Synthesis of Magnetic Metal-Organic Frame Material and Its Application in Food Sample Preparation
Abstract
:1. Introduction
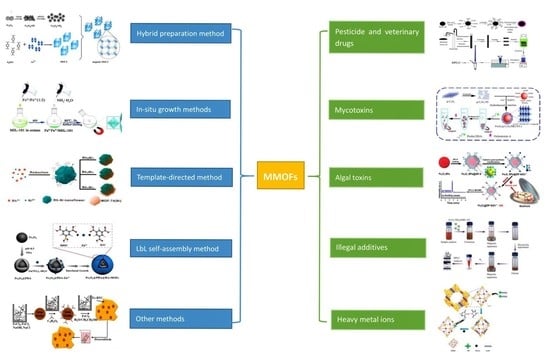
2. Synthesis Strategies of MMOFs Composite
2.1. Hybrid Preparation Method
2.2. In-Situ Growth Method
2.2.1. In-Situ Growth of MOFs on MNPs
2.2.2. In-Situ Growth of MNPs on MOFs
2.3. Template-Directed Method
2.3.1. Self-Sacrificial Template Method
2.3.2. Emulsion Template Method
2.4. Layer by Layer Self-Assembly Method
2.5. Other Strategies for MMOFs Synthesis
3. Applications of Magnetic MOFs for Food Contaminants Extraction
3.1. Residues of Pesticide and Veterinary Drugs
3.2. Toxins
3.2.1. Mycotoxins
3.2.2. Algal Toxins
3.3. Illegal Additives
3.4. Heavy Metal Ions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kantiani, L.; Llorca, M.; Sanchis, J.; Farre, M.; Barcelo, D. Emerging food contaminants: A review. Anal. Bioanal. Chem. 2010, 398, 2413–2427. [Google Scholar] [PubMed]
- Alexander, J.; Barlow, S.; Benford, D.; Bolger, M.; Cantrill, R.; Cressey, P.; De Nijs, M.; Edwards, S.; Feeley, M.; Mueller, U.; et al. Evaluation of certain food additives and contaminants. WHO Tech. Rep. Ser. 2017, 1002, 1–166. [Google Scholar]
- Callen, C.; Bhatia, J.; Czerkies, L.; Klish, W.J.; Gray, G.M. Challenges and considerations when balancing the risks of contaminants with the benefits of fruits and vegetables for infants and toddlers. Nutrients 2018, 10, 1572. [Google Scholar]
- Rotariu, L.; Lagarde, F.; Jaffrezic-Renault, N.; Bala, C. Electrochemical biosensors for fast detection of food contaminants trends and perspective. Trends Anal. Chem. 2016, 79, 80–87. [Google Scholar]
- Raeisossadati, M.J.; Danesh, N.M.; Borna, F.; Gholamzad, M.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Lateral flow based immunobiosensors for detection of food contaminants. Biosens. Bioelectron. 2016, 86, 235–246. [Google Scholar]
- Frenich, A.G.; Romero-Gonzalez, R.; Aguilera-Luiz, M.D. Comprehensive analysis of toxics (pesticides, veterinary drugs and mycotoxins) in food by UHPLC-MS. Trends Anal. Chem. 2014, 63, 158–169. [Google Scholar]
- Justino, C.I.L.; Duarte, K.R.; Freitas, A.C.; Panteleitchouk, T.S.L.; Duarte, A.C.; Rocha-Santos, T.A.P. Contaminants in aquaculture: Overview of analytical techniques for their determination. Trends Anal. Chem. 2016, 80, 293–310. [Google Scholar]
- Li, D.A.; Lv, D.Y.; Zhu, Q.X.; Li, H.; Chen, H.; Wu, M.M.; Chai, Y.F.; Lu, F. Chromatographic separation and detection of contaminants from whole milk powder using a chitosan-modified silver nanoparticles surface-enhanced Raman scattering device. Food Chem. 2017, 224, 382–389. [Google Scholar] [PubMed]
- Chiou, J.C.; Leung, A.H.H.; Lee, H.W.; Wong, W.T. Rapid testing methods for food contaminants and toxicants. J. Integr. Agric. 2015, 14, 2243–2264. [Google Scholar]
- Huang, X.L.; Aguilar, Z.P.; Xu, H.Y.; Lai, W.H.; Xiong, Y.H. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosens. Bioelectron. 2016, 75, 166–180. [Google Scholar]
- Xu, M.L.; Gao, Y.; Han, X.X.; Zhao, B. Detection of pesticide residues in food using surface-enhanced Raman spectroscopy: A review. J. Agric. Food Chem. 2017, 65, 6719–6726. [Google Scholar] [PubMed]
- Naing, N.N.; Lee, H.K. Microextraction and analysis of contaminants adsorbed on atmospheric fine particulate matter: A review. J. Chromatogr. A 2020, 1627, 461433. [Google Scholar] [PubMed]
- Zhang, Y.Y.; Li, G.L.; Wu, D.; Li, X.T.; Yu, Y.X.; Luo, P.J.; Chen, J.; Dai, C.J.; Wu, Y.N. Recent advances in emerging nanomaterials based food sample pretreatment methods for food safety screening. Trends Anal. Chem. 2019, 121, 115669. [Google Scholar]
- Burato, J.S.D.; Medina, D.A.V.; de Toffoli, A.L.; Maciel, E.V.S.; Lancas, F.M. Recent advances and trends in miniaturized sample preparation techniques. J. Sep. Sci. 2020, 43, 202–225. [Google Scholar]
- Xin, J.H.; Wang, X.; Li, N.; Liu, L.; Lian, Y.J.; Wang, M.L.; Zhao, R.S. Recent applications of covalent organic frameworks and their multifunctional composites for food contaminant analysis. Food Chem. 2020, 330, 127255. [Google Scholar]
- Li, G.Q.; Ma, G.H. Recent applications of solid-phase extraction techniques for analysis of trace residues and contaminants in food. Chin. J. Chromatogr. 2011, 29, 606–612. [Google Scholar]
- Li, Z.; Yu, B.; Cong, H.L.; Yuan, H.; Peng, Q.H. Recent development and application of solid phase extraction materials. Rev. Adv. Mater. Sci. 2017, 49, 87–111. [Google Scholar]
- Mashile, G.P.; Nomngongo, P.N. Recent application of solid phase based techniques for extraction and preconcentration of cyanotoxins in environmental matrices. Crit. Rev. Anal. Chem. 2017, 47, 119–126. [Google Scholar]
- Farajzadeh, M.A.; Dabbagh, M.S. Development of a dispersive solid phase extraction method based on in situ formation of adsorbent followed by dispersive liquid-liquid microextraction for extraction of some pesticide residues in fruit juice samples. J. Chromatogr. A 2020, 1627, 461398. [Google Scholar]
- Yu, X.; Yu, L.; Ma, F.; Li, P.W. Quantification of phenolic compounds in vegetable oils by mixed-mode solid-phase extraction isotope chemical labeling coupled with UHPLC-MS/MS. Food Chem. 2021, 334, 127572. [Google Scholar]
- Fumes, B.H.; Silva, M.R.; Andrade, F.N.; Nazario, C.E.D.; Lanças, F.M. Recent advances and future trends in new materials for sample preparation. Trends Anal. Chem. 2015, 71, 9–25. [Google Scholar]
- Maciel, E.V.S.; Toffoli, A.L.; Neto, E.S.; Nazario, C.E.D.; Lancas, F.M. New materials in sample preparation: Recent advances and future trends. Trends Anal. Chem. 2019, 119, 115633. [Google Scholar]
- Chen, Q.L.; Zhu, L.; Chen, J.X.; Jiang, T.; Ye, H.Z.; Ji, H.; Tsang, S.W.; Zhao, Z.Z.; Yi, T.; Chen, H.B. Recent progress in nanomaterial-based assay for the detection of phytotoxins in foods. Food Chem. 2019, 277, 162. [Google Scholar]
- Herrero-Latorre, C.; Barciela-Garcia, J.; Garcia-Martin, S.; Pena-Crecente, R.M.; Otarola-Jimenez, J. Magnetic solid-phase extraction using carbon nanotubes as sorbents: A review. Anal. Chim. Acta 2015, 892, 10–26. [Google Scholar]
- He, M.; Huang, L.J.; Zhao, B.S.; Chen, B.B.; Hu, B. Advanced functional materials in solid phase extraction for ICP-MS determination of trace elements and their species—A review. Anal. Chim. Acta 2017, 973, 1–24. [Google Scholar]
