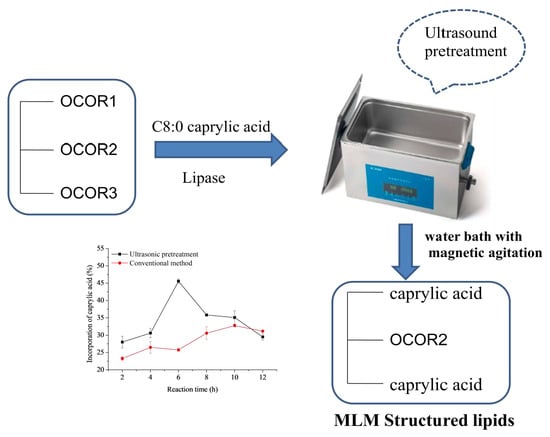
Ultrasonic Pretreatment in Synthesis of Caprylic-Rich Structured Lipids by Lipase-Catalyzed Acidolysis of Corn Oil in Organic System and Its Physicochemical Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lipase-Catalyzed Acidolysis with Ultrasonic Pretreatment
2.3. Removal of Free Fatty Acids (FFA)
2.4. Triacylglycerol Isolation by TLC
2.5. Fatty Acids Composition Analysis
2.6. Pancreatic Lipase Catalyzed sn-2 Position
2.7. TAG Composition Analysis by Ultra-HPLC
2.8. FTIR Analysis
2.9. DSC Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Screening of Commercial Immobilized Lipases
3.2. Effect of Ultrasonic Pretreatment Parameters on the Acidolysis Reaction
3.3. Fatty Acid Composition and Positional Distribution
3.4. TAG Composition Analysis
3.5. FTIR Spectra and X-Ray Spectra Analysis
3.6. Crystallization and Melting Profiles by DSC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roth, G.A.; Huffman, M.D.; Moran, A.E.; Valery, F.; Mensah, G.A.; Mohsen, N.; Murray, C.J.L. Global and Regional Patterns in Cardiovascular Mortality From 1990 to 2013. Circulation 2015, 132, 1667–1678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Liu, Y.; Wang, J.; Xu, Q.; Yu, X.; Yang, X.; Liu, Z.; Xue, C. Medium-chain triglycerides promote macrophage reverse cholesterol transport and improve atherosclerosis in ApoE-deficient mice fed a high-fat diet. Nutr. Res. 2016, 36, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, H.; Cheong, L.Z.; Tan, T.; Xu, X. Enzymatic Production of ABA-Type Structured Lipids Containing Omega-3 and Medium-Chain Fatty Acids: Effects of Different Acyl Donors on the Acyl Migration Rate. Food Bioprocess Technol. 2012, 5, 541–547. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Tang, T.K.; Phuah, E.T.; Karim, N.A.A.; Alwi, S.M.M.; Lai, O.M. Palm-based medium-and-long-chain triacylglycerol (P-MLCT): Production via enzymatic interesterification and optimization using response surface methodology (RSM). J. Food Sci. Technol. 2015, 52, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Lu, Z.; Bie, X.; Lu, F.; Liu, Z. Lipase catalyzed acidolysis of lard with capric acid in organic solvent. J. Food Eng. 2007, 78, 41–46. [Google Scholar] [CrossRef]
- Abed, S.M.; Wei, W.; Ali, A.H.; Korma, S.A.; Mousa, A.H.; Hassan, H.M.; Jin, Q.; Wang, X. Synthesis of structured lipids enriched with medium-chain fatty acids via solvent-free acidolysis of microbial oil catalyzed by Rhizomucormiehei lipase. LWT Food Sci. Technol. 2018, 93, 306–315. [Google Scholar] [CrossRef]
- Verdasco-Martin, C.M.; Corchado-Lopo, C.; Fernandez-Lafuente, R.; Otero, C. Rapid and high yield production of phospholipids enriched in CLA via acidolysis: The critical role of the enzyme immobilization protocol. Food Chem. 2019, 296, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, Y.; Zheng, L.; Jin, Q.; Wang, X. Synthesis of 1,3-distearoyl-2-oleoylglycerol by enzymatic acidolysis in a solvent-free system. Food Chem. 2017, 228, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Mallouchos, A.; Efthymiou, M.N.; Gardeli, C.; Kopsahelis, N.; Aguieiras, E.C.G.; Freire, D.M.G.; Papanikolaou, S.; Koutinas, A.A. Production of wax esters via microbial oil synthesis from food industry waste and by-product streams. Bioresour. Technol. 2017, 245, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Fernandes, K.V.; Chatzifragkou, A.; Aguieiras, E.C.G.; da Silva, J.A.C.; Fernandez-Lafuente, R.; Papanikolaou, S.; Koutinas, A.; Freire, D.M.G. Bioprocess development for biolubricant production using microbial oil derived via fermentation from confectionery industry wastes. Bioresour. Technol. 2018, 267, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Kopsahelis, N.; Mallouchos, A.; Mandala, I.; Koutinas, A.A. Bioprocess development for the production of novel oleogels from soybean and microbial oils. Food Res. Int. 2019, 126, 108684. [Google Scholar] [CrossRef]
