Characterization of High-Ornithine-Producing Weissella koreensis DB1 Isolated from Kimchi and Its Application in Rice Bran Fermentation as a Starter Culture
Abstract
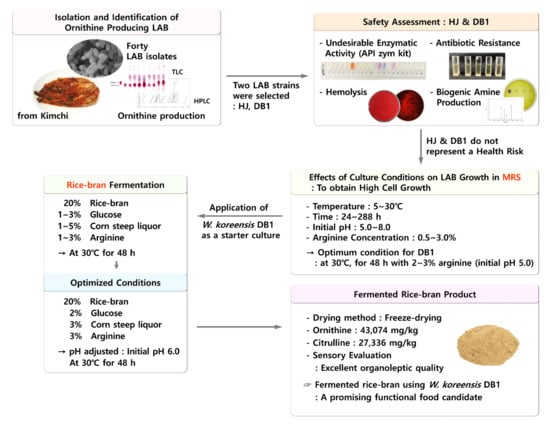
:1. Introduction
2. Materials and Methods
2.1. Kimchi Sampling and Isolation of LAB
2.2. Identification of Isolates
2.3. Thin Layer Chromatography (TLC) and High-Performance Liquid Chromatography (HPLC)
2.4. Effects of Culture Conditions on Growth
2.4.1. Temperature
2.4.2. pH
2.4.3. Arginine (Precursor) Concentrations
2.5. Safety Assessment
2.5.1. Harmful Enzyme Activities
2.5.2. Antibiotic Susceptibility and Hemolysis
2.5.3. Production of Biogenic Amines
2.6. Preparation of Starter Culture and Rice Bran Fermentation
2.7. Characterization of Fermented Rice Bran
2.7.1. pH and LAB Counts
2.7.2. Amino Acid Contents
2.7.3. Sensory Evaluation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of Ornithine-Producing LAB
3.2. Safety Assessment
3.2.1. Undesirable Enzymatic Activities
3.2.2. Antibiotic Resistance and Hemolysis
3.2.3. Biogenic Amine Production
3.3. Effects of Culture Conditions on LAB Growth
3.3.1. Effect of Temperature
3.3.2. Effect of pH
3.3.3. Effect of Arginine Concentration
3.4. Characteristics of the Fermented Rice Bran
3.4.1. LAB Growth and Ornithine Production
3.4.2. Sensory Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rakhimuzzaman, M.; Noda, M.; Danshiitsoodol, N.; Sugiyama, M. Development of a system of high ornithine and citrulline production by a plant-derived lactic acid bacterium, Weissella confusa K-28. Biol. Pharm. Bull. 2019, 42, 1581–1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, M.; Kirisako, T.; Kokubo, T.; Miura, Y.; Morishita, K.; Okamura, H.; Tsuda, A. Randomised controlled trial of the effects of L-ornithine on stress markers and sleep quality in healthy workers. Nutr. J. 2014, 13, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konishi, Y.; Koosaka, Y.; Maruyama, R.; Imanishi, K.; Kasahara, K.; Matsuda, A.; Akiduki, S.; Hishida, Y.; Kurata, Y.; Shibamoto, T.; et al. L-Ornithine intake affects sympathetic nerve outflows and reduces body weight and food intake in rats. Brain Res. Bull. 2015, 111, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Kurata, K.; Nagasawa, M.; Tomonaga, S.; Aoki, M.; Akiduki, S.; Morishita, K.; Denbow, D.M.; Furuse, M. Orally administered L-ornithine reduces restraint stress-induced activation of the hypothalamic-pituitary-adrenal axis in mice. Neurosci. Lett. 2012, 506, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.P.; Fishel, R.S.; Efron, D.T.; Williams, J.Z.; Fishel, M.H.; Barbul, A. Effect of supplemental ornithine on wound healing. J. Surg. Res. 2002, 106, 299–302. [Google Scholar] [CrossRef]
- Yu, J.J.; Oh, S.H. Isolation and characterization of lactic acid bacteria strains with ornithine producing capacity from natural sea salt. J. Microbiol. 2010, 48, 467–472. [Google Scholar] [CrossRef]
- Uchisawa, H.; Sato, A.; Ichita, J.; Matsue, H.; Ono, T. Influence of low-temperature processing of the brackish-water bivalve, Corbicula japonica, on the ornithine content of its extract. Biosci. Biotechnol. Biochem. 2004, 68, 1228–1234. [Google Scholar] [CrossRef] [Green Version]