- Er, E.O.; Bozyigit, G.D.; Buyukpinar, C.; Bakirdere, S. Magnetic nanoparticles based solid phase extraction methods for the determination of trace elements. Crit. Rev. Anal. Chem. 2020, 18, 1–18. [Google Scholar]
- Capriotti, A.L.; Cavaliere, C.; Foglia, P.; La Barbera, G.; Samperi, R.; Ventura, S.; Lagana, A. Mycoestrogen determination in cow milk: Magnetic solid-phase extraction followed by liquid chromatography and tandem mass spectrometry analysis. J. Sep. Sci. 2016, 39, 4794–4804. [Google Scholar]
- Patino-Ropero, M.J.; Diaz-Alvarez, M.; Martin-Esteban, A. Molecularly imprinted core-shell magnetic nanoparticles for selective extraction of triazines in soils. J. Mol. Recognit. 2017, 30, e2593. [Google Scholar]
- Mohamed, F.A.; Khashaba, P.Y.; El-Wekil, M.M.; Shahin, R.Y. Fabrication of water compatible and biodegradable super-paramagnetic molecularly imprinted nanoparticles for selective separation of memantine from human serum prior to its quantification: An efficient and green pathway. Int. J. Biol. Macromol. 2019, 140, 140–148. [Google Scholar]
- Wierucka, M.; Biziuk, M. Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. Trends Anal. Chem. 2014, 59, 50–58. [Google Scholar]
- Rais, S.; Islam, A.; Ahmad, I.; Kumar, S.; Chauhan, A.; Javed, H. Preparation of a new magnetic ion-imprinted polymer and optimization using Box-Behnken design for selective removal and determination of Cu (II) in food and wastewater samples. Food Chem. 2021, 334, 127563. [Google Scholar]
- Pan, L.; Liu, G.; Li, H.; Meng, S.; Han, L.; Shang, J.; Chen, B.; Platero-Prats, A.E.; Lu, W.; Zou, X.D.; et al. A resistance-switchable and ferroelectric metal-organic framework. J. Am. Chem. Soc. 2014, 136, 17477–17483. [Google Scholar]
- Qu, B.T.; Lai, J.C.; Liu, S.; Liu, F.; Gao, Y.D.; You, X.Z. Cu- and Ag-based metal-organic frameworks with 4-Pyranone-2,6-dicarboxylic acid: Syntheses, crystal structures, and dielectric properties. Cryst. Growth Des. 2015, 15, 1707–1713. [Google Scholar]
- Wang, C.H.; Liu, X.L.; Demir, N.K.; Chen, J.P.; Li, K. Applications of water stable metal–organic frameworks. Chem. Soc. Rev. 2016, 45, 5107–5134. [Google Scholar]
- Zhang, M.W.; Chen, Y.P.; Bosch, M.; Gentle, T.; Wang, K.C.; Feng, D.W.; Wang, Z.Y.U.; Zhou, H.C. Symmetry-guided synthesis of highly porous metal-organic frameworks with fluorite topology. Angew. Chem. 2014, 126, 834–837. [Google Scholar]
- Banerjee, D.; Cairns, A.J.; Liu, J.; Motkuri, R.K.; Nune, S.K.; Fernandez, C.A.; Krishna, R.; Strachan, D.M.; Thallapally, P.K. Potential of metal-organic frameworks for separation of Xenon and Krypton. Acc. Chem. Res. 2015, 48, 211–219. [Google Scholar]
- Xu, Y.X.; Li, Q.; Xue, H.G.; Pang, H. Metal-organic frameworks for direct electrochemical applications. Coordin. Chem. Rev. 2018, 376, 292–318. [Google Scholar]
- Liu, C.S.; Li, J.J.; Pang, H. Metal-organic framework-based materials as an emerging platform for advanced electrochemical sensing. Coordin. Chem. Rev. 2020, 213222. [Google Scholar] [CrossRef]
- Seo, J.S.; Whang, D.; Lee, H.; Jun, S.I.; Oh, J.; Jeon, Y.J.; Kim, K. A homochiral metal-organic porous material for enantioselective separation and catalysis. Nature 2000, 404, 982–986. [Google Scholar]
- Horcajada, P.; Chalati, T.; Serre, C.; Gillet, B.; Sebrie, C.; Baati, T.; Eubank, J.F.; Heurtaux, D.; Clayette, P.; Kreuz, C.; et al. Porous metal-organic-framework nanoscale carriers as a potential platform for drug delivery and imaging. Nat. Mater. 2010, 2, 172–178. [Google Scholar]
- Ghaemi, F.; Amiri, A. Microcrystalline cellulose/metal-organic framework hybrid as a sorbent for dispersive micro-solid phase extraction of chlorophenols in water samples. J. Chromatog. A 2020, 1626, 461386. [Google Scholar]
- Giliopoulos, D.; Zamboulis, A.; Giannakoudakis, D.; Bikiaris, D.; Triantafyllidis, K. Polymer/metal organic framework (MOF) nanocomposites for biomedical applications. Molecules 2020, 25, 185. [Google Scholar] [CrossRef] [Green Version]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar]
- Gu, Z.Y.; Yang, C.X.; Chang, N.; Yan, X.P. Metal-organic frameworks for analytical chemistry: From sample collection to chromatographic separation. Acc. Chem. Res. 2012, 45, 734–745. [Google Scholar]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar]
- Zhu, Q.L.; Xu, Q. Metal–organic framework composites. Chem. Soc. Rev. 2014, 43, 5468–5512. [Google Scholar]
- Wang, R.Y.; Zhang, C.Y.; Wang, S.P.; Zhou, Y.Y. Synthesis and application of magnetic metal-organic frameworks. Prog. Chem. 2015, 27, 945–952. [Google Scholar]
- Hashemi, B.; Zohrabi, P.; Raza, N.; Kim, K.H. Metal organic frameworks as advanced sorbents for the extraction and determination of pollutants from environmental, biological, and food media. Trends Anal. Chem. 2017, 97, 65–82. [Google Scholar]
- Wang, D.; Zeng, F.; Hu, X.; Li, C.; Su, Z. Synthesis of a magnetic 2D Co@NC-600 material by designing a MOF precursor for efficient catalytic reduction of water pollutants. Inorg. Chem. 2020, 59, 12672–12680. [Google Scholar]
- Mohamed, A.K.; Mahmoud, M.E. Encapsulation of starch hydrogel and doping nanomagnetite onto metal -organic frameworks for efficient removal of fluvastatin antibiotic from water. Carbohyd. Polym. 2020, 245, 116438. [Google Scholar]
- Huang, X.D.; Liu, Y.N.; Liu, H.F.; Liu, G.Y.; Xu, X.M.; Li, L.Y.; Lv, J.; Gao, H.X.; Xu, D.H. Magnetic solid-phase extraction of pyrethroid insecticides from tea infusions using ionic liquid-modified magnetic zeolitic imidazolate framework-8 as an adsorbent. RES Adv. 2019, 9, 39272–39281. [Google Scholar]
- Han, Q.; Sun, Y.; Ding, K.; Chen, X.; Han, T. Preparation of multitarget immunomagnetic beads based on metal-organic frameworks and their application in food samples. J. Chromatogr. B 2020, 1158, 122341. [Google Scholar]
- Li, S.Q.; Zhang, X.D.; Huang, Y.M. Zeolitic imidazolate framework-8 derived nanoporous carbon as an effective and recyclable adsorbent for removal of ciprofloxacin antibiotics from water. J. Hazard. Mater. 2017, 321, 711–719. [Google Scholar] [PubMed]
- Liang, C.H.; Zhang, X.D.; Feng, P.; Chai, H.X.; Huang, Y.M. ZIF-67 derived hollow cobalt sulfide as superior adsorbent for effective adsorption removal of ciprofloxacin antibiotics. Chem. Eng. J. 2018, 344, 95–104. [Google Scholar]
