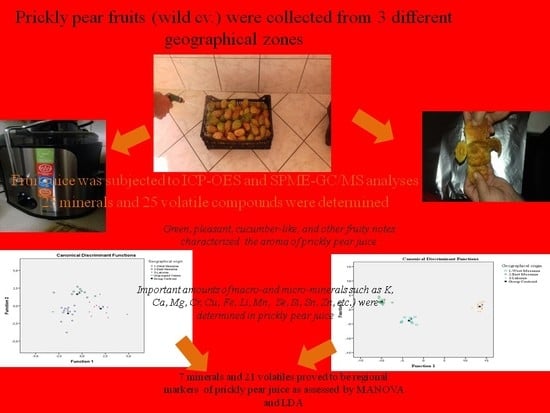
Valorization of Prickly Pear Juice Geographical Origin Based on Mineral and Volatile Compound Contents Using LDA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Prickly Pear Juice Samples
2.2. Chemicals and Multi-Element Standard
2.3. Determination of Mineral Content in Soil Samples
2.4. Determination of Mineral Content in Prickly Pear Juice Samples
2.5. ICP-OES Instrumentation and Method Analytical Characteristics
2.6. HS-SPME/GC-MS Analysis
2.6.1. Method Optimization
2.6.2. Extraction of Volatile Compounds
2.6.3. GC/MS Instrumentation and Analysis Conditions
2.6.4. Identification of Volatile Compounds
2.7. Statistical Treatment of Data
3. Results and Discussion
3.1. Mineral Content Analysis of Soil Samples
3.2. Mineral Content Analysis of Prickly Pear Juice Samples
3.3. Volatile Profile of Prickly Pear Juice
3.4. Valorization of Prickly Pear Juice Geographical Origin Based on Mineral Content Using LDA
3.5. Valorization of Prickly Pear Juice Geographical Origin Based on Semi-Quantitative Data of Volatile Compounds Using LDA
3.6. Summary Regarding the Most Effective Predictors of Prickly Pear Juice Geographical Origin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Quattrocchi, U. CRC World Dictionary of Plant Names; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2000; Volume III, p. 1885. [Google Scholar]
- United States Department of Agriculture (USDA). Food Description: “09287, Prickly Pears, Raw”. 2017. Available online: https://www.usda.gov (accessed on 24 September 2018).
- Butera, D.; Tesoriere, L.; Di Gaudio, F.; Bongiorno, A.; Allegra, M.; Pintaudi, A.M.; Kohen, R.; Livrea, A.M. Antioxidant activities of sicilian prickly pear (Opuntia ficus indica) fruit extracts and reducing properties of its betalains: Betanin and indicaxanthin. J. Agric. Food Chem. 2002, 50, 6895–6901. [Google Scholar] [CrossRef]
- Guzmán-Maldonado, S.H.; Morales-Montelongo, A.L.; Mondragón-Jacobo, C.; Herrera-Hernández, G.; Guevara-Lara, F.; Reynoso-Camacho, R. Physicochemical, nutritional, and functional characterization of fruits xoconostle (Opuntia matudae) pears from Central-México Region. J. Food Sci. 2010, 75, C485–C492. [Google Scholar] [CrossRef] [PubMed]
- Frati, A.C.; Xilotl Díaz, N.; Altamirano, P.; Ariza, R.; López-Ledesma, R. The effect of two sequential doses of Opuntia streptacantha upon glycemia. Archivos De Investigación Médica 1991, 22, 333–336. [Google Scholar] [PubMed]
- Franke, A.A.; Cooney, R.V.; Henning, S.M.; Custer, L.J. Bioavailability and antioxidant effects of orange juice components in humans. J. Agric. Food Chem. 2005, 53, 5170–5178. [Google Scholar] [CrossRef] [Green Version]
- Zielinski, A.A.F.; Haminium, C.W.I.; Nunes, C.A.; Schnitzler, E.; van Ruth, S.M.; Granato, D. Chemical composition, sensory properties, provenance, and bioactivity of fruit juices as assessed by chemometrics: A critical review and guideline. Compr. Rev. Food Sci. Food Saf. 2014, 13, 300–316. [Google Scholar] [CrossRef]
