Crocetin Isolated from the Natural Food Colorant Saffron Reduces Intracellular Fat in 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Isolation of CCT
2.2. 3T3-L1 Cell Culture and Adipocyte Differentiation
2.3. Quantification of the Intracellular Fat by Oil Red O Staining
2.4. Determination of the Number and Size of Lipid Droplets
2.5. Quantification of Cellular Viability
2.5.1. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Assay
2.5.2. Trypan Blue Assay
2.6. Expression of Main Genes Related to Early and Late Differentiation
2.7. Data Analysis
3. Results
3.1. Crocetin Reduced Intracellular Fat in 3T3-L1 Adipocytes
3.2. Crocetine Did Not Affect the Total Number of Lipid Drops or Their Size
3.3. Crocetin Did Not Affect 3T3-L1 Cell Viability
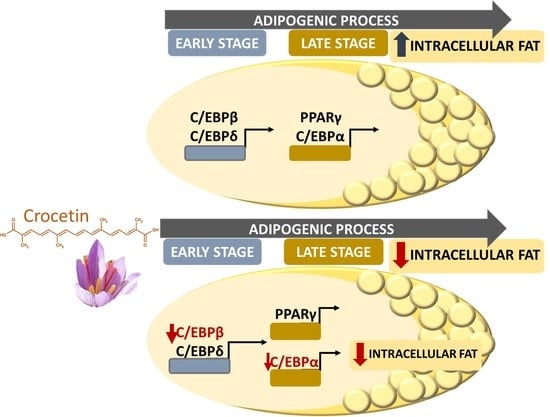
3.4. Crocetin Altered the Expression of Early and Late Genes during the Adipogenic Process
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Forward | Reverse | |
|---|---|---|
| β-Actin | AGGGAAATCGTGCGTGACAT | GGAAAAGAGCCTCAGGGCAT |
| aP2 | TCACCTGGAAGACAGCTCCT | AATCCCCATTTACGCTGATG |
| C/EBPβ | TTATAAACCTCCCGCTCGGC | CTCAGCTTGTCCACCGTCTT |
| C/EBPδ | AGAACCCGCGGCCTTCTAC | GTCGTACATGGCAGGAGTCG |
| C/EBPα | CCCTTGCTTTTTGCACCTCC | TGCCCCCATTCTCCATGAAC |
| PPARy | CCAGAGTCTGCTGATCTGCG- | GCCACCTCTTTGCTCTGCTC |
References
- Clydesdale, F.M. Color as a factor in food choice. Crit. Rev. Food Sci. Nutr. 1993, 33, 83–101. [Google Scholar] [CrossRef] [PubMed]
- Wrolstad, R.E.; Culver, C.A. Alternatives to those artificial FD&C food colorants. Annu. Rev. Food Sci. Technol. 2012, 3, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.A.; Abdel Hameid, H.; Abd Elsttar, A.H. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chem. Toxicol. 2010, 48, 2994–2999. [Google Scholar] [CrossRef] [PubMed]
- Moutaouakkil, A.; Zeroual, Y.; Zohra Dzayri, F.; Talbi, M.; Lee, K.; Blaghen, M. Purification and partial characterization of azoreductase from Enterobacter agglomerans. Arch. Biochem. Biophys. 2003, 413, 139–146. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources Added to Food. Scientific Opinion on the re-evaluation Tartrazine (E 102). EFSA J. 2009, 7, 1331–1383. [Google Scholar] [CrossRef]
- Nowik, W. DYES|Liquid Chromatography. In Encyclopedia of Separation Science; Wilson, I.D., Ed.; Academic Press: Oxford, UK, 2000; pp. 2602–2618. [Google Scholar] [CrossRef]
- Ulbricht, C.; Conquer, J.; Costa, D.; Hollands, W.; Iannuzzi, C.; Isaac, R.; Jordan, J.K.; Ledesma, N.; Ostroff, C.; Serrano, J.M.; et al. An evidence-based systematic review of saffron (Crocus sativus) by the Natural Standard Research Collaboration. J. Diet. Suppl. 2011, 8, 58–114. [Google Scholar] [CrossRef]
- Mehedi, N.; Ainad-Tabet, S.; Mokrane, N.; Addou, S.; Zaoui, C.; Kheroua, O.; Saidi, D. Reproductive Toxicology of Tartrazine (FD and C Yellow No. 5) in Swiss Albino Mice. Am. J. Pharmacol. Toxicol. 2009, 4, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Stevens, L.J.; Kuczek, T.; Burgess, J.R.; Stochelski, M.A.; Arnold, L.E.; Galland, L. Mechanisms of behavioral, atopic, and other reactions to artificial food colors in children. Nutr. Rev. 2013, 71, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Corkey, B.E.; Shirihai, O. Metabolic master regulators: Sharing information among multiple systems. Trends Endrocrinol. Metab. 2012, 23, 594–601. [Google Scholar] [CrossRef] [Green Version]