- Maya, F.; Cabello, C.P.; Frizzarin, R.M.; Estela, J.M.; Palomino, G.T.; Cerda, V. Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. Trends Anal. Chem. 2017, 90, 142–152. [Google Scholar]
- Jiang, H.L.; Li, N.; Cui, L.; Wang, X.; Zhao, R.S. Recent application of magnetic solid phase extraction for food safety analysis. Trends Anal. Chem. 2019, 120, 115632. [Google Scholar]
- Gao, Y.H.; Liu, G.; Gao, M.K.; Huang, X.D.; Xu, D.H. Recent advances and applications of magnetic metal-organic frameworks in adsorption and enrichment removal of food and environmental pollutants. Crit. Rev. Anal. Chem. 2020, 50, 472–484. [Google Scholar]
- Pan, S.D.; Chen, X.H.; Li, X.H.; Jin, M.C. Nonderivatization method for determination of glyphosate, glufosinate, bialaphos, and their main metabolites in environmental waters based on magnetic metal-organic framework pretreatment. J. Sep. Sci. 2019, 42, 1045–1050. [Google Scholar]
- Aghayi-Anaraki, M.; Safarifard, V. Fe3O4@MOF magnetic nanocomposites: Synthesis and applications. Eur. J. Inorg. Chem. 2020, 20, 1916–1937. [Google Scholar]
- He, K.; Tsui, C.; Yeung, K. Flow synthesis of magnetic metal-organic frameworks. Abstr. Pap. Am. Chem. Soc. 2015, 250, 174. [Google Scholar]
- Li, H.Q.; Sadiq, M.M.; Suzuki, K.; Falcaro, P.; Hill, A.J.; Hill, M.R. Magnetic induction framework synthesis: A general route to the controlled growth of metal-organic frameworks. Chem. Mater. 2017, 29, 6186–6190. [Google Scholar]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 43, 933–969. [Google Scholar]
- Ma, Y.J.; Jiang, X.X.; Lv, Y.K. Recent advances in preparation and applications of magnetic framework composites. Chem. Asian J. 2019, 14, 3515–3530. [Google Scholar] [PubMed]
- Abazari, R.; Mahjoub, A.R.; Molaie, S.; Ghaffarifar, F.; Ghasemi, E.; Slawin, A.M.Z.; Carpenter-Warren, C.L. The effect of different parameters under ultrasound irradiation for synthesis of new nanostructured Fe3O4@bio-MOF as an efficient anti-leishmanial in vitro and in vivo conditions. Ultrason. Sonochem. 2018, 43, 248–261. [Google Scholar]
- Du, F.Y.; Qin, Q.; Deng, J.C.; Ruan, G.H.; Yang, X.Q.; Li, L.H.; Li, J.P. Magnetic metal-organic framework MIL-100(Fe) microspheres for the magnetic solid-phase extraction of trace polycyclic aromatic hydrocarbons from water samples. J. Sep. Sci. 2016, 39, 2356–2364. [Google Scholar]
- Zhang, S.L.; Yao, W.X.; Ying, J.B.; Zhao, H.T. Polydopamine-reinforced magnetization of zeolitic imidazolate framework ZIF-7 for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from the air-water environment. J. Chromatogr. A 2016, 1452, 18–26. [Google Scholar] [PubMed]
- Jiang, Z.W.; Dai, F.Q.; Huang, C.Z.; Li, Y.F. Facile synthesis of a Fe3O4/MIL-101(Fe) composite with enhanced catalytic performance. RSC Adv. 2016, 6, 86443–86446. [Google Scholar]
- Hu, Y.L.; Huang, Z.L.; Liao, J.; Li, G.K. Chemical bonding approach for fabrication of hybrid magnetic metal–organic framework-5: High efficient adsorbents for magnetic enrichment of trace analytes. Anal. Chem. 2013, 85, 6885–6893. [Google Scholar]
- Pena-Mendez, E.M.; Mawale, R.M.; Conde-Gonzalez, J.E.; Socas-Rodriguez, B.; Havel, J.; Ruiz-Perez, C. Metal organic framework composite, nano-Fe3O4@Fe-(benzene-1,3,5-tricarboxylic acid), for solid phase extraction of blood lipid regulators from water. Talanta 2020, 207, 120275. [Google Scholar]
- Li, L.N.; Chen, Y.L.; Yang, L.; Wang, Z.T.; Liu, H.W. Recent advances in applications of metal–organic frameworks for sample preparation in pharmaceutical analysis. Coordin. Chem. Rev. 2020, 411, 213235. [Google Scholar]
- Xiang, W.L.; Gebhardt, S.; Glaser, R.; Liu, C.J. Millimeter-scale magnetic spherical metal-organic framework core-shell structured composites for recyclable catalytic applications. Micropor. Mescopor. Mat. 2020, 300, 110152. [Google Scholar]
- Cao, C.H.; Xiao, L.; Chen, C.H.; Shi, X.W.; Cao, Q.H.; Gao, L. In situ preparation of magnetic Fe3O4/chitosan nanoparticles via a novel reduction-precipitation method and their application in adsorption of reactive azo dye. Powder Technol. 2014, 260, 90–97. [Google Scholar]
- Shi, Z.N.; Xu, C.; Guan, H.; Li, L.; Fan, L.; Wang, Y.X.; Liu, L.; Meng, Q.T.; Zhang, R. Magnetic metal organic frameworks (MOFs) composite for removal of lead and malachite green in wastewater. Colloids Surf. A 2018, 539, 382–390. [Google Scholar]
- Bhattacharjee, A.; Gumma, S.; Purkait, M.K. Fe3O4 promoted metal organic framework MIL-100(Fe) for the controlled release of doxorubicin hydrochloride. Micropor. Mesopor. Mat. 2018, 259, 203–210. [Google Scholar]
- Liu, J.F.; Qiu, H.J.; Zhang, F.; Li, Y. Zeolitic imidazolate framework-8 coated Fe3O4@SiO2 composites for magnetic solid-phase extraction of bisphenols. New J. Chem. 2020, 44, 5324–5332. [Google Scholar]
- Zhou, Z.X.; Gao, Z.J.; Shen, H.; Li, M.Q.; He, W.T.; Su, P.; Song, J.Y.; Yang, Y. Metal−organic framework in situ post-encapsulating DNA−enzyme composites on a magnetic carrier with high stability and reusability. ACS Appl. Mater. Inter. 2020, 12, 7510–7517. [Google Scholar]
- Xu, J.Y.; Zhai, X.P.; Gao, L.F.; Chen, P.; Zhao, M.; Yang, H.B.; Cao, D.F.; Wang, Q.; Zhang, H.L. In situ preparation of a MOF-derived magnetic carbonaceous catalyst for visible-light-driven hydrogen evolution. RSC Adv. 2016, 6, 2011–2018. [Google Scholar]
- Wu, Y.N.; Zhou, M.M.; Li, S.; Li, Z.H.; Li, J.; Wu, A.Z.; Li, G.T.; Li, F.T.; Guan, X.H. Magnetic metal-organic frameworks: Gamma-Fe2O3@MOFs via confined in situ pyrolysis method for drug delivery. Small 2014, 10, 2927–2936. [Google Scholar]
- Liu, Y.; Gong, C.S.; Lin, L.S.; Zhou, Z.J.; Liu, Y.J.; Yang, Z.; Shen, Z.Y.; Yu, G.C.; Wang, Z.T.; Wang, S.; et al. Core-shell metal-organic frameworks with fluorescence switch to trigger an enhanced photodynamic therapy. Theranostics 2019, 9, 2791–2799. [Google Scholar] [PubMed]
- Yang, Q.X.; Zhao, Q.Q.; Ren, S.S.; Lu, Q.Q.; Guo, X.M.; Chen, Z.J. Fabrication of core-shell Fe3O4@MIL-100(Fe) magnetic microspheres for the removal of Cr(VI) in aqueous solution. J. Solid State Chem. 2016, 244, 25–30. [Google Scholar]
- Jiang, Z.W.; Li, Y.F. Facile synthesis of magnetic hybrid Fe3O4/MIL-101 via heterogeneous coprecipitation assembly for efficient adsorption of anionic dyes. J. Taiwan Inst. Chem. E. 2016, 59, 373–379. [Google Scholar]
- Imaz, I.; Hernando, J.; Ruiz-Molina, D.; Maspoch, D. Metal–organic spheres as functional systems for guest encapsulation. Angew. Chem. Int. Ed. 2009, 48, 2325–2329. [Google Scholar]
