Natural Red Pigment Production by Monascus Purpureus in Submerged Fermentation Systems Using a Food Industry Waste: Brewer’s Spent Grain
Abstract
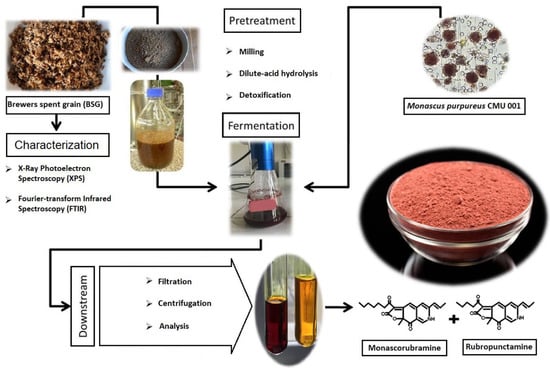
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Media
2.2. Preparation of BSG Hydrolysate
2.3. Cultivation and Fermentation Conditions
2.4. Analytical Methods
2.5. Statistical Analysis
3. Results and Discussion
3.1. The Chemical Composition and Characterization of Brewer’s Spent Grain(BSG)
3.1.1. Chemical Composition
3.1.2. Fourier-Transform Infrared Spectroscopy (FTIR)
3.1.3. X-ray Photoelectron Spectroscopy (XPS)
3.2. Effect of Acid Concentration
3.3. Effect of Solid: Liquid Ratio
3.4. Effect of Shaking Speed and Medium Volume on Pigment Synthesis
3.5. Effect of Initial pH
3.6. Effect of Inoculation Ratio
3.7. Effect of Nitrogen Source
3.8. Selecting the Important Medium Components by Plackett-Burman Design
3.9. Kinetics of Red Pigment Synthesis by Monascus Purpureus
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sharmila, G.; Nidhi, B.; Muthukumaran, C. Sequential statistical optimization of red pigment production by Monascus purpureus (MTCC 369) using potato powder. Ind. Crops Prod. 2013, 44, 158–164. [Google Scholar] [CrossRef]
- Haque, M.A.; Kachrimanidou, V.; Koutinas, A.; Lin, C.S.K. Valorization of bakery waste for biocolorant and enzyme production by Monascus purpureus. J. Biotechnol. 2016, 231, 55–64. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Oh, H.J.; Shin, C.S. Morphology control of Monascus cells and scale-up of pigment fermentation. Process Biochem. 2002, 38, 649–655. [Google Scholar] [CrossRef]
- Kalaivani, M.; Sabitha, R.; Kalaiselvan, V.; Rajasekaran, A. Health benefits and clinical impact of major nutrient, red yeast rice: A review. Food Bioprocess Technol. 2010, 3, 333–339. [Google Scholar] [CrossRef]
- Babitha, S.; Soccol, C.R.; Pandey, A. Jackfruit seed—A novel substrate for the production of Monascus pigments through solid-state fermentation. Food Technol. Biotechnol. 2006, 44, 465–471. [Google Scholar]
- Dufossé, L.; Galaup, P.; Yaron, A.; Arad, S.M.; Blanc, P.; Murthy, K.N.C.; Ravishankar, G.A. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality? Trends Food Sci. Technol. 2005, 16, 389–406. [Google Scholar] [CrossRef]
- Babitha, S.; Soccol, C.R.; Pandey, A. Solid-state fermentation for the production of Monascus pigments from jackfruit seed. Bioresour. Technol. 2007, 98, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Silveira, S.T.; Daroit, D.J.; Brandelli, A. Pigment production by Monascus purpureus in grape waste using factorial design. LWT Food Sci. Technol. 2008, 41, 170–174. [Google Scholar] [CrossRef]
- Hilares, R.T.; de Souza, R.A.; Marcelino, P.F.; da Silva, S.S.; Dragone, G.; Mussatto, S.I.; Santos, J.C. Sugarcane bagasse hydrolysate as a potential feedstock for red pigment production by Monascus ruber. Food Chem. 2018, 245, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Silveira, S.T.; Daroit, D.J.; Sant’Anna, V.; Brandelli, A. Stability Modeling of Red Pigments Produced by Monascus purpureus in Submerged Cultivations with Sugarcane Bagasse. Food Bioprocess Technol. 2013, 6, 1007–1014. [Google Scholar] [CrossRef]
- Domínguez-Espinosa, R.M.; Webb, C. Submerged fermentation in wheat substrates for production of Monascus pigments. World J. Microbiol. Biotechnol. 2003, 19, 329–336. [Google Scholar] [CrossRef]
- Srivastav, P.; Yadav, V.K.; Govindasamy, S.; Chandrasekaran, M. Red pigment production by Monascus purpureus using sweet potato-based medium in submerged fermentation. Nutrafoods. 2015, 14, 159–167. [Google Scholar] [CrossRef]