- Papadaki, A.; Cipolatti, E.P.; Aguieiras, E.C.G.; Pinto, M.C.C.; Kopsahelis, N.; Freire, D.M.G.; Mandala, I.; Koutinas, A.A.J.F.; Technology, B. Development of Microbial Oil Wax-Based Oleogel with Potential Application in Food Formulations. Food Bioprocess Technol. 2019, 12, 899–909. [Google Scholar] [CrossRef]
- Kopsahelis, N.; Dimou, C.; Papadaki, A.; Xenopoulos, E.; Kyraleou, M.; Kallithraka, S.; Kotseridis, Y.; Papanikolaou, S.; Koutinas, A.A. Biotechnology. Refining of wine lees and cheese whey for the production of microbial oil, polyphenol-rich extracts and value-added co-products. J. Chem. Technol. Biotechnol. 2018, 93, 257–268. [Google Scholar] [CrossRef]
- Asadi, F.; Shahriari, A.; Chahardah-Cheric, M. Effect of long-term optional ingestion of canola oil, grape seed oil, corn oil and yogurt butter on serum, muscle and liver cholesterol status in rats. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48, 2454–2457. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, T.; Ustun, G.; Aksoy, H.A. Production of medium-chain triacylglycerols from corn oil: Optimization by response surface methodology. Bioresour. Technol. 2010, 101, 7456–7461. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.E.; Vinay, J.C.; Brieva, R.; Hill, C.G.; Garcia, H.S. Lipase-catalyzed acidolysis of corn oil with conjugated linoleic acid in hexane. J. Food Lipids 2010, 10, 11–24. [Google Scholar] [CrossRef]
- Liu, S.L.; Dong, X.Y.; Wei, F.; Wang, X.; Lv, X.; Zhong, J.; Wu, L.; Quek, S.Y.; Chen, H. Ultrasonic pretreatment in lipase-catalyzed synthesis of structured lipids with high 1,3-dioleoyl-2-palmitoylglycerol content. Ultrason. Sonochem. 2015, 23, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, Q.; Qin, Z.; Qian, L.; Kong, B. Ultrasonic pretreatment promotes diacylglycerol production from lard by lipase-catalysedglycerolysis and its physicochemical properties. Ultrason. Sonochem. 2018, 48, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bashari, M.; Eibaid, A.; Wang, J.; Tian, Y.; Xu, X.; Jin, Z. Influence of low ultrasound intensity on the degradation of dextran catalyzed by dextranase. Ultrason. Sonochem. 2013, 20, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Kumar, D.; Johari, R.; Singh, C.P. Enzymatic transesterification of Jatrophacurcas oil assisted by ultrasonication. Ultrason. Sonochem. 2011, 18, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Li, T.; Hao, W.; Song, W.; Ye, W.; Ma, D.; Fang, X. Ultrasonic irradiation with vibration for biodiesel production from soybean oil by Novozym 435. Process Biochem. 2010, 45, 519–525. [Google Scholar] [CrossRef]
- Santin, C.M.T.; Michelin, S.; Scherer, R.P.; Valério, A.; Di Luccio, M.; Oliveira, D.; Vladimir Oliveira, J. Comparison of macauba and soybean oils as substrates for the enzymatic biodiesel production in ultrasound-assisted system. Ultrason. Sonochem. 2017, 35, 525–528. [Google Scholar] [CrossRef] [PubMed]
- Wanga, Y.; Xu, X.; Liang, X.; Duan, Z. Lipase-catalyzed acidolysis of canola oil with caprylic acid to produce medium-, long- and medium-chain-type structured lipids. Food Bioprocess Process. 2012, 90, 707–712. [Google Scholar] [CrossRef]