- Sugino, T.; Shirai, T.; Kajimoto, Y.; Kajimoto, O. L-ornithine supplementation attenuates physical fatigue in healthy volunteers by modulating lipid and amino acid metabolism. Nutr. Res. 2008, 28, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.A.; Chang, H.C. Cholesterol-lowering effects of a putative probiotic strain Lactobacillus plantarum EM isolated from kimchi. LWT Food. Sci. Technol. 2015, 62, 210–217. [Google Scholar] [CrossRef]
- Ryu, E.H.; Chang, H.C. In vitro study of potentially probiotic lactic acid bacteria strains isolated from kimchi. Ann. Microbiol. 2013, 63, 1387–1395. [Google Scholar] [CrossRef]
- Ryu, E.H.; Yang, E.J.; Woo, E.R.; Chang, H.C. Purification and characterization of antifungal compounds from Lactobacillus plantarum HD1 isolated from kimchi. Food Microbiol. 2014, 41, 19–26. [Google Scholar] [CrossRef]
- Jo, S.Y.; Choi, E.A.; Lee, J.J.; Chang, H.C. Characterization of starter kimchi fermented with Leuconostoc kimchii GJ2 and its cholesterol‐lowering effects in rats fed a high‐fat and high‐cholesterol diet. J. Sci. Food Agrc. 2015, 95, 2750–2756. [Google Scholar] [CrossRef]
- Lee, S.H.; Chang, H.C. Isolation of antifungal activity of Leuconostoc mesenteroides TA from kimchi and characterization of its antifungal compounds. Food Sci. Biotechnol. 2016, 25, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.B.; Lee, J.J.; Chang, H.C. Characterization of juice fermented with Lactobacillus plantarum EM and its cholesterol‐lowering effects on rats fed a high‐fat and high‐cholesterol diet. Food Sci. Nutr. 2019, 7, 3622–3634. [Google Scholar] [CrossRef] [Green Version]
- Mun, S.Y.; Chang, H.C. Characterization of Weissella koreensis SK isolated from kimchi fermented at low temperature (around 0 °C) based on complete genome sequence and corresponding phenotype. Microorganisms 2020, 8, 1147. [Google Scholar] [CrossRef]
- Arena, M.E.; Saguir, F.M.; De Nadra, M.M. Arginine, citrulline and ornithine metabolism by lactic acid bacteria from wine. Int. J. Food Microbiol. 1999, 52, 155–161. [Google Scholar] [CrossRef]
- Hwang, H.; Lee, J.H. Characterization of arginine catabolism by lactic acid bacteria isolated from kimchi. Molecules 2018, 23, 3049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, S.H.; Kim, C.R.; Lee, P.W.; Chang, H.C. Study on the establishment of standard for mukeunji product through characteristic analysis of commercial mukeunji products. Korean J. Community Living Sci. 2019, 30, 33–51. [Google Scholar] [CrossRef]
- Lee, H.H.; Kim, G.H. Changes in the levels of γ-aminobutyric acid and free amino acids during kimchi fermentation. Korean J. Food Cook. Sci. 2013, 29, 671–677. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.J.; Chang, H.C. Antifungal activity of Lactobacillus plantarum isolated from kimchi. Microbiol. Biotechnol. Lett. 2008, 36, 276–284. [Google Scholar]
- Padonou, S.W.; Schillinger, U.; Nielsen, D.S.; Franz, C.M.; Hansen, M.; Hounhouigan, J.D.; Nago, M.C.; Jakobsen, M. Weissella beninensis sp. nov., a motile lactic acid bacterium from submerged cassava fermentations, and emended description of the genus Weissella. Int. J. Syst. Evol. Microbiol. 2010, 60, 2193–2198. [Google Scholar] [CrossRef]
