials of black gram (Vigna mungo L.) and its milled by-products. Food Res. Int. 2012, 46, 370–377. [Google Scholar] [CrossRef]
- Rose, D.R.; Chaudet, M.M.; Jones, K. Structural studies of the intestinal α-glucosidases, maltase-glucoamylase and sucrase-isomaltase. J. Pediatr. Gastroenterol. Nutr. 2018, 66, S11–S13. [Google Scholar] [CrossRef]
- Peytam, F.; Adib, M.; Shourgeshty, R.; Mohammadi-Khanaposhtani, M.; Jahani, M.; Imanparast, S.; Faramarzi, M.A.; Mahdavi, M.; Moghadamnia, A.A.; Rastegar, H.; et al. Design and synthesis of new imidazo[1,2-b]pyrazole derivatives, in vitro α-glucosidase inhibition, kinetic and docking studies. Mol. Divers. 2019. [Google Scholar] [CrossRef]
- Silva, C.P.D.; Soares-Freitas, R.A.M.; Sampaio, G.R.; Santos, M.C.B.; do Nascimento, T.P.; Cameron, L.C.; Ferreira, M.S.L.; Arêas, J.A.G. Identification and action of phenolic compounds of Jatobá-do-cerrado (Hymenaea stignocarpa Mart.) on α-amylase and α-glucosidase activities and flour effect on glycemic response and nutritional quality of breads. Food Res. Int. 2019, 116, 1076–1083. [Google Scholar] [CrossRef]
- Simsek, M.; Quezada-Calvillo, R.; Nichols, B.L.; Hamaker, B.R. Phenolic compounds increase the transcription of mouse intestinal maltase-glucoamylase and sucrase-isomaltase. Food Funct. 2017, 8, 1915–1924. [Google Scholar] [CrossRef]
- Toda, M.; Kawabata, J.; Kasai, T. α-Glucosidase inhibitors from clove (Syzgium aromaticum). Biosci. Biotechnol. Biochem. 2000, 64, 294–298. [Google Scholar] [CrossRef]
- Kawakami, K.; Li, P.; Uraji, M.; Hatanaka, T.; Ito, H. Inhibitory effects of pomegranate extracts on recombinant human maltase-glucoamylase. J. Food Sci. 2014, 79, H1848–H1853. [Google Scholar] [CrossRef]
- Lee, B.H.; Hamaker, B.R. Maltase has most versatile α-hydrolytic activity among the mucosal α-glucosidases of the small intestine. J. Pediatr. Gastroenterol. Nutr. 2018, 66, S7–S10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.G.; Shentu, X.P.; Shen, Y.C. Inhibition of porcine small intestinal sucrose by valienamine. J. Enzyme Inhib. Med. Chem. 2005, 20, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Nijpels, G.; Boorsma, W.; Dekker, J.M.; Kostense, P.J.; Bouter, L.M.; Heine, R.J. A study of the effects of acarbose on glucose metabolism in patients predisposed to developing diabetes: The Dutch acarbose intervention study in persons with impaired glucose tolerance (DAISI). Diabetes Metab. Res. Rev. 2008, 24, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.I.; Jang, H.D.; Shetty, K. Evaluation of Rhodiola crenulata and Rhodiola rosea for management of type II diabetes and hypertension. Asia Pac. J. Clin. Nutr. 2006, 15, 425–432. [Google Scholar] [PubMed]
- Chu, Y.H.; Wu, S.H.; Hsieh, J.F. Isolation and characterization of α-glucosidase inhibitory constituents from Rhodiola crenulata. Food Res. Int. 2014, 57, 8–14. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Chantarasinlapin, P.; Thammarat, H.; Yibchok-Anun, S. A series of cinnamic acid derivatives and their inhibitory activity on intestinal α-glucosidase. J. Enzyme Inhib. Med. Chem. 2009, 24, 1194–2000. [Google Scholar] [CrossRef] [PubMed]
- Akkarachiyasit, S.; Charoenlertkul, P.; Yibchok-anun, S.; Adisakwattana, S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010, 11, 3387–3396. [Google Scholar] [CrossRef]
- Cornish-Bowden, A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem. J. 1974, 137, 143–144. [Google Scholar] [CrossRef]