- Nunes, P.A.; Pires-Cabral, P.; Ferreira-Dias, S. Production of olive oil enriched with medium chain fatty acids catalysed by commercial immobilised lipases. Food Chem. 2011, 127, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Luddy, F.E.; Barford, R.A.; Herb, S.F.; Magidman, P.; Riemenschneider, R.W. Pancreatic lipase hydrolysis of triglycerides by a semimicro technique. J. Am. Oil Chem. Soc. 1964, 41, 693–696. [Google Scholar] [CrossRef]
- Lu, J.; Jin, Q.; Wang, X.; Wang, X. Preparation of medium and long chain triacylglycerols by lipase-catalyzed interesterification in a solvent-free system. Process Biochem. 2017, 54, 89–95. [Google Scholar] [CrossRef]
- Elzey, B.; Pollard, D.; Fakayode, S.O. Determination of Adulterated Neem and Flaxseed Oil Compositions by FTIR Spectroscopy and Multivariate Regression Analysis. Food Control 2016, 68, 303–309. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Kodali, S.; Balle, T.; Chen, B.; Guo, Z. Liquid lipases for enzymatic concentration of n-3 polyunsaturated fatty acids in monoacylglycerols via ethanolysis: Catalytic specificity and parameterization. Bioresour. Technol. 2017, 224, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Poppe, J.K.; Garcia-Galan, C.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Optimization of synthesis of fatty acid methyl esters catalyzed by lipase B from Candida antarctica immobilized on hydrophobic supports. J. Mol. Catal. B Enzym. 2013, 94, 51–56. [Google Scholar] [CrossRef]
- Zhu, K.; Liu, H.; Han, P.; Wei, P. Study of ultrasound-promoted, lipase-catalyzed synthesis of fructose ester. Front. Chem. Eng. China 2010, 4, 367–371. [Google Scholar] [CrossRef]
- Bansode, S.R.; Rathod, V.K. An Investigation of lipase catalysedsonochemical synthesis: A review. Ultrason. Sonochem. 2017, 38, 503–529. [Google Scholar] [CrossRef] [PubMed]
- Suganya, T.; Renganathan, S. Optimization and kinetic studies on algal oil extraction from marine macroalgaeUlvalactuca. Bioresour. Technol. 2012, 107, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Higuera-Barraza, O.A.; Toro-Sanchez, C.L.D.; Ruiz-Cruz, S.; Márquez-Ríos, E. Effects of high-energy ultrasound on the functional properties of proteins. Ultrason. Sonochem. 2016, 31, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Subhedar, P.B.; Gogate, P.R. Ultrasound assisted intensification of biodiesel production using enzymatic interesterification. Ultrason. Sonochem. 2016, 29, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Gupta, M.N. The effect of ultrasonic pre-treatment on the catalytic activity of lipases in aqueous and non-aqueous media. Chem. Cent. J. 2008, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Zhang, C. Propylsulfonic and arenesulfonic functionalized SBA-15 silica as an efficient and reusable catalyst for the acidolysis of soybean oil with medium-chain fatty acids. Food Chem. 2016, 211, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.P.; Calvo, L.; Alba, C.; Leitgeb, M.; Primožič, M.; Knez, Ž. Enzymatic synthesis of isoamyl acetate with immobilized Candida antarctica lipase in supercritical carbon dioxide. J. Supercrit. Fluids 2005, 33, 77–84. [Google Scholar] [CrossRef]