- Kang, E.S.; Ford, K.; Grokulsky, G.; Wang, Y.B.; Chiang, T.M.; Acchiardo, S.R. Normal circulating adult human red blood cells contain inactive NOS proteins. J. Lab. Clin. Med. 2000, 135, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.R.; Chang, J.Y.; Chang, H.C. Production of gamma-aminobutyric acid (GABA) by Lactobacillus buchneri isolated from kimchi and its neuroprotective effect on neuronal cells. J. Microbiol. Biotechnol. 2007, 17, 104–109. [Google Scholar] [PubMed]
- Henderson, J.W.; Ricker, R.D.; Bidlingmeyer, B.A.; Woodward, C. Rapid, accurate, sensitive, and reproducible HPLC analysis of amino acids. Agil. Appl. Note 2000, 1100, 1–10. [Google Scholar]
- Moon, S.H.; Mun, S.Y.; Chang, H.C. Characterization of fermented rice-bran using the lactic acid bacteria Weissella koreensis DB1 derived from kimchi. Korean J. Community Living Sci. 2019, 30, 543–551. [Google Scholar] [CrossRef]
- Moon, S.H.; Kim, C.R.; Chang, H.C. Heterofermentative lactic acid bacteria as a starter culture to control kimchi fermentation. LWT Food Sci. Technol. 2018, 88, 181–188. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- Jeong, D.W.; Lee, J.H. Antibiotic resistance, hemolysis and biogenic amine production assessments of Leuconostoc and Weissella isolates for kimchi starter development. LWT Food. Sci. Technol. 2015, 64, 1078–1084. [Google Scholar] [CrossRef]
- Özdestan, Ö.; Üren, A. A method for benzoyl chloride derivatization of biogenic amines for high performance liquid chromatography. Talanta 2009, 78, 1321–1326. [Google Scholar] [CrossRef]
- Chang, M.; Chang, H.C. Development of a screening method for biogenic amine producing Bacillus spp. Int. J. Food Microbiol. 2012, 153, 269–274. [Google Scholar] [CrossRef]
- Liong, M.T. Safety of probiotics: Translocation and infection. Nut. Rev. 2008, 66, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Moon, S.H.; Mun, S.Y.; Lee, J.J. Method for Preparing Fermented Rice Bran by Using Weissella koreensis and Fermented Rice Bran Prepared Thereby. K. R. Patent 10-2019-0131979, 23 October 2019. [Google Scholar]
- European Food Safety Authority (EFSA). Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 10: Suitability of taxonomic units notified to EFSA until March 2019. EFSA J. 2019, 17, 5753. [Google Scholar]
- Collins, M.D.; Samelis, J.; Metaxopoulos, J.; Wallbanks, S. Taxonomic studies on some leuconostoc‐like organisms from fermented sausages: Description of a new genus Weissella for the Leuconostoc paramesenteroides group of species. J. Appl. Bacteriol. 1993, 75, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Anandharaj, M.; Sivasankari, B.; Santhanakaruppu, R.; Manimaran, M.; Rani, R.P.; Sivakumar, S. Determining the probiotic potential of cholesterol-reducing Lactobacillus and Weissella strains isolated from gherkins (fermented cucumber) and south Indian fermented koozh. Res. Microbiol. 2015, 166, 428–439. [Google Scholar] [CrossRef]
- Choi, A.R.; Patra, J.K.; Kim, W.J.; Kang, S.S. Antagonistic activities and probiotic potential of lactic acid bacteria derived from a plant-based fermented food. Front. Microbiol. 2018, 9, 1963. [Google Scholar] [CrossRef]
- Abriouel, H.; Lerma, L.L.; Casado Muñoz, M.D.C.; Montoro, B.P.; Kabisch, J.; Pichner, R.; Cho, G.S.; Neve, H.; Fusco, V.; Franz, C.M.A.P.; et al. The controversial nature of the Weissella genus: Technological and functional aspects versus whole genome analysis-based pathogenic potential for their application in food and health. Front. Microbiol. 2015, 6, 1197. [Google Scholar] [CrossRef]