- Mangas, J.J.; Suarez, B.; Picinelli, A.; Moreno, J.; Blanco, D. Differentiation by phenolic profile of apple juices prepared according to membrane techniques. J. Agric. Food Chem. 1997, 45, 4777–4784. [Google Scholar] [CrossRef]
- Simpkins, W.A.; Louie, H.; Wu, M.; Harrison, M.; Goldberg, D. Trace elements in Australian orange juices and others products. Food Chem. 2000, 71, 423–433. [Google Scholar] [CrossRef]
- Reid, L.M.; O’Donnell, C.P.; Kelly, J.D.; Downey, G. Preliminary studies for the differentiation of apple juice samples by chemometric analysis of solid-phase microextraction-gas chromatographic data. J. Agric. Food Chem. 2004, 52, 6891–6896. [Google Scholar] [CrossRef]
- He, J.; Rodrigues-Saona, L.E.; Giusti, M. Mid-infrared spectroscopy for juice authentication-rapid differentiation of commercial juices. J. Agric. Food Chem. 2007, 55, 4443–4452. [Google Scholar] [CrossRef]
- Martina, V.; Ionescu, K.; Pigani, L.; Terzi, F.; Ulrici, A.; Zanardi, C.; Seeber, R. Development of an electronic tongue based on a PEDOT-modified voltammetric sensor. Anal. Bioanal. Chem. 2007, 387, 2101–2110. [Google Scholar] [CrossRef]
- Pellerano, R.G.; Mazza, S.S.; Marigliano, R.A.; Marchevsky, E.J. Multielement analysis of Argentinean lemon juices by instrumental neutronic activation analysis and their classification according to geographical origin. J. Agric. Food Chem. 2008, 56, 5222–5225. [Google Scholar] [CrossRef]
- Longobardi, F.; Ventrella, A.; Bianco, A.; Catucci, L.; Cafagna, I.; Gallo, V.; Mastrorilli, P.; Agostiano, A. Non-targeted 1H NMR fingerprinting and multivariate statistical analyses for the characterization of the geographical origin of Italian sweet cherries. Food Chem. 2013, 141, 3028–3033. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, P.A.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and geographical discrimination of Greek pine and thyme honeys based on their mineral content, using chemometrics. Eur. Food Res. Technol. 2017, 243, 101–113. [Google Scholar] [CrossRef]
- AOAC Official Method 990.08. Metals in Solid Wastes, Inductively Coupled Plasma Atomic Emission Spectrometric Method; AOAC International: Rockville, MD, USA, 1993. [Google Scholar]
- Karabagias, I.K.; Louppis, P.A.; Karabournioti, S.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and geographical discrimination of commercial Citrus spp. honeys produced in different Mediterranean countries based on minerals, volatile compounds and physicochemical parameters, using chemometrics. Food Chem. 2017, 217, 445–455. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterisation and classification of Greek pine honeys according to their geographical origin based on volatiles, physicochemical parameters and chemometrics. Food Chem. 2014, 146, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.K. Volatile metabolites or pollen characteristics as regional markers of monofloral thyme honey? Sep. Sci. Plus 2018, 1, 83–92. [Google Scholar] [CrossRef]
- Louppis, P.A.; Karabagias, I.K.; Kontakos, S.; Kontominas, M.G.; Papastephanou, C. Botanical discrimination of Greek unifloral honeys based on mineral content in combination with physicochemical parameter analysis, using a validated chemometric approach. Microchem. J. 2017, 135, 180–189. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 6th ed.; Pearson Education Limited: Essex, UK, 2010; pp. 1–278. [Google Scholar]