- Simsek, M.; Quezada-Calvillo, R.; Ferruzzi, M.G.; Nichols, B.L.; Hamaker, B.R. Dietary phenolic compounds selectively inhibit the individual subunits of maltase-glucoamylase and sucrase-isomaltase with the potential of modulating glucose release. J. Agric. Food Chem. 2015, 63, 3873–3879. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, M.J.; Choi, H.N.; Jeong, S.M.; Lee, Y.M.; Kim, J.I. Quercetin attenulates fasting and postprandial hyperglycemia in animal models of diavetes mellitus. Nutr. Res. Pract. 2011, 5, 107–111. [Google Scholar] [CrossRef]
- Wang, H.; Du, Y.J.; Song, H.C. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010, 123, 6–13. [Google Scholar] [CrossRef]
- Pyner, A.; Nyambe-Silavwe, H.; Williamson, G. Inhibition of human and rat sucrase and maltase activities to assess antiglycemic potential: Optimization of the assay using acarbose and polyphenols. J. Agric. Food Chem. 2017, 65, 8643–8651. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Murata, Y.; Inui, H.; Sugiura, M.; Nakano, Y. Triterpene glycosides of Siraitia grosvenori inhibit rat intestinal maltase and suppress the rise in blood glucose level after a single oral administration of maltose in rats. J. Agric. Food Chem. 2005, 53, 2941–2946. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Nguyen, T.H.; Kurihara, H.; Kim, S.M. α-Glucosidase inhibitory activity of bromophenol purified from the red alga Polyopes lancifolia. J. Food Sci. 2010, 75, H145–H150. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Jung, S.H.; Lee, S.; Ryu, H.J.; Kang, H.K.; Moon, Y.H.; Kim, Y.M.; Kimura, A.; Kim, D. Inhibitory effects of epigallocatechin gallate and its glucoside on the human intestinal maltase inhibition. Biotechnol. Bioprocess Eng. 2012, 17, 966–971. [Google Scholar] [CrossRef]
- Cortés, A.; Cascante, M.; Cárdenas, M.L.; Cornish-Bowden, A. Relationships between inhibition constants, inhibitor concentrations for 50% inhibition and types of inhibition: New ways of analysing data. Biochem. J. 2001, 357, 263–268. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Yibchok-anun, S.; Charoenlertkul, P.; Wongsasiripat, N. Cyanidin-3-rutinoside alleviates postprandial hyperglycemia and its synergism with acarbose by inhibition of intestinal α-glucosidase. J. Clin. Biochem. Nutr. 2011, 49, 36–41. [Google Scholar] [CrossRef]
- Abdelhady, M.I.; Shaheen, U.; Bader, A.; Youns, M.A. A new sucrase enzyme inhibitor from Azadirachta indica. Pharmacogn. Mag. 2016, 12, S293–S296. [Google Scholar] [CrossRef]







| Inhibitor | Structure | Molecular Weight | Molecular Formula | IC50 (μM) a | |
|---|---|---|---|---|---|
| Maltase | Sucrase | ||||
| Epicatechin-(4β,8)-epicatechingallate (B2-3’-O-gallate) |  | 731.16 | C37H30O16 | 1.73 ± 1.37 | 6.91 ± 3.41 |
| Epicatechin gallate (ECG) |  | 442.37 | C22H18O10 | 3.64 ± 2.99 | 18.27 ± 3.99 |
| Epicatechin (EC) |  | 290.27 | C15H14O6 | 6.25 ± 1.84 | 18.91 ± 3.66 |
| Quercetin |  | 302.24 | C15H10O7 | 8.33 ± 3.91 | 18.98 ± 2.53 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-T.; Chuang, Y.-H.; Hsieh, J.-F. Characterization of Maltase and Sucrase Inhibitory Constituents from Rhodiola crenulata. Foods 2019, 8, 540. https://doi.org/10.3390/foods8110540
Li W-T, Chuang Y-H, Hsieh J-F. Characterization of Maltase and Sucrase Inhibitory Constituents from Rhodiola crenulata. Foods. 2019; 8(11):540. https://doi.org/10.3390/foods8110540
Chicago/Turabian StyleLi, Wen-Tai, Yu-Hsuan Chuang, and Jung-Feng Hsieh. 2019. "Characterization of Maltase and Sucrase Inhibitory Constituents from Rhodiola crenulata" Foods 8, no. 11: 540. https://doi.org/10.3390/foods8110540
APA StyleLi, W. -T., Chuang, Y. -H., & Hsieh, J. -F. (2019). Characterization of Maltase and Sucrase Inhibitory Constituents from Rhodiola crenulata. Foods, 8(11), 540. https://doi.org/10.3390/foods8110540