- Benkerroum, N. Biogenic amines in dairy products: Origin, incidence, and control means. Compr. Rev. Food Sci. Food Saf. 2016, 15, 801–826. [Google Scholar] [CrossRef]
- Barbieri, F.; Montanari, C.; Gardini, F.; Tabanelli, G. Biogenic amine production by lactic acid bacteria: A review. Foods 2019, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Noens, E.E.; Lolkema, J.S. Convergent evolution of the arginine deiminase pathway: The ArcD and ArcE arginine/ornithine exchangers. MicrobiologyOpen 2017, 6, e00412. [Google Scholar] [CrossRef]
- Schillinger, U.; Holzapfel, W.H.; Björkroth, K.J. 20-Lactic acid bacteria. In Food Spoilage Microorganisms; De W Blackburn, C., Ed.; Woodhead Publishing: Cambridge, UK, 2006; pp. 541–578. [Google Scholar]
- Papadia, C.; Osowska, S.; Cynober, L.; Forbes, A. Citrulline in health and disease. Review on human studies. Clin. Nutr. 2018, 37, 1823–1828. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, A.; Wong, A.; Jaime, S.J.; Gonzales, J.U. Influence of L-citrulline and watermelon supplementation on vascular function and exercise performance. Curr. Opin. Clin. Nutr. 2017, 20, 92–98. [Google Scholar] [CrossRef]
- Aktaş, K.; Akın, N. Influence of rice bran and corn bran addition on the selected properties of tarhana, a fermented cereal based food product. LWT Food. Sci. Technol. 2020, 129, 109574. [Google Scholar] [CrossRef]
- Kato, H.; Rhue, M.R.; Nishimura, T. Role of Free Amino Acids and Peptides in Food Taste. In Flavor Chemistry Trends and Developments; Teranishi, R., Buterry, R.G., Shahidi, F., Eds.; American Chemical Society: Washington, DC, USA, 1989; Volume 388, pp. 158–175. [Google Scholar]
- Moongngarm, A.; Daomukda, N.; Khumpika, S. Chemical compositions, phytochemicals, and antioxidant capacity of rice bran, rice bran layer, and rice germ. Apcbee Procedia 2012, 2, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Demirci, T.; Aktaş, K.; Sözeri, D.; Öztürk, H.İ.; Akın, N. Rice bran improve probiotic viability in yoghurt and provide added antioxidative benefits. J. Funct. Foods. 2017, 36, 396–403. [Google Scholar] [CrossRef]
- Kurdi, P.; Hansawasdi, C. Assessment of the prebiotic potential of oligosaccharide mixtures from rice bran and cassava pulp. LWT Food. Sci. Technol. 2015, 63, 1288–1293. [Google Scholar] [CrossRef]




| Amino Acid (mg/L) | Weissella koreensis | ||||
|---|---|---|---|---|---|
| GL | DB1 | CM | CGM1 | HJ | |
| Arginine | 93.35 ± 0.92 b | 55.46 ± 2.07 c | 248.98 ± 26.13 a | 102.03 ± 1.02 b | 60.69 ± 0.71 c |
| Ornithine | 4963.02 ± 23.18 c | 6581.64 ± 115.09 a | 3904.86 ± 101.73 d | 4922.22 ± 86.31 c | 5975.50 ± 126.94 b |
| Enzyme (nmol) | Activity of W. koreensis | |
|---|---|---|
| DB1 | HJ | |
| Alkaline phosphate | 0 | 0 |
| Esterase (C4) | 0 | 0 |
| Esterase lipase (C8) | 0 | 0 |
| Lipase (C14) | 0 | 0 |
| Leucine arylamidase | 0 | 10 |
| Valine arylamidase | 0 | 0 |
| Cystine arylamidase | 0 | 0 |
| Trypsin | 0 | 0 |
| α-Chymotrypsin | 0 | 0 |
| Acid phosphatase | 0 | 5 |
| Naphthol-AS-BI-phosphohydrolase | 5 | 5 |
| A-Galactosidase | 0 | 0 |
| β-Galactosidase | 10 | 0 |
| β-Glucuronidase | 0 | 0 |
| α-Glucosidase | 0 | 0 |
| β-Glucosidase | 0 | 0 |
| N-Acetyl-β-glucosaminidase | 0 | 0 |
| α-Mannosidase | 0 | 0 |
| α-Fucosidase | 0 | 0 |
| Antibiotics (μg/mL) | Breakpoints for Leuconostocs 1 | MICs for Other Weissella koreensis 2 | W. koreensis | |