- Kim, H.R.; Hou, C.T.; Lee, K.T.; Kim, B.H.; Kim, I.H. Enzymatic synthesis of structured lipids using a novel cold-active lipase from Pichialynferdii NRRL Y-7723. Food Chem. 2010, 122, 846–849. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.D.; Zhao, X.Y.; Liu, X.; Dong, T.; Wu, F.A. From microalgae oil to produce novel structured triacylglycerols enriched with unsaturated fatty acids. Bioresour. Technol. 2015, 184, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.H.; Meng, Z.; Cao, P.R.; Liang, X.Y.; Piatko, M.; Campbell, S.; Lo, S.K.; Liu, Y.F. Influence of indigenous minor components on fat crystal network of fully hydrogenated palm kernel oil and fully hydrogenated coconut oil. Food Chem. 2018, 255, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, Y.; Ma, X.; Wang, E.; Liu, M.; Yan, R. Characterisation and oxidation stability of monoacylglycerols from partially hydrogenated corn oil. Food Chem. 2015, 173, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.W.; Weng, H.T.; Zhang, X.; Wu, H.; Li, B. Mechanistic insight into the relationship between triacylglycerol and crystallization of lipase-catalyzed interesterified blend of palm stearin and vegetable oil. Food Chem. 2018, 260, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, C.A.; Akoh, C.C. Preparation of Infant Formula Fat Analog Containing Capric Acid and Enriched with DHA and ARA at the sn-2 Position. J. Am. Oil Chem. Soc. 2016, 93, 531–542. [Google Scholar] [CrossRef]





| Fatty Acids | Corn oil | SLs-U | SLs-N | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Sn-2 | Sn-1,3 | Total | Sn-2 | Sn-1,3 | Total | Sn-2 | Sn-1,3 | |
| C8:0 | ND | ND | ND | 45.55 ± 0.65 a | 7.01 ± 0.02 a | 64.82 ± 0.43 a | 32.75 ± 0.29 b | 6.36 ± 0.03 b | 45.95 ± 0.78 b |
| C16:0 | 11.72 ± 0.21 a | 4.24 ± 0.05 b | 15.46 ± 0.08 a | 5.31 ± 0.1 c | 4.42 ± 0.05 a | 5.76 ± 0.2 c | 6.44 ± 0.2 b | 4.43 ± 0.1 a | 7.45 ± 0.6 b |
| C18:0 | 1.53 ± 0.2 a | 0.47 ± 0.04 a | 2.08 ± 0.2 a | 0.62 ± 0.1 c | 0.42 ± 0.03 b | 0.72 ± 0.6 c | 0.84 ± 0.5 b | 0.46 ± 0.2 a | 1.03 ± 0.6 b |
| C18:1 | 25.60 ± 0.6 a | 24.16 ± 0.7 b | 26.32 ± 0.8 a | 13.23 ± 0.12 c | 31.76 ± 2.18 a | 3.97 ± 0.3 c | 16.54 ± 1.02 b | 33.88 ± 2.64 a | 7.87 ± 0.3 b |
| C18:2 | 47.66 ± 0.8 a | 61.35 ± 0.8 a | 40.82 ± 1.42 a | 25.32 ± 1.14 c | 51.64 ± 2.06 b | 12.16 ± 1.2 c | 32.16 ± 0.8 b | 59.07 ± 0.7 a | 18.71 ± 0.5 b |
| C18:3 | 0.51 ± 0.04 a | 0.73 ± 0.04 a | 0.40 ± 0.03 a | 0.33 ± 0.03 c | 0.35 ± 0.01 c | 0.32 ± 0.02 b | 0.42 ± 0.02 b | 0.51 ± 0.03 b | 0.37 ± 0.02 a |
| C20:0 | 0.48 ± 0.03 a | 0.64 ± 0.04 a | 0.40 ± 0.03 a | 0.39 ± 0.01 c | 0.61 ± 0.01 b | 0.28 ± 0.03 c | 0.42 ± 0.04 b | 0.65 ± 0.01 a | 0.31 ± 0.01 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, C.; Ben, H.; Wang, J.; Li, T.; Yu, G. Ultrasonic Pretreatment in Synthesis of Caprylic-Rich Structured Lipids by Lipase-Catalyzed Acidolysis of Corn Oil in Organic System and Its Physicochemical Properties. Foods 2019, 8, 566. https://doi.org/10.3390/foods8110566
Yue C, Ben H, Wang J, Li T, Yu G. Ultrasonic Pretreatment in Synthesis of Caprylic-Rich Structured Lipids by Lipase-Catalyzed Acidolysis of Corn Oil in Organic System and Its Physicochemical Properties. Foods. 2019; 8(11):566. https://doi.org/10.3390/foods8110566
Chicago/Turabian StyleYue, Chonghui, Hongyan Ben, Junwen Wang, Tiantian Li, and Guoping Yu. 2019. "Ultrasonic Pretreatment in Synthesis of Caprylic-Rich Structured Lipids by Lipase-Catalyzed Acidolysis of Corn Oil in Organic System and Its Physicochemical Properties" Foods 8, no. 11: 566. https://doi.org/10.3390/foods8110566
APA StyleYue, C., Ben, H., Wang, J., Li, T., & Yu, G. (2019). Ultrasonic Pretreatment in Synthesis of Caprylic-Rich Structured Lipids by Lipase-Catalyzed Acidolysis of Corn Oil in Organic System and Its Physicochemical Properties. Foods, 8(11), 566. https://doi.org/10.3390/foods8110566