- Huberty, C.J.; Olejnik, S. Applied MANOVA and Discriminant Analysis, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006; pp. 355–356. [Google Scholar]
- Krishnamoorthy, K. Statistical Tolerance Regions: Theory, Applications, and Computation; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 1–6. [Google Scholar]
- Dehbi, F.; Hasib, A.; Ouatmane, A.; Elbatal, H.; Jaouad, A. Physicochemical characteristics of Moroccan prickly pear juice (Opuntia ficus indica L.). Int. J. Adv. Res. Technol. 2014, 4, 300–306. [Google Scholar]
- Mohamed, S.A.; Hussein, A.M.S.; Ibraheim, G.E. Physicochemical, sensorial, antioxidant and volatile of juice from prickly pear with guava or mandarin. Int. J. Food Sci. Nutr. 2014, 3, 44–53. [Google Scholar]
- Berger, R.F. Flavours and Fragrances, Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–649. [Google Scholar]
- Yilmaz, E.; Baldwin, E.A.; Shewfelt, R.L. Enzymatic modification of tomato Homogenate and its effect on volatile flavor Compounds. J. Food Sci. 2002, 67, 2122–2125. [Google Scholar] [CrossRef]
- Haslbeck, F.; Grosch, W. HPLC analysis of all positional isomers of the monohydroperoxides formed by soybean lipoxygenases during oxidation of linoleic acid. J. Food Biochem. 2007, 9, 1–14. [Google Scholar] [CrossRef]
- Chan, H.W.S. Autoxidation of Unsaturated Lipids; Academic Press: London, UK, 1987; pp. 1–296. [Google Scholar]
- Engel, K.H.; Ramming, D.W.; Flath, R.A.; Teranishi, R. Investigation of volatile constituents in nectarines. 2. Changes in aroma composition during nectarine maturation. J. Agric. Food Chem. 1988, 36, 1003–1006. [Google Scholar] [CrossRef]
- Aubert, C.; Günata, Z.; Ambid, C.; Baumes, R. Changes in physicochemical characteristics and volatile constituents of yellow- and white-fleshed nectarines during maturation and artificial ripening. J. Agric. Food Chem. 2003, 51, 3083–3091. [Google Scholar] [CrossRef]
- Arena, E.; Campisi, S.; Fallico, B.; Lanza, M.C.; Maccarone, E. Aroma value of volatile compounds of prickly pear (Opuntia ficus indica L. Mill. Cactaceae). Int. J. Food Sci. Nutr. 2001, 3, 311–319. [Google Scholar]
- Oumato, J.; Zrira, S.; Petretto, G.L.; Saidi, B.; Salaris, M.; Pintore, G. Volatile constituents and polyphenol composition of Opuntia ficus indica (L.) Mill from Morocco. Dir. Open Access J. 2016, 4, 5–11. [Google Scholar]
- Schieberle, P.; Ofner, S.; Grosch, W. Evaluation of potent odorants in cucumbers (Cucumis sativus) and Muskmelons (Cucumis melo) by aroma extract dilution analysis. J. Food Sci. 1990, 55, 193–195. [Google Scholar] [CrossRef]
- Guler, Z.; Candir, E.; Yetisir, H.; Karaca, F.; Solmaz, I. Volatile organic compounds in watermelon (Citrullus lanatus) grafted onto 21 local and two commercial bottle gourd (Lagenaria siceraria) rootstocks. J. Hortic. Sci. Biotechnol. 2014, 89, 448–452. [Google Scholar] [CrossRef]
- Maarse, H. Volatile Compounds in Fruits and Beverages; Dekker: New York, NY, USA, 1991. [Google Scholar]