|---|---|---|---|---|
| DB1 | HJ | |||
| Ampicillin | 2 | 1~>10 | 0.5 | 1 |
| Vancomycin | N.R 3 | 1024 | 512 | 512 |
| Gentamycin | 16 | >10–32 | 2 | 4 |
| Kanamycin | 16 | 30–512 | 8 | 8 |
| Streptomycin | 64 | 128 | 8 | 4 |
| Erythromycin | 1 | 1~>15 | 0.06 | 0.25 |
| Tetracycline | 8 | 1 | 0.5 | 0.5 |
| Chloramphenicol | 4 | 4~>30 | 4 | 4 |
| Fermented Rice Bran | Characteristics | Fermentation time | |
|---|---|---|---|
| 0 h | 48 h | ||
| A | pH | 6.00 ± 0.01 a | 5.54 ± 0.20 b |
| Viable cells (log CFU/mL) | 6.07 ± 0.12 b | 8.61 ± 0.09 a | |
| Arginine (mg/kg) | 27,986.04 ± 279.01 a | 169.33 ± 82.95 b | |
| Citrulline (mg/kg) | 74.10 ± 9.10 b | 5444.79 ± 364.49 a | |
| Ornithine (mg/kg) | 17.10 ± 2.10 b | 19,972.33 ± 1255.01 a | |
| Lactic acid (mg/kg) | 16,253.65 ± 477.16 b | 58,743.83 ± 673.83 a | |
| Acetic acid (mg/kg) | 1723.24 ± 50.59 b | 2209.00 ± 25.34 a | |
| B | pH | 6.00 ± 0.01 b | 6.37 ± 0.02 a |
| Viable cells (log CFU/mL) | 6.06 ± 0.06 b | 8.72 ± 0.15 a | |
| Arginine (mg/kg) | 64,950.08 ± 425.02 a | 256.26 ± 25.79 b | |
| Citrulline (mg/kg) | 127.23 ± 6.90 b | 16,113.25 ± 2321.96 a | |
| Ornithine (mg/kg) | 68.02 ± 7.03 b | 32,747.80 ± 1421.15 a | |
| Lactic acid (mg/kg) | 16,253.65 ± 477.16 b | 52,493.50 ± 1100.15 a | |
| Acetic acid (mg/kg) | 1723.24 ± 50.59 b | 3736.31 ± 1113.09 a | |
| C | pH | 6.00 ± 0.00 b | 7.16 ± 0.10 a |
| Viable cells (log CFU/mL) | 6.07 ± 0.09 b | 8.65 ± 0.10 a | |
| Arginine (mg/kg) | 91,813.04 ± 608.01 a | 464.14 ± 33.94 b | |
| Citrulline (mg/kg) | 188.07 ± 8.05 b | 27,336.37 ± 4870.86 a | |
| Ornithine (mg/kg) | 442.10 ± 55.06 b | 43,074.13 ± 2457.05 a | |
| Lactic acid (mg/kg) | 16,253.65 ± 477.16 b | 45,380.99 ± 1258.69 a | |
| Acetic acid (mg/kg) | 1723.24 ± 50.59 b | 4687.37 ± 183.08 a | |
| Items | RRB | SRB | FRB |
|---|---|---|---|
| Bitterness | 3.8 ± 0.8 a | 1.8 ± 0.4 b | 3.5 ± 0.8 a |
| Saltiness | 1.9 ± 0.7 b | 3.5 ± 0.8 a | 3.8 ± 0.4 a |
| Savory flavor | 2.3 ± 0.9 c | 3.3 ± 0.6 b | 4.5 ± 0.5 a |
| Hay smell | 1.5 ± 0.5 c | 3.6 ± 0.5 b | 4.2 ± 0.6 a |
| Mouthfeel texture | 1.7 ± 0.5 c | 3.7 ± 0.8 b | 4.3 ± 0.5 a |
| Overall acceptability | 1.7 ± 0.5 c | 2.8 ± 0.8 b | 4.4 ± 0.5 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeong, M.S.; Hee, M.S.; Choon, C.H. Characterization of High-Ornithine-Producing Weissella koreensis DB1 Isolated from Kimchi and Its Application in Rice Bran Fermentation as a Starter Culture. Foods 2020, 9, 1545. https://doi.org/10.3390/foods9111545
Yeong MS, Hee MS, Choon CH. Characterization of High-Ornithine-Producing Weissella koreensis DB1 Isolated from Kimchi and Its Application in Rice Bran Fermentation as a Starter Culture. Foods. 2020; 9(11):1545. https://doi.org/10.3390/foods9111545
Chicago/Turabian StyleYeong, Mun So, Moon Song Hee, and Chang Hae Choon. 2020. "Characterization of High-Ornithine-Producing Weissella koreensis DB1 Isolated from Kimchi and Its Application in Rice Bran Fermentation as a Starter Culture" Foods 9, no. 11: 1545. https://doi.org/10.3390/foods9111545
APA StyleYeong, M. S., Hee, M. S., & Choon, C. H. (2020). Characterization of High-Ornithine-Producing Weissella koreensis DB1 Isolated from Kimchi and Its Application in Rice Bran Fermentation as a Starter Culture. Foods, 9(11), 1545. https://doi.org/10.3390/foods9111545