- Lohe, M.R.; Gedrich, K.; Freudenberg, T.; Kockrick, E.; Dellmann, T.; Kaskel, S. Heating and separation using nanomagnet-functionalized metal-organic frameworks. Chem. Commun. 2011, 47, 3075–3077. [Google Scholar]
- Fang, Q.L.; Shen, Y.; Chen, B.L. Synthesis, decoration and properties of three-dimensional graphene-based macrostructures: A review. Chem. Eng. J. 2015, 264, 753–771. [Google Scholar]
- Liu, Y.D.; Goebl, J.; Yin, Y.D. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2013, 42, 2610–2653. [Google Scholar]
- Cheng, J.J.; Mallet, N.; Baaziz, W.; Ersen, O.; Gombart, E.; Alard, V.; Majimel, J.; Delville, M.H.; Treguer-Delapierre, M.; Duguet, E. Template-directed synthesis of titania nanocages with four tetrahedrally arranged open windows. Chem. Eur. J. 2018, 24, 6917–6921. [Google Scholar]
- Yue, Y.F.; Fulvio, P.F.; Dai, S. Hierarchical metal–organic framework hybrids: Perturbation-assisted nanofusion synthesis. Acc. Chem. Res. 2015, 48, 3044. [Google Scholar]
- Chen, C.; Feng, N.J.; Guo, Q.R.; Li, Z.; Li, X.; Ding, J.; Wang, L.; Wan, H.; Guan, G.F. Template-directed fabrication of MIL-101(Cr)/mesoporous silica composite: Layer-packed structure and enhanced performance for CO2 capture. J. Colloid Interf. Sci. 2018, 513, 891–902. [Google Scholar]
- Yang, X.J.; Liang, T.; Sun, J.X.; Zaworotko, M.J.; Chen, Y.; Cheng, P.; Zhang, Z.J. Template-directed synthesis of photocatalyst-encapsulating metal-organic frameworks with boosted photocatalytic activity. ACS Catal. 2019, 9, 7486–7493. [Google Scholar]
- Ma, K.K.; Wang, Y.F.; Chen, Z.J.; Islamoglu, T.; Lai, C.L.; Wang, X.W.; Fei, B.; Farha, O.K.; Xin, J.H. Facile and scalable coating of metal–organic frameworks on fibrous substrates by a coordination replication method at room temperature. ACS Appl. Mater. Inter. 2019, 11, 22714–22721. [Google Scholar]
- Ding, B.; Wang, J.; Chang, Z.; Xu, G.Y.; Hao, X.D.; Shen, L.F.; Dou, H.; Zhang, X.G. Self-sacrificial template-directed synthesis of metal–organic framework-derived porous carbon for energy-storage devices. ChemElectroChem 2016, 3, 668–674. [Google Scholar]
- Huang, Y.F.; Zhang, L.; Ma, L.; Li, Y.; Zhong, C.L. Fe3O4@Cu/C and Fe3O4@CuO composites derived from magnetic metal–organic frameworks Fe3O4@HKUST-1 with improved peroxidase-like catalytic activity. Catal. Lett. 2019, 150, 815–825. [Google Scholar]
- Chen, L.N.; Li, H.Q.; Yan, M.W.; Yuan, C.F.; Zhan, W.W.; Jiang, Y.Q.; Xie, Z.X.; Kuang, Q.; Zheng, L.S. Ternary alloys encapsulated within different MOFs via a self-sacrificing template process: A potential platform for the investigation of size-selective catalytic performances. Small 2017, 13, 1700683. [Google Scholar]
- Liu, T.; Li, P.; Yao, N.; Kong, T.G.; Cheng, G.Z.; Chen, S.L.; Luo, W. Self-sacrificial template-directed vapor-phase growth of MOF assemblies and surface vulcanization for efficient water splitting. Adv. Mater. 2019, 31, 1806672. [Google Scholar]
- Huang, L.J.; He, M.; Chen, B.B.; Hu, B. A designable magnetic MOF composite and facile coordination-based post-synthetic strategy for the enhanced removal of Hg2+ from water. J. Mater. Chem. A 2015, 21, 11587–11595. [Google Scholar]
- Chen, L.N.; Li, H.Q.; Zhan, W.W.; Cao, Z.M.; Chen, J.Y.; Jiang, Q.R.; Jiang, Y.Q.; Xie, Z.X.; Kuang, Q.; Zheng, L.S. Controlled encapsulation of flower-like Rh-Ni alloys with MOFs via tunable template dealloying for enhanced selective hydrogenation of alkyne. ACS Appl. Mater. Inter. 2016, 8, 31059–31066. [Google Scholar]
- El-Feky, S.A.; Al-Sherbini, E.A. Fabrication and optical absorption properties of gold-silver and gold-platinum alloy nanoparticles formed by laser ablation. Curr. Nanosci. 2013, 9, 192–196. [Google Scholar]
- Kumar, M.K.; Do, J.Y.; Reddy, A.K.; Kang, M. Natural solar light-driven preparation of plasmonic resonance-based alloy and core-shell catalyst for sustainable enhanced hydrogen production: Green approach and characterization. Appl. Catal. B Environ. 2018, 231, 137–150. [Google Scholar]
- Li, L.; Wang, Y.Z.; Wang, X.X.; Song, K.K.; Jian, X.D.; Qan, P.; Bai, Y.; Su, Y.J. Size and stoichiometry effect of FePt bimetal nanoparticle catalyst for CO oxidation: A DFT study. J. Phys. Chem. C 2020, 124, 8706–8715. [Google Scholar]
- Zhou, L.; Mao, J.Y.; Ren, Y.; Yang, J.Q.; Zhang, S.R.; Zhou, Y.; Liao, Q.F.; Zeng, Y.J.; Shan, H.Q.; Xu, Z.X.; et al. Biological spiking synapse constructed from solution processed bimetal core-shell nanoparticle based composites. Small 2018, 14, 1800288. [Google Scholar]
- Ma, R.Q.; Jiang, H.Q.; Wang, C.; Zhao, C.B.; Deng, H.X. Multivariate MOFs for laser writing of alloy nanoparticle patterns. Chem. Commun. 2020, 56, 2715–2718. [Google Scholar]
- Yuan, L.; Li, X.Y.; Ge, L.M.; Jia, X.Q.; Lei, J.F.; Mu, C.D.; Li, D.F. Emulsion template method for the fabrication of gelatin-based scaffold with controllable pore structure. ACS Appl. Mater. Inter. 2019, 11, 269–277. [Google Scholar]
- Chen, J.; Azhar, U.; Wang, Y.K.; Liang, J.H.; Geng, B. Preparation of fluoropolymer materials with different porous morphologies by an emulsion template method using supercritical carbon dioxide as a medium. RSC Adv. 2019, 9, 11331–11340. [Google Scholar]
- Kim, H.; Lah, M.S. Templated and template-free fabrication strategies for zero-dimensional hollow MOF superstructures. Dalton Trans. 2017, 46, 6146–6158. [Google Scholar]
- Zhu, H.; Zhang, Q.; Zhu, S.P. MOFsome via transient pickering emulsion template. Adv. Mater. Interfaces 2016, 3. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, M.J.; Zhang, S.Y.; Shi, L.; Yang, Y.M.; Liu, Z.; Ju, X.J.; Xie, R.; Wang, W.; Chu, L.Y. Simple and continuous fabrication of self-propelled micromotors with photocatalytic metal−organic frameworks for enhanced synergistic environmental remediation. ACS Appl. Mater. Inter. 2020, 12, 35120–35131. [Google Scholar]
- Jin, P.; Tan, W.L.; Huo, J.; Liu, T.T.; Liang, Y.; Wang, S.Y.; Bradshaw, D. Hierarchically porous MOF/polymer composites via interfacial nanoassembly and emulsion polymerization. J. Mater. Chem. A 2018, 6, 20473–20479. [Google Scholar]
- Chen, L.J.; Ding, X.; Huo, J.; El Hankari, S.; Bradshaw, D. Facile synthesis of magnetic macroporous polymer/MOF composites as separable catalysts. J. Mater. Sci. 2019, 54, 370–382. [Google Scholar]