- Hamdi, M.; Blanc, P.J.; Goma, G. Effect of aeration conditions on the production of red pigments by Monascus purpureus growth on prickly pear juice. Process Biochem. 1996, 31, 543–547. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Fernandes, M.; Mancilha, I.M.; Roberto, I.C. Effects of medium supplementation and pH control on lactic acid production from brewer’s spent grain. Biochem. Eng. J. 2008, 40, 437–444. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.C.; Lopes, S.; Parajó, J.C.; Pereira, H.; Gírio, F.M. Evaluation of the detoxification of brewery’s spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Process Biochem. 2005, 40, 1215–1223. [Google Scholar] [CrossRef]
- Van Soest, P.J. Use of Detergents in the Analysis of Fibrous Feeds. II. A Rapid Method for the Determination of Fiber and Lignin. J. AOAC Int. 1963, 46, 829–835. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D. (Eds.) Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environmental Federation: Alexandria, VA, USA, 2017. [Google Scholar]
- Kjeldahl, J.G.C. En ny Methode til Kvaelstofvestemmelse i organiske Stoffer. Z. Anal. Chem. 1883, 22, 366–382. [Google Scholar] [CrossRef]
- Miller, G.L. Use of DinitrosaIicyIic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Plackett, R.L.; Burman, J.P. The Design of Optimum Multifactorial Experiments. Biometrika 1946, 33, 305–325. [Google Scholar] [CrossRef]
- Xiros, C.; Topakas, E.; Katapodis, P.; Christakopoulos, P. Hydrolysis and fermentation of brewer’s spent grain by Neurospora crassa. Bioresour. Technol. 2008, 99, 5427–5435. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Roberto, I.C. Chemical characterization and liberation of pentose sugars from brewer’s spent grain. J. Chem. Technol. Biotechnol. 2006, 81, 268–274. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Esteves, M.P.; Parajó, J.C.; Pereira, H.; Gírio, F.M. Production of oligosaccharides by autohydrolysis of brewery’s spent grain. Bioresour. Technol. 2004, 91, 93–100. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. A comparative analysis of pretreatment strategies on the properties and hydrolysis of brewers’ spent grain. Bioresour. Technol. 2018, 248, 272–279. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Borel, L.D.M.S.; Lira, T.S.; Ribeiro, J.A.; Ataíde, C.H.; Barrozo, M.A.S. Pyrolysis of brewer’s spent grain: Kinetic study and products identification. Ind. Crops Prod. 2018, 121, 388–395. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Acid hydrolysis and fermentation of brewer’s spent grain to produce xylitol. J. Sci. Food Agric. 2005, 85, 2453–2460. [Google Scholar] [CrossRef]
- Liu, Y.S.; Wu, J.Y.; Ho, K.P. Characterization of oxygen transfer conditions and their effects on Phaffia rhodozyma growth and carotenoid production in shake-flask cultures. Biochem. Eng. J. 2006, 27, 331–335. [Google Scholar] [CrossRef]
- Mantzouridou, F.; Roukas, T.; Achatz, B. Effect of oxygen transfer rate on β-carotene production from synthetic medium by Blakeslea trispora in shake flask culture. Enzyme Microb. Technol. 2005, 37, 687–694. [Google Scholar] [CrossRef]
- Prajapati, V.S.; Soni, N.; Trivedi, U.B.; Patel, K.C. An enhancement of red pigment production by submerged culture of Monascus purpureus MTCC 410 employing statistical methodology. Biocatal. Agric. Biotechnol. 2014, 3, 140–145. [Google Scholar] [CrossRef]
- Kang, B.; Zhang, X.; Wu, Z.; Qi, H.; Wang, Z. Effect of pH and nonionic surfactant on profile of intracellular and extracellular Monascus pigments. Process Biochem. 2013, 48, 759–767. [Google Scholar] [CrossRef]
- Orozco, S.F.B.; Kilikian, B.V. Effect of pH on citrinin and red pigments production by Monascus purpureus CCT3802. World J. Microbiol. Biotechnol. 2008, 24, 263–268. [Google Scholar] [CrossRef]
- Tucker, K.G.; Thomas, C.R. Inoculum effects on fungal morphology: Shake flasks vs agitated bioreactors. Biotechnol. Tech. 1994, 8, 153–156. [Google Scholar] [CrossRef]