- Lavilla, T.; Recasens, I.; Lopez, M.L.; Puy, J. Multivariate analysis of maturity stages, including quality and aroma, in ‘Royal Glory’ peaches and ‘Big Top’ nectarines. J. Sci. Food Agric. 2002, 82, 1842–1849. [Google Scholar] [CrossRef]
- Jakobsen, H.B.; Hansen, M.; Chrisyensen, M.R.; Brockhoff, P.B.; Olsen, C.E. Aroma volatiles of blanched green peas (Pisum sativum L.). J. Agric. Food Chem. 1998, 46, 3727–3734. [Google Scholar] [CrossRef]
- Mann, J.C.; Hobbs, J.B.; Banthorpe, D.V.; Harborne, J.B. Natural Products: Their Chemistry and Biological Significance; Longman Scientific & Technical: Essex, UK, 1994; pp. 308–309. [Google Scholar]
- American Industrial Hygiene Association (AIHA). Odor Thresholds for Chemicals with Established Health Standards, 2nd ed.; AIHA: Fairfax, VA, USA, 2013. [Google Scholar]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 5th ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–1864. [Google Scholar]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–988. [Google Scholar]
- Vallverdú-Queralt, A.; Bendini, A.; Tesini, F.; Valli, E.; Lamuela-Raventos, R.M.; Toschi, T.G. Chemical and sensory analysis of commercial tomato juices present on the Italian and Spanish markets. J. Agric. Food Chem. 2013, 61, 1044–1050. [Google Scholar] [CrossRef] [PubMed]



| Mineral (mg/kg)/Region | Al | B | Ca | Cu | Fe | K | Li | Mg | Mn | Na | Ni | P | Se | Si | Sn | Zn | TMC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Western Messinia (n = 12) | |||||||||||||||||
| Average | 0.26 a | 2.49 b | 83.78 c | 0.52 f | 1.21 g | 1869.70 h | 0.12 k | 93.92 l | 1.69 m | 21.36 o | 0.28 r | 127.52 t | 1.23 w | 0.24 y | 0.40 aa | 1.30 ac | 2206.07 ad |
| ±SD | 0.26 | 0.50 | 9.68 | 0.35 | 0.79 | 205.64 | 0.10 | 11.80 | 2.04 | 17.41 | 0.25 | 17.70 | 2.05 | 0.46 | 0.47 | 0.56 | 214.77 |
| Eastern Messinia (n = 12) | |||||||||||||||||
| Average | 0.45 a | 2.24 b | 59.61 d | 0.48 f | 1.36 g | 2175.97 i | 0.13 k | 100.20 l | 2.83 n | 48.14 p | 0.74 s | 186.13 u | 0.77 x | 0.08 z | 0.14 ab | 0.79 ad | 2580.11 ae |
| ±SD | 1.15 | 0.45 | 20.72 | 0.21 | 2.33 | 161.25 | 0.07 | 10.18 | 0.96 | 25.94 | 0.21 | 27.57 | 0.42 | 0.18 | 0.29 | 0.22 | 206.06 |
| Lakonia (n = 12) | |||||||||||||||||
| Average | 0.27 a | 2.63 b | 89.66 e | 0.67 f | 0.76 g | 2398.60 j | 0.10 k | 108.50 l | 1.39 m | 33.87 q | 0.35 r | 188.53 v | 0.70 x | 0.24 y | 0.37 aa | 1.27 ac | 2827.98 af |
| ± SD | 0.38 | 0.81 | 33.85 | 0.39 | 0.70 | 252.36 | 0.10 | 14.74 | 2.43 | 31.10 | 0.48 | 28.94 | 0.38 | 0.34 | 0.40 | 0.33 | 310.65 |
| LOD | 4.90 | 0.32 | 4.06 | 1.40 | 1.96 | 1.47 | 0.12 | 5.18 | 0.80 | 1.45 | 0.74 | 1.50 | 1.49 | 0.11 | 8.84 | 0.40 | |
| LOQ | 14.70 | 1.06 | 12.17 | 4.19 | 5.87 | 5.41 | 0.40 | 15.53 | 2.40 | 5.47 | 2.21 | 5.00 | 4.48 | 0.36 | 26.53 | 1.20 | |
| CV (n = 36) | 2.15 | 0.25 | 0.34 | 0.59 | 1.31 | 0.14 | 0.78 | 0.13 | 0.99 | 0.79 | 0.84 | 0.23 | 1.35 | 1.83 | 1.32 | 0.40 |
| RT (min) | Volatile Compounds (μg/L) | KI a | Western Messinia (Avg ± SD) | Eastern Messinia (Avg ± SD) | Lakonia (Avg ± SD) | Method of Identification | MS Qualification (%) | Orthonasal Threshold (μg/L) b | Odour Note c |