- Zhu, H.; Zhang, Q.; Zhu, S.P. Assembly of a metal–organic framework into 3D hierarchical porous monoliths using a pickering high internal phase emulsion template. Chem. Eur. J. 2016, 22, 8751–8755. [Google Scholar]
- Iost, R.M.; Crespilho, F.N. Layer-by-layer self-assembly and electrochemistry: Applications in biosensing and bioelectronics. Biosens. Bioelectron. 2012, 31, 1–10. [Google Scholar]
- Ariga, K.; Yamauchi, Y.; Rydzek, G.; Ji, Q.M.; Yonamine, Y.; Wu, K.C.W.; Hill, J.P. Layer-by-layer nanoarchitectonics: Invention, innovation, and evolution. Chem. Lett. 2014, 45, 36–68. [Google Scholar]
- Rawtani, D.; Agrawal, Y.K. Emerging strategies and applications of layer-by-layer self-assembly. Nanobiomedicine 2014, 1, 8. [Google Scholar]
- Ohhashi, T.; Tsuruoka, T.; Nawafune, H.; Akamatsu, K. Controlled synthesis of metal-organic framework films on metal nanoparticles by the versatile layer-by-layer assembly approach. Tetrahedron 2014, 39, 153–156. [Google Scholar]
- Wang, N.X.; Liu, T.J.; Shen, H.P.; Ji, S.L.; Li, J.R.; Zhang, R. Ceramic tubular mof hybrid membrane fabricated through in situ layer-by-layer self-assembly for nanofiltration. AIChE J. 2016, 62, 538–546. [Google Scholar]
- So, M.C.; Jin, S.; Son, H.J.; Wiederrecht, G.P.; Farha, O.K.; Hupp, J.T. Layer-by-layer fabrication of oriented porous thin films based on porphyrin-containing metal-organic frameworks. J. Am. Chem. Soc. 2013, 135, 15698. [Google Scholar]
- Liu, S.C.; Ma, Y.; Gao, L.; Pan, J.M. pH-responsive magnetic metal-organic framework nanocomposite: A smart porous adsorbent for highly specific enrichment of c is-diol containing luteolin. Chem. Eng. J. 2018, 341. [Google Scholar] [CrossRef]
- Liu, J.; Yang, F.; Zhang, Q.L.; Chen, W.; Gu, Y.F.; Chen, Q. Construction of hierarchical Fe3O4@HKUST-1/MIL-100(Fe) microparticles with large surface area through layer-by-layer deposition and epitaxial growth methods inorganic chemistry. Inorg. Chem. 2019, 58, 3564–3568. [Google Scholar]
- Miao, Z.; Shu, X.; Ramella, D. Synthesis of a Fe3O4@P4VP@metal–organic framework core–shell structure and studies of its aerobic oxidation reactivity. RSC Adv. 2017, 7, 2773–2779. [Google Scholar]
- Rice, C.M.; Davis, Z.H.; McKay, D.; Bignami, G.P.M.; Chitac, R.G.; Dawson, D.M.; Morris, R.E.; Ashbrook, S.E. Following the unusual breathing behaviour of (17)O-enriched mixed-metal (Al,Ga)-MIL-53 using NMR crystallography. Phys. Chem. Chem. Phys. 2020, 22, 14514–14526. [Google Scholar] [PubMed]
- Lee, Y.R.; Jang, M.S.; Cho, B.Y.; Kwon, H.J.; Kim, S.; Ahn, W.S. ZIF-8: A comparison of synthesis methods. Chem. Eng. J. 2015, 271, 276–280. [Google Scholar]
- Tan, P.; Xie, X.Y.; Liu, X.Q.; Pan, T.; Gu, C.; Chen, P.F.; Zhou, J.Y.; Pan, Y.C.; Sun, L.B. Fabrication of magnetically responsive HKUST-1/Fe3O4 composites by dry gel conversion for deep desulfurization and denitrogenation. J. Hazard. Mater. 2017, 321, 344–352. [Google Scholar]
- Rocio-Bautista, P.; Pino, V.; Ayala, J.H.; Pasan, J.; Ruiz-Perez, C.; Afonso, A.M. A magnetic-based dispersive micro-solid-phase extraction method using the metal-organic framework HKUST-1 and ultra-high-performance liquid chromatography with fluorescence detection for determining polycyclic aromatic hydrocarbons in waters and fruit tea infusions. J. Chromatogr. A 2016, 1436, 42–50. [Google Scholar]
- Gokpinar, S.; Diment, T.; Janiak, C. Environmentally benign dry-gel conversions of Zr-based UiO metal–organic frameworks with high yield and the possibility of solvent re-use. Dalton Trans. 2017, 46, 9895–9900. [Google Scholar] [PubMed]
- Bellusci, M.; Guglielmi, P.; Masi, A.; Padella, F.; Singh, G.; Yaacoub, N.; Peddis, D.; Secci, D. Magnetic metal–organic framework composite by fast and facile mechanochemical process. Inorg. Chem. 2018, 57, 1806–1814. [Google Scholar]
- Zhang, R.; Tao, C.A.; Chen, R.; Wu, L.F.; Zou, X.X.; Wang, J.F. Ultrafast synthesis of Ni-MOF in one minute by ball milling. Nanomaterials 2018, 8, 1067. [Google Scholar]
- Sun, J.K.; Chen, C.; Cai, L.X.; Ren, C.X.; Tan, B.; Zhang, J. Mechanical grinding of a single-crystalline metal-organic framework triggered emission with tunable violet-to-orange luminescence. Chem. Commun. 2014, 50, 15956–15959. [Google Scholar]
- Park, K.M.; Kim, H.; Murray, J.; Koo, J.; Kim, K. A facile preparation method for nanosized MOFs as a multifunctional material for cellular imaging and drug delivery. Supramol. Chem. 2016, 29, 441–445. [Google Scholar]
- Zhou, J.W.; Zou, X.M.; Song, S.H.; Chen, G.H. Quantum dots applied to methodology on detection of pesticide and veterinary drug residues. J. Agric. Food Chem. 2018, 66, 1307–1319. [Google Scholar]
- Kumar, A.; Bhattacharyya, A.; Shinde, R.; Dhanshetty, M.; Elliott, C.T.; Banerjee, K. Development and validation of a multiresidue method for pesticides and selected veterinary drugs in animal feed using liquid- and gas chromatography with tandem mass spectrometry. J. Chromatogr. A 2020, 1627, 461416. [Google Scholar]
- Ninga, E.; Sapozhnikova, Y.; Lehotay, S.J.; Lightfield, A.R.; Monteiro, S.H. High-throughput mega-method for the analysis of pesticides, veterinary drugs, and environmental contaminants by ultra-high-performance liquid chromatography-tandem mass spectrometry and robotic mini-solid-phase extraction cleanup + low-pressure gas chromatography-tandem mass spectrometry, part 2: Catfish. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef]
- Jadhav, M.R.; Pudale, A.; Raut, P.; Utture, S.; Shabeer, T.P.A.; Banerjee, K. A unified approach for high-throughput quantitative analysis of the residues of multi-class veterinary drugs and pesticides in bovine milk using LC-MS/MS and GC-MS/MS. Food Chem. 2019, 272, 292–305. [Google Scholar]
- Byzova, N.A.; Serchenya, T.S.; Vashkevich, I.I.; Zherdev, A.V.; Sviridov, O.V.; Dzantiev, B.B. Lateral flow immunoassay for rapid qualitative and quantitative control of the veterinary drug bacitracin in milk. Microchem. J. 2020, 156, 104884. [Google Scholar]
- Li, B.; Zhou, X.Z.; Cheng, F.S.; Wei, X.J.; Wang, W.W.; Zhang, J.Y. Research progress on analysis and detection techniques of veterinary drug residues in animal foods. Agric. Biotechnol. 2019, 8, 60–69. [Google Scholar]
- Wu, Z.L.; Wang, Y.P.; Xiong, Z.K.; Ao, Z.M.; Pu, S.Y.; Yao, G.; Lai, B. Core-shell magnetic Fe3O4@Zn/Co-ZIFs to activate peroxymonosulfate for highly efficient degradation of carbamazepine. Appl. Catal. B Environ. 2020, 277, 119136. [Google Scholar]