- Hamano, P.S.; Kilikian, B.V. Production of red pigments by Monascus ruber in culture media containing corn steep liquor. Braz. J. Chem. Eng. 2006, 23, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Meinicke, R.M.; Vendruscolo, F.; Moritz, D.E.; de Oliveira, D.; Schmidell, W.; Samohyl, R.W.; Ninow, J.L. Potential use of glycerol as substrate for the production of red pigments by Monascus ruber in submerged fermentation. Biocatal. Agric. Biotechnol. 2012, 1, 238–242. [Google Scholar] [CrossRef]
- Bau, Y.S.; Wong, H.C. Zinc Effects on Growth, Pigmentation and Antibacterial Activity of Monascus purpureus. Physiol. Plant. 1979, 46, 63–67. [Google Scholar] [CrossRef]






| Code | Variable | Low Level (−) | High Level (+) | # | A | B | C | D | E | F | Pigment Production (UA500) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | MSG | 800 | 8000 | 1 | − | − | + | − | + | + | 21.76 |
| B | K2HPO4 | 500 | 5000 | 2 | + | − | + | + | − | + | 17.39 |
| C | KH2PO4 | 500 | 5000 | 3 | − | + | + | − | + | + | 21.25 |
| D | MgSO4·7H2O | 10 | 1 | 4 | + | + | − | − | − | + | 7.14 |
| E | CaCl2 | 10 | 1 | 5 | + | − | − | − | + | − | 12.94 |
| F | ZnSO4·7H2O | 10 | 1 | 6 | − | + | − | + | + | − | 5.71 |
| 7 | + | + | − | + | + | + | 13.94 | ||||
| 8 | + | − | + | + | + | − | 6.30 | ||||
| 9 | − | − | − | − | − | − | 15.62 | ||||
| 10 | − | − | − | + | − | + | 19.32 | ||||
| 11 | + | + | + | − | − | − | 4.58 | ||||
| 12 | − | + | + | + | − | − | 20.41 |
| Term | Effect | Coeff | t | p | % Contribution |
|---|---|---|---|---|---|
| Constant | 15.146 | 5.93 | 0.0000 | ||
| A) MSG | −6.96 | −9.67 | 14.38 | 0.0192 | 31.57 |
| B) K2HPO4 | −3.38 | −7.51 | 3.39 | 0.1395 | 7.44 |
| C) KH2PO4 | 2.84 | 6.3 × 10−4 | 2.38 | 0.1976 | 5.23 |
| D) MgSO4·7H2O | −3.5 × 10−2 | −3.89 × 10−3 | 3.63 × 10−4 | 0.9857 | 7.97 × 10−4 |
| E) CaCl2 | −0.43 | −0.04744 | 0.054 | 0.8276 | 0.12 |
| F) ZnSO4·7H2O | 5.87 | 0.6526 | 10.22 | 0.0330 | 22.45 |
| Compound | Amount (%) | Analysis/Method |
|---|---|---|
| HEMI | 53.09 | Van Soest [16] |
| CELL | 19.24 | |
| LIGN | 8.53 | |
| SOLU | 19.15 | |
| Total | 100 | |
| Ash | 3.68 | TS/VS [17] |
| TS | 94.53 | |
| VS | 90.05 | |
| Nitrogen | 2.76 | Kjeldahl [18] |
| Protein | 17.25 * | |
| Carbon | 81.63 ± 1.53 | XPS |
| Nitrogen | 2.00 ± 0.59 | |
| Oxygen | 15.99 ± 1.23 | |
| Phosphorus | 0.66 ± 0.08 |
| Element | Binding Energy (eV) | Functional Group |
|---|---|---|
| P2p | 134.1 | P*-O, Phosphate |
| C1s | 285.3 | C-C* |
| N1s | 400.11 | C-NH2 |
| O1s | 532.92 | C-O*-H |
| C1s | 284.99 | C-C* |
| C1s | 284.36 | C=C* |
| C1s | 286.09 | C-OH, C-O-C, C-N |
| C1s | 288.26 | C=O |
| N1s | 400.44 | C-NH2 |
| O1s | 532.4 | C-O*-H |
| O1s | 532.86 | C=O* |
| P2p | 133.25 | P*-O, Phosphate |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silbir, S.; Goksungur, Y. Natural Red Pigment Production by Monascus Purpureus in Submerged Fermentation Systems Using a Food Industry Waste: Brewer’s Spent Grain. Foods 2019, 8, 161. https://doi.org/10.3390/foods8050161
Silbir S, Goksungur Y. Natural Red Pigment Production by Monascus Purpureus in Submerged Fermentation Systems Using a Food Industry Waste: Brewer’s Spent Grain. Foods. 2019; 8(5):161. https://doi.org/10.3390/foods8050161
Chicago/Turabian StyleSilbir, Selim, and Yekta Goksungur. 2019. "Natural Red Pigment Production by Monascus Purpureus in Submerged Fermentation Systems Using a Food Industry Waste: Brewer’s Spent Grain" Foods 8, no. 5: 161. https://doi.org/10.3390/foods8050161
APA StyleSilbir, S., & Goksungur, Y. (2019). Natural Red Pigment Production by Monascus Purpureus in Submerged Fermentation Systems Using a Food Industry Waste: Brewer’s Spent Grain. Foods, 8(5), 161. https://doi.org/10.3390/foods8050161