|---|---|---|---|---|---|---|---|---|---|
| Alcohols | |||||||||
| 11.66 | 1-Pentanol | 702 | 192.54 ± 90.69 | 178.18 ± 54.94 | 314.94 ± 105.62 | MS | 90 | 0.0055-305 | Fermented, green |
| 15.27 | 3-Hexen-1-ol | 787 | 32.26 ± 15.86 | 41.02 ± 31.12 | 47.81 ± 21.01 | MS | 94 | na | Woody, green, leafy |
| 15.89 | 2-Hexen-1-ol | 802 | 2486.91 ± 593.02 | 2681.84 ± 1280.63 | 3022.18 ± 1101.85 | MS/KI | 91 | na | Green, fruity, leafy |
| 16.02 | 1-Hexanol | 804 | 2372.75 ± 1341.20 | 3885.41 ± 941.84 | 3429.98 ± 1149.45 | MS/KI | 90 | 500-2500 | Green, fruity |
| 16.86 | 3,5-Hexadien-1-ol | 823 | 120.61 ± 57.82 | 189.66 ± 53.18 | 180.42 ± 67.70 | MS/KI | 92 | na | |
| 24.79 | 1-Octanol | 999 | ni | ni | 55.27 ± 46.57 | MS/KI | 90 | 190 | Herbal, green, penetrating |
| 28.67 | 2,6 Nonadien-1-ol | 1090 | 318.22 ± 265.20 | 270.39 ± 222.85 | 797.17 ± 337.37 | MS/KI | 91 | na | Green, cucumber-like |
| 28.75 | 2-Nonen-1-ol | 1091 | 443.17 ± 240.80 | 564.97 ± 124.63 | 921.67 ± 240.39 | MS/KI | 92 | na | Fatty |
| Aldehydes | |||||||||
| 7.63 | 2-Butenal | 520 | ni | ni | 41.58 ± 19.44 | MS | 90 | na | Floral |
| 9.13 | Pentanal | 620 | ni | ni | 19.87 ± 18.71 | MS | 86 | na | Bready, fruity, berry-like |
| 13.17 | Hexanal | 737 | 431.69 ± 218.41 | 288.86 ± 241.71 | 692.64 ± 156.50 | MS | 96 | 9.18-10.50 | Green, grassy, floral |
| 15.51 | 2-Hexenal | 793 | 512.87 ± 263.34 | 627.65 ± 411.57 | 1129.38 ± 258.88 | MS | 96 | 24.2 | Soapy, fatty, green |
| 17.63 | Heptanal | 840 | 26.20 ± 17.26 | ni | 43.79 ± 46.45 | MS/KI | 90 | na | Fruity, oily-greasy |
| 18.14 | 2,4-Hexadienal | 851 | ni | ni | 27.72 ± 23.07 | MS/KI | 92 | na | Green, fruity, waxy |
| 20.11 | 2-Heptenal | 894 | 29.28 ± 28.21 | ni | 55.93 ± 22.71 | MS/KI | 97 | na | Green, fatty, oily, fruity |
| 24.48 | 2-Octenal | 992 | 64.02 ± 49.55 | ni | 181.08 ± 48.33 | MS/KI | 91 | na | Sweet, green, fatty, brothy |
| 26.34 | Nonanal | 1035 | 86.00 ± 35.76 | 96.35 ± 20.97 | 187.25 ± 58.03 | MS/KI | 91 | 2.53-5.00 | Soapy, floral |
| 28.37 | 2,6-Nonadienal | 1082 | 78.21 ± 70.68 | 80.75 ± 68.90 | 378.80 ± 152.75 | MS/KI | 90 | na | Cucumber-like, green |
| 34.37 | 2,4-Decadienal | 1243 | ni | ni | 54.09 ± 47.75 | MS/KI | 93 | 0.2 | Fatty, waxy, green |
| Hydrocarbons | |||||||||
| 6.95 | 2,4-Hexadiene | 473 | ni | ni | 12.66 ± 13.81 | MS | 90 | na | na |
| 21.52 | 1-Decene | 926 | ni | 75.67 ± 65.39 | ni | MS/KI | 95 | 6.45 | Pleasant |
| 21.89 | Decane | 934 | ni | 22.57 ± 20.78 | ni | MS/KI | 93 | na | na |
| 29.73 | 1-Dodecene | 1116 | ni | 64.01 ± 60.12 | ni | MS/KI | 95 | na | na |
| Terpenoids | |||||||||
| 23.56 | dl-Limonene | 971 | 23.07 ± 49.07 | 14.85 ± 9.83 | ni | MS/KI | 98 | 0.0018-0.31 | Lemon, citrus |
| Furan derivatives | |||||||||
| 21.58 | Furan, 2-pentyl- | 927 | 40.71 ± 35.39 | ni | 109.03 ± 81.45 | MS/KI | 91 | 10.06 | Strong |
| Minerals | Wilks’ Lambda | F | df1 | df2 | p | Function 1 | Function 2 |
|---|---|---|---|---|---|---|---|
| Ca | 0.748 | 5.397 | 2 | 32 | 0.0010 | 0.132 | 0.371 * |