- Yi, L.H.; Yan, Y.H.; Tang, K.Q.; Ding, C.F. Facile preparation of polymer grafted ZIF-8 modified magnetic nanosphere for effective identification and capture phosphorylated peptides and glycosylated peptides. Anal. Methods UK 2020, 38. [Google Scholar] [CrossRef]
- Ghiasi, A.; Malekpour, A.; Mahpishanian, S. Metal-organic framework MIL101 (Cr)-NH2 functionalized magnetic graphene oxide for ultrasonic-assisted magnetic solid phase extraction of neonicotinoid insecticides from fruit and water samples. Talanta 2020, 217, 121120. [Google Scholar]
- Shakourian, M.; Yamini, Y.; Safari, M. Facile magnetization of metal–organic framework TMU-6 for magnetic solid-phase extraction of organophosphorus pesticides in water and rice samples. Talanta 2020, 218, 121139. [Google Scholar]
- Li, D.D.; He, M.; Chen, B.B.; Hu, B. Metal organic frameworks-derived magnetic nanoporous carbon for preconcentration of organophosphorus pesticides from fruit samples followed by gas chromatography-flame photometric detection. J. Chromatogr. A 2019, 1583, 19–27. [Google Scholar]
- Yang, J.H.; Cui, C.X.; Qu, L.B.; Chen, J.; Zhou, X.M.; Zhang, Y.P. Preparation of a monolithic magnetic stir bar for the determination of sulfonylurea herbicides coupled with HPLC. Microchem. J. 2018, 141, 369–376. [Google Scholar]
- Yamini, Y.; Safari, M. Magnetic Zink-based metal organic framework as advance and recyclable adsorbent for the extraction of trace pyrethroids. Microchem. J. 2019, 146, 134–141. [Google Scholar]
- Gordi, Z.; Ghorbani, M.; Khakhiyani, M.A. Adsorptive removal of enrofloxacin with magnetic functionalized graphene oxide@ metal-organic frameworks employing D-optimal mixture design. Water Environ. Res. 2020, 92, 1935–1947. [Google Scholar]
- Lian, L.L.; Zhang, X.Y.; Hao, J.; Lv, J.Y.; Wang, X.Y.; Zhu, B.; Lou, D.W. Magnetic solid-phase extraction of fluoroquinolones from water samples using titanium-based metal-organic framework functionalized magnetic microspheres. J. Chromatogr. A 2018, 1579, 1–8. [Google Scholar]
- Mendiola-Alvarez, S.Y.; Palomino, G.T.; Guzman-Mar, J.; Hernandez-Ramirez, A.; Hinojosa-Reyes, L.; Cabello, C.P. Magnetic porous carbons derived from cobalt(II)-based metal-organic frameworks for the solid-phase extraction of sulfonamides. Dalton Trans. 2020, 49, 8959–8966. [Google Scholar]
- Bagheri, A.R.; Ghaedi, M. Magnetic metal organic framework for pre-concentration of ampicillin from cow milk samples. J. Pharm. Anal. 2020, 10, 365–375. [Google Scholar]
- Wang, Q.Q.; Liu, W.H.; Hao, L.; Wang, C.; Wu, Q.H.; Wang, Z. Fabrication of magnetic porous organic framework for effective enrichment and assay of nitroimidazoles in chicken meat. Food Chem. 2020, 332, 127427. [Google Scholar]
- Wang, X.M.; Ma, X.M.; Huang, P.F.; Wang, J.; Du, T.T.; Du, X.Z.; Lu, X.Q. Magnetic Cu-MOFs embedded within graphene oxide nanocomposites for enhanced preconcentration of benzenoid-containing insecticides. Talanta 2018, 181, 112–117. [Google Scholar]
- Du, F.Y.; Sun, L.S.; Tan, W.; Wei, Z.Y.; Nie, H.G.; Huang, Z.J.; Ruan, G.H.; Li, J.P. Magnetic stir cake sorptive extraction of trace tetracycline antibiotics in food samples: Preparation of metal-organic framework-embedded polyHIPE monolithic composites, validation and application. Anal. Bioanal. Chem. 2019, 411, 2239–2248. [Google Scholar] [PubMed]
- Liang, L.; Wang, X.H.; Sun, Y.; Ma, P.Y.; Li, X.P.; Piao, H.L.; Jiang, Y.X.; Song, D.Q. Magnetic solid-phase extraction of triazine herbicides from rice using metal-organic framework MIL-101(Cr) functionalized magnetic particles. Talanta 2018, 179, 512–519. [Google Scholar]
- Duo, H.X.; Lu, X.F.; Wang, S.; Wang, L.C.; Guo, Y.; Liang, X.J. Synthesis of magnetic metal-organic framework composites, Fe3O4-NH2@MOF-235, for the magnetic solid-phase extraction of benzoylurea insecticides from honey, fruit juice and tap water samples. New J. Chem. 2019, 43, 12563–12569. [Google Scholar]
- D’Mello, J.P.F.; Macdonald, A.M.C.; Postel, D.; Dijksma, W.T.P.; Dujardin, A.; Placinta, C.M. Pesticide use and mycotoxin production in fusarium and aspergillus phytopathogens. Eur. J. Plant Pathol. 1998, 104, 741–751. [Google Scholar]
- Bata, A.; Lasztity, R. Detoxification of mycotoxin-contaminated food and feed by microorganisms. Trends Food Sci. Technol. 1999, 10, 223–228. [Google Scholar]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to prevent mycotoxin contamination of food and animal feed: A review. Crit. Rev. Food Sci. 2006, 46, 593–619. [Google Scholar]
- Xu, B.J.; Jia, X.Q.; Gu, L.J.; Sung, C.K. Review on the qualitative and quantitative analysis of the mycotoxin citrinin. Food Control 2006, 17, 271–285. [Google Scholar]
- Li, C.Y.; Liu, J.M.; Wang, Z.H.; Lv, S.W.; Zhao, N.; Wang, S. Integration of Fe3O4@UiO-66-NH2@MON core-shell structured adsorbents for specific preconcentration and sensitive determination of aflatoxins against complex sample matrix. J. Hazard. Mater. 2020, 384, 121348. [Google Scholar]
- Durmus, Z.; Kurt, B.Z.; Gazioglu, I.; Sevgi, E.; Hancer, C.K. Spectrofluorimetric determination of Aflatoxin B1 in winter herbal teas via magnetic solid phase extraction method by using metal–organic framework (MOF) hybrid structures anchored with magnetic nanoparticles. Appl. Organomet. Chem. 2020, 34, e5375. [Google Scholar]
- Sabeghi, M.B.; Ghasempour, H.R.; Koohi, M.K.; Karimi, N. Synthesis and application of a novel functionalized magnetic MIL-101(Cr) nanocomposite for determination of aflatoxins in pistachio samples. Res. Chem. Intermed. 2020, 46, 4099–4111. [Google Scholar]
- Hu, S.S.; Ouyang, W.J.; Guo, L.H.; Lin, Z.Y.; Jiang, X.H.; Qiu, B.; Chen, G.N. Facile synthesis of Fe3O4/g-C3N4/HKUST-1 composites as a novel biosensor platform for ochratoxin A. Biosens. Bioelectron. 2017, 92, 718–723. [Google Scholar]
- Huang, C.H.; Qiao, X.Z.; Sun, W.M.; Chen, H.; Chen, X.Y.; Zhang, L.; Wang, T. Effective extraction of domoic acid from seafood based on postsynthetic-modified magnetic zeolite imidazolate framework-8 particles. Anal. Chem. 2019, 91, 2418–2424. [Google Scholar]
- Hallegraeff, G.M. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar]
- Christensen, V.G.; Khan, E. Freshwater neurotoxins and concerns for human, animal, and ecosystem health: A review of anatoxin-a and saxitoxin. Sci. Total Environ. 2020, 736, 139515. [Google Scholar] [PubMed]
- Li, Z.J.; Gong, C.C.; Huo, P.P.; Deng, C.H.; Pu, S.Z. Synthesis of magnetic core-shell Fe3O4@PDA@Cu-MOFs composites for enrichment of microcystin-LR by MALDI-TOF MS analysis. RSC Adv. 2020, 10, 29061–29067. [Google Scholar]
- Piatkowska, M.; Jedziniak, P.; Zmudzki, J. Multiresidue method for the simultaneous determination of veterinary medicinal products, feed additives and illegal dyes in eggs using liquid chromatography-tandem mass spectrometry. Food Chem. 2016, 197, 571–580. [Google Scholar]
- Zhang, T.; Li, Z.H.; Li, Y.; Liu, N.; Liu, K. A combined raman and DFT study of illegal food additives methenamine. J. Insp. Quar. 2013, 23, 36–47. [Google Scholar]
- Zhang, N.; Huang, C.H.; Tong, P.; Feng, Z.M.; Wu, X.P.; Zhang, L. Moisture stable Ni-Zn MOF/g-C3N4 nanoflowers: A highly efficient adsorbent for solid-phase microextraction of PAHs. J. Chromatogr. A 2015, 1556, 37–46. [Google Scholar]
- Ventura, K.; Arrieta, R.A.; Marcos-Hernandez, M.; Jabbari, V.; Powell, C.D.; Turley, R.; Lounsbury, A.W.; Zimmerman, J.B.; Gardea-Torresdey, J.; Wong, M.S.; et al. Superparamagnetic MOF@GO Ni and Co based hybrid nanocomposites as efficient water pollutant adsorbents. Sci. Total Environ. 2020, 738, 139213. [Google Scholar]
- Yuan, Y.; Zheng, X.W.; Lin, H.Z.; Li, Y.Y.; Yang, M.; Liu, X.L.; Deng, C.H.; Fan, Z.Q. Development of a hydrophilic magnetic amino-functionalized metal-organic framework for the highly efficient enrichment of trace bisphenols in river water samples. Talanta 2020, 221, 120713. [Google Scholar]
- Lu, Y.J.; Wang, B.C.; Yan, Y.H.; Liang, H.Z. Location-controlled synthesis of hydrophilic magnetic metal-organic frameworks for highly efficient recognition of phthalates in beverages. Chem. Select 2018, 3, 12440–12445. [Google Scholar]
- Yamini, Y.; Safari, M.; Morsali, A.; Safarifard, V. Magnetic frame work composite as an efficient sorbent for magnetic solid-phase extraction of plasticizer compounds. J. Chromatogr. A 2018, 1570, 38–46. [Google Scholar]
- Shi, X.R.; Chen, X.L.; Hao, Y.L.; Li, L.; Xu, H.J.; Wang, M.M. Magnetic metal-organic frameworks for fast and efficient solid-phase extraction of six Sudan dyes in tomato sauce. J. Chromatogr. B 2018, 1086, 146–152. [Google Scholar]
- Zhou, Z.H.; Fu, Y.Q.; Qin, Q.; Lu, X.; Shi, X.Z.; Zhao, C.X.; Xu, G.W. Synthesis of magnetic mesoporous metal-organic framework-5 for the effective enrichment of malachite green and crystal violet in fish samples. J Chromatogr. B 2018, 1560, 19–25. [Google Scholar]
- Zhong, X.; Chen, Z.W.; Li, Y.Y.; Ding, K.B.; Liu, W.S.; Liu, Y.; Yuan, Y.Q.; Zhang, M.Y.; Baker, A.J.M.; Yang, W.J.; et al. Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 2020, 400, 123289. [Google Scholar]
- Leng, J.S.; Gao, X.M.; Wang, L.X. Hazards of heavy metal pollution in food and progress in study on analysis technologies. Farm Prod. Process. 2015, 23, 50–53. [Google Scholar]
- Ma, J.F.; Chen, Y.P.; Antoniadis, V.; Wang, K.B.; Huang, Y.Z.; Tian, H.W. Assessment of heavy metal(loid)s contamination risk and grain nutritional quality in organic waste-amended soil. J. Hazard. Mater. 2020, 399, 123095. [Google Scholar]
- Zhang, X.Y.; Zhong, T.Y.; Liu, L.; Ouyang, X.Y. Impact of soil heavy metal pollution on food safety in China. PLoS ONE 2015, 10, e0135182. [Google Scholar]
- Li, J.; Yue, X.L.; Cheng, Y.H.; Bao, L.Y.; Yu, Y.Y.; Ren, X.M. Research progress on heavy metal pollution in soil and the effect on heavy metal residues in vegetables. J. Food Saf. Food Qual. 2019, 10, 5299–5305. [Google Scholar]
- Zhang, H.K.; Wang, Z.Y.; Cai, S.S. Research progress of ICP-MS in determination of heavy metal elements in food and relative products. Food Res. Dev. 2016, 37, 195–200. [Google Scholar]
- Manousi, N.; Giannakoudakis, D.A.; Rosenberg, E.; Zachariadis, G.A. Extraction of metal ions with metal-organic frameworks. Molecules 2019, 24, 4605. [Google Scholar]
- Abolhasani, J.; Khanmiri, R.H.; Babazadeh, M.; Ghorbani-Kalhor, E.; Edjlali, L.; Hassanpour, A. Determination of Hg(II) ions in sea food samples after extraction and preconcentration by novel Fe3O4@SiO2@polythiophene magnetic nanocomposite. Environ. Monit. Assess. 2015, 187, 554. [Google Scholar]
- Ghorbani-Kalhor, E.; Hosseinzadeh-Khanmiri, R.; Abolhasani, J.; Babazadeh, M.; Hassanpour, A. Determination of mercury(II) ions in seafood samples after extraction and preconcentration by a novel functionalized magnetic metal-organic framework nanocomposite. J. Sep. Sci. 2015, 38, 1179–1186. [Google Scholar]
- Yang, J.; Dong, X.; Zhen, X.T.; Chen, Y.; Wang, Y.; Wang, Q.Y.; Hu, Y.H.; Xie, T.; Wang, S.L.; Cao, J. Metal organic framework assisted in situ complexation for miniaturized solid phase extraction of organic mercury in fish and Dendrobium officinale. Talanta 2020, 209, 120598. [Google Scholar] [PubMed]
- Esmaeilzadeh, M. Ultrasound-assisted dispersive magnetic solid phase extraction based on metal-organic framework/1-(2-pyridylazo)-2-naphthol modified magnetite nanoparticle composites for speciation analysis of inorganic tin. New J. Chem. 2019, 43, 4929–4936. [Google Scholar]
- Hassanpour, A.; Hosseinzadeh-Khanmiri, R.; Babazadeh, M.; Abolhasani, J.; Ghorbani-Kalhor, E. Determination of heavy metal ions in vegetable samples using a magnetic metal-organic framework nanocomposite sorbent. Food Addit. Contam. A 2015, 32, 725–736. [Google Scholar]
- Ghorbani-Kalhor, E.; Hosseinzadeh-Khanmiri, R.; Babazadeh, M.; Abolhasani, J.; Hassanpour, A. Synthesis and application of a novel magnetic metal-organic framework nanocomposite for determination of Cd, Pb, and Zn in baby food samples. Can. J. Chem. 2015, 93, 518–525. [Google Scholar]
- Mahmoud, M.E.; Amira, M.F.; Selim, S.M.; Mohamed, A.K. Amino-decorated magnetic metal-organic framework as a potential novel platform for selective removal of chromium (VI), cadmium (II) and lead (II). J. Hazard. Mater. 2020, 381, 120979. [Google Scholar]
- Mehraban, M.; Manoochehri, M.; Taromi, F.A. Trace amount determination of Cd(II), Pb(II) and Ni(II) ions in agricultural and seafood samples after magnetic solid phase extraction by MIL-101(Cr)/phenylthiosemicarbazide-functionalized magnetite nanoparticle composite. New J. Chem. 2018, 42, 17636–17643. [Google Scholar]