| K | 0.458 | 18.938 | 2 | 32 | <0.001 | 0.463 * | −0.334 |
| Mg | 0.797 | 4.070 | 2 | 32 | 0.027 | 0.230 * | −0.086 |
| Na | 0.770 | 4.768 | 2 | 32 | 0.015 | 0.025 | −0.397 * |
| Ni | 0.746 | 5.439 | 2 | 32 | 0.009 | −0.047 | −0.419 * |
| P | 0.428 | 21.397 | 2 | 32 | <0.001 | 0.381 | −0.600 * |
| Zn | 0.704 | 6.741 | 2 | 32 | 0.004 | 0.081 | 0.457 * |
| Volatile Compounds (μg/L) | Wilks’ Lambda | F | df1 | df2 | p | Function 1 | Function 2 |
|---|---|---|---|---|---|---|---|
| 2-Butenal | 0.231 | 54.909 | 2 | 33 | <0.001 | 0.171 ** | 0.165 |
| 2,4-Decadienal | 0.517 | 15.401 | 2 | 33 | <0.001 | 0.091 ** | 0.088 |
| Decanal | 0.538 | 14.158 | 2 | 33 | <0.001 | −0.064 | 0.220 ** |
| Decene | 0.507 | 16.070 | 2 | 33 | <0.001 | −0.069 | 0.234 * |
| 1-Dodecene | 0.548 | 13.604 | 2 | 33 | <0.001 | −0.063 | 0.215 * |
| Heptanal | 0.699 | 7.120 | 2 | 33 | 0.003 | 0.060 | −0.079 ** |
| 2-Heptenal | 0.434 | 21.488 | 2 | 33 | <0.001 | 0.107 ** | −0.107 |
| 2,4-Hexadienal | 0.488 | 17.326 | 2 | 33 | <0.001 | 0.096 ** | 0.093 |
| 2,4-Hexadiene | 0.620 | 10.094 | 2 | 33 | <0.001 | 0.073 ** | 0.071 |
| 3,5-Hexadien-1-ol | 0.778 | 4.703 | 2 | 33 | 0.016 | 0.007 | 0.179 ** |
| Hexanal | 0.588 | 11.554 | 2 | 33 | <0.001 | 0.081 ** | −0.024 |
| 1-Hexanol | 0.753 | 5.410 | 2 | 33 | 0.009 | −0.003 | 0.194 ** |
| 2-Hexenal | 0.566 | 12.657 | 2 | 33 | <0.001 | 0.077 | 0.128 ** |
| 2,6-Nonadienal | 0.337 | 32.509 | 2 | 33 | <0.001 | 0.132 ** | 0.131 |
| 2,6-Nonadien-1-ol | 0.558 | 13.067 | 2 | 33 | <0.001 | 0.085 ** | 0.057 |
| Nonanal | 0.429 | 21.969 | 2 | 33 | <0.001 | 0.105 | 0.139 ** |
| 2-Nonen-1-ol | 0.493 | 16.955 | 2 | 33 | <0.001 | 0.086 | 0.170 ** |
| 1-Octanol | 0.494 | 16.903 | 2 | 33 | <0.001 | 0.095 ** | 0.092 |
| 2-Octenal | 0.207 | 63.348 | 2 | 33 | <0.001 | 0.190 ** | −0.056 |
| Pentanal | 0.549 | 13.541 | 2 | 33 | <0.001 | 0.085 ** | 0.082 |
| Pentanol | 0.645 | 9.079 | 2 | 33 | 0.001 | 0.071 ** | 0.044 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karabagias, V.K.; Karabagias, I.K.; Louppis, A.; Badeka, A.; Kontominas, M.G.; Papastephanou, C. Valorization of Prickly Pear Juice Geographical Origin Based on Mineral and Volatile Compound Contents Using LDA. Foods 2019, 8, 123. https://doi.org/10.3390/foods8040123
Karabagias VK, Karabagias IK, Louppis A, Badeka A, Kontominas MG, Papastephanou C. Valorization of Prickly Pear Juice Geographical Origin Based on Mineral and Volatile Compound Contents Using LDA. Foods. 2019; 8(4):123. https://doi.org/10.3390/foods8040123
Chicago/Turabian StyleKarabagias, Vassilios K., Ioannis K. Karabagias, Artemis Louppis, Anastasia Badeka, Michael G. Kontominas, and Chara Papastephanou. 2019. "Valorization of Prickly Pear Juice Geographical Origin Based on Mineral and Volatile Compound Contents Using LDA" Foods 8, no. 4: 123. https://doi.org/10.3390/foods8040123
APA StyleKarabagias, V. K., Karabagias, I. K., Louppis, A., Badeka, A., Kontominas, M. G., & Papastephanou, C. (2019). Valorization of Prickly Pear Juice Geographical Origin Based on Mineral and Volatile Compound Contents Using LDA. Foods, 8(4), 123. https://doi.org/10.3390/foods8040123