- Marieeswaran, M.; Panneerselvam, P. A magnetic nanoscale metal–organic framework (MNMOF) as a viable fluorescence quencher material for ssDNA and for the detection of mercury ions via a novel quenching–quenching mechanism. RSC Adv. 2020, 10, 3705–3714. [Google Scholar]
- Taghizadeh, M.; Asgharinezhad, A.A.; Pooladi, M.; Barzin, M.; Abbaszadeh, A.; Tadjarodi, A. A novel magnetic metal organic framework nanocomposite for extraction and preconcentration of heavy metal ions, and its optimization via experimental design methodology. Microchim. Acta 2013, 180, 1073–1084. [Google Scholar]
- Li, W.T.; Shi, W.; Hu, Z.J.; Yang, T.; Chen, M.L.; Zhao, B.; Wang, J.H. Fabrication of magnetic Fe3O4@metal organic framework@covalent organic framework composite and its selective separation of trace copper. Appl. Surf. Sci. 2020, 530, 147254. [Google Scholar]
- Liu, G.Y.; Li, L.Y.; Gao, Y.H.; Gao, M.K.; Huang, X.D.; Lv, J.; Xu, D.H. A beta-cyclodextrin- functionalized magnetic metal organic framework for efficient extraction and determination of prochloraz and triazole fungicides in vegetables samples. Ecotox. Environ. Saf. 2019, 183, 109546. [Google Scholar]
- Xia, L.; Liu, L.J.; Lv, X.X.; Qu, F.; Li, G.L.; You, J.M. Towards the determination of sulfonamides in meat samples: A magnetic and mesoporous metal-organic framework as an efficient sorbent for magnetic solid phase extraction combined with high-performance liquid chromatography. J. Chromatogr. A 2017, 1500, 24–31. [Google Scholar]











| Methods | Advantages | Disadvantages |
|---|---|---|
| Hybrid preparation method | Simple; suitable for most MMOFs | Easy to fall off |
| In-situ growth of MOFs on MNPs | Simple; synthesis at room temperature | Direct nucleation and growth of MOFs in solution |
| In-situ growth of MNPs on MOFs | More complete MOF structure; good adsorption property; remarkable magnetic response | Complex preparation process |
| Self-sacrificial template method | Core-shell structures; easy to operate; synthesis under mild conditions | Need a suitable precursor |
| Emulsion template method | Avoid embedding MNPs in MOFs tunnels; obtaining macroporous polymers | Require large amount of inorganic solvent; complex preparation process |
| LbL self-assembly method | Core-shell structure; precisely control the thickness and properties of MMOFs; synthesis at room temperature | Long preparation time Lack of self-assembled MOFs ligands |
| Dry gel conversion method | Avoid MNPs embedding in MOFs tunnels; reduce the loss of organic solvents | Not easily controlled uniformity of MOF growth |
| Mechanical grinding method | Synthesis at room temperature | Limited types of MOFs ligands; uneven MMOFs performance |
| Materials | Analyte | Instrument | Sample | Conditions (Sorbent Amount; Eluent) | LOD | Linear Range | RSD | Recovery | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Fe3O4/ZIF-8/IL | Pyrethroids | GC-MS/MS | Tea infusions (2 g) | 10 mg; 0.8 mL of acetonitrile | 0.0065–0.1017 μg/L | 0.5–50 μg/L | ≤9.70% | 81.5–98.1% | [51] |
| Fe3O4@TGA@TMU-6 | Phosalone Chlorpyrifos Profenofos | HPLC-UV | Rice (20 g) | 2 mg; 0.1 mL of 1-butanol | 0.15–0.87 µg/L | 7.5–75 μg/L 10–100 μg/L 10–150 μg/L | 4.8–7.3% | 88–107.2% | [137] |
| MNPCs-Zn/Co-MOF | OPPs | GC-FPD | Fruit (1 g) | 10 mg; 0.2 mL of acetone/ethyl acetate (1:1, v/v) | 0.018–0.045 μg/L | 0.1-100 μg/L | 3.5–9.7% | 84–116% | [138] |
| Nd2Fe14B-UIO-66, (Zr)-NH2 | Sulfonylurea | HPLC | Water (5 mL) | 5 mg; 0.2 mL of methanol-acetic acid (9:1, v/v) | 0.04–0.84 μg/L | 10–700 μg/L | ≤13.8% | 68.8–98.1% | [139] |
| Fe3O4@UiO-66-NH2@MON | Aflatoxins | HPLC | Corn, rice, millet (5 g) | 10 mg; 6 mL of acetonitrile | 1 μg/L | 0.15–0.87 μg/L | – | 87.3–101.8% | [154] |
| MIL53(Al)-SiO2@Fe3O4 | Aflatoxin B1 | FTIR | Herbal teas (no indication) | 100 mg; 2 mL of Me2CO/MeCN/CH2Cl2 (1:1:2, v/v) | 0.5 ng/mL | 0.5–150 ng/mL | <4.3% | 70.7–96.5% | [155] |
| Fe3O4/g-C3N4/HKUST-1 | Ochratoxin A | Fluorescent biosensor | Corn (no indication) | – | 2.57 ng/mL | 5.0–160.0 ng/mL | <2.5% | 96.5–101.4% | [157] |
| Fe3O4@PDA@Cu-MOFs | Microcystin | MALDI-TOF-MS | Water (80 μL) | 20 μg; 10 μL of NH4HCO3 (0.25 mol/L) | 0.015 mg/L | 0.05–4 mg/L | 6.1–8.2% | 98.67–106.15% | [160] |
| Fe3O4 SPs@ZIF-8/Zn2+ | Domoic acid | LC-MS | Shellfish (5 g) | 1.0 mg; 0.4 mL of aqueous histidine solution (3 mmol/L) | 0.2 pg/mL | 1.0–1000 pg/mL | ≤3.4% | 93.1–102.3% | [161] |
| magG@PDA@ZIF-8 | Phthalates | HPLC | Beverages (1 mL) | 20 mg; 1 mL of acetone | 0.003–0.09 ng/mL | 50–8000 ng/mL | <4.5% | 91.5–104.7% | [167] |
| Fe3O4-NH2@MIL-101 | Sudan dyes | HPLC | Tomato (4 g) | 3 mg; 1 mL × 2 ethyl acetate | 0.5–2.5 μg/kg | 0.01–25 μg/mL | ≤9.2% | 69.6–92.6% | [169] |
| Fe3O4@ 1-phenylthiosemicarbazide/MIL-101(Cr) | Cd2+; Pb2+; Ni2+ | FAAS | Shrimp, Cucumber, Tomato, Parsley (1 g) | 13 mg; 2.2 mL of 0.85 mol/L HCl | 0.07–0.5 mg/kg | 0.07–0.5 mg/kg 0.2–2.0 mg/kg 0.25–250 mg/kg | 4.5–7.3% | 97.5–99.0% | [185] |
| Fe3O4-NH2@MIL-101(Fe) | Hg2+ | Fluorescent biosensor | Water (no indication) | – | 8 nm | 2–20 nm | -- | About 70% | [186] |
| M-MOF/β-CD | Triazole Fungicides | HPLC-MS/MS | Lettuce, tomato (10 g) | 10 mg; 4 mL of acetone-sodium citrate buffer | 0.25–1.0 mg/L | 0.25–1.0 μg/L | 1.5–7.3% | 86.44–119.83% | [189] |
| Fe3O4@JUC-48 | Sulfonamides | HPLC | Chicken, pork, shrimp (5 g) | 25 mg; 0.8 mL of methanol | 3.97–1000 ng/g | 3.97–1000 ng/g | <4.5% | 76.1–102.6% | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Wang, Y.; Pan, M.; Xie, X.; Liu, K.; Hong, L.; Wang, S. Synthesis of Magnetic Metal-Organic Frame Material and Its Application in Food Sample Preparation. Foods 2020, 9, 1610. https://doi.org/10.3390/foods9111610
Yang J, Wang Y, Pan M, Xie X, Liu K, Hong L, Wang S. Synthesis of Magnetic Metal-Organic Frame Material and Its Application in Food Sample Preparation. Foods. 2020; 9(11):1610. https://doi.org/10.3390/foods9111610
Chicago/Turabian StyleYang, Jingying, Yabin Wang, Mingfei Pan, Xiaoqian Xie, Kaixin Liu, Liping Hong, and Shuo Wang. 2020. "Synthesis of Magnetic Metal-Organic Frame Material and Its Application in Food Sample Preparation" Foods 9, no. 11: 1610. https://doi.org/10.3390/foods9111610
APA StyleYang, J., Wang, Y., Pan, M., Xie, X., Liu, K., Hong, L., & Wang, S. (2020). Synthesis of Magnetic Metal-Organic Frame Material and Its Application in Food Sample Preparation. Foods, 9(11), 1610. https://doi.org/10.3390/foods9111610