- Axon, A.; May, F.E.B.; Gaughan, L.E.; Williams, F.M.; Blain, P.G.; Wright, M.C. Tartrazine and sunset yellow are xenoestrogens in a new screening assay to identify modulators of human oestrogen receptor transcriptional activity. Toxicology 2012, 298, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Guyot, S.; Serrand, S.; Le Quéré, J.M.; Sanoner, P.; Renard, C.M.G.C. Enzymatic synthesis and physicochemical characterisation of phloridzin oxidation products (POP), a new water-soluble yellow dye deriving from apple. Innov. Food Sci. Emerg. Technol. 2007, 8, 443–450. [Google Scholar] [CrossRef]
- Mortensen, A. Carotenoids and other pigments as natural colorant. Pure Appl. Chem. 2006, 78, 1477–1491. [Google Scholar] [CrossRef]
- Bagur, M.J.; Alonso Salinas, G.L.; Jiménez-Monreal, A.M.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, G.L. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules 2017, 23, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmet, P.; Alberti, K.G.; Shaw, J. Global and societal implications of the diabetes epidemic. Nature 2001, 414, 782–787. [Google Scholar] [CrossRef]
- Sethi, J.K.; Vidal-Puig, A.J. Thematic review series: Adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J. Lipid Res. 2007, 48, 1253–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashemi, M.; Hosseinzadeh, H. A comprehensive review on biological activities and toxicology of crocetin. Food Chem. Toxicol. 2019, 130, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Lillie, R.D. Harold Joel Conn 1886-1975. Stain Technol. 1977, 52, 3–4. [Google Scholar] [CrossRef] [PubMed]
- Giaccio, M. Crocetin from saffron: An active component of an ancient spice. Crit. Rev. Food Sci. Nutr. 2004, 44, 155–172. [Google Scholar] [CrossRef]
- Festuccia, C.; Mancini, A.; Gravina, G.L.; Scarsella, L.; Llorens, S.; Alonso, G.L.; Tatone, C.; Di Cesare, E.; Jannini, E.A.; Lenzi, A.; et al. Antitumor Effects of Saffron-Derived Carotenoids in Prostate Cancer Cell Models. BioMed Res. Int. 2014, 2014, 135048. [Google Scholar] [CrossRef]
- Llorens, S.; Mancini, A.; Serrano-Díaz, J.; D’Alessandro, A.M.; Nava, E.; Alonso, G.L.; Carmona, M. Effects of Crocetin Esters and Crocetin from Crocus sativus L. on Aortic Contractility in Rat Genetic Hypertension. Molecules 2015, 20, 17570–17584. [Google Scholar] [CrossRef] [Green Version]
- Mancini, A.; Serrano-Díaz, J.; Nava, E.; D’Alessandro, A.M.; Alonso, G.L.; Carmona, M.; Llorens, S. Crocetin, a Carotenoid Derived from Saffron (Crocus sativus L.), Improves Acetylcholine-Induced Vascular Relaxation in Hypertension. J. Vasc. Res. 2014, 51, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, F.M.; Smas, C.M.; Sul, H.S. Understanding Adipocyte Differentiation. Physiol. Rev. 1998, 78, 783–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payne, V.A.; Au, W.S.; Lowe, C.E.; Rahman, S.M.; Friedman, J.E.; O’Rahilly, S.; Rochford, J.J. C/EBP transcription factors regulate SREBP1c gene expression during adipogenesis. Biochem. J. 2009, 425, 215–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosen, E.D.; MacDougald, O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006, 7, 885–896. [Google Scholar] [CrossRef]
- White, U.A.; Stephens, J.M. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell Endocrinol. 2010, 318, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Palomer, X.; Pérez, A.; Blanco-Vaca, F. Adiponectina: Un nuevo nexo entre obesidad, resistencia a la insulina y enfermedad cardiovascular. Med. Clin. 2005, 124, 388–395. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- McGregor, R.A.; Choi, M.S. microRNAs in the regulation of adipogenesis and obesity. Curr. Mol. Med. 2011, 11, 304–316. [Google Scholar] [CrossRef]
- Sheng, L.; Qian, Z.; Shi, Y.; Yang, L.; Xi, L.; Zhao, B.; Xu, X.; Ji, H. Crocetin improves the insulin resistance induced by high-fat diet in rats. Br. J. Pharmacol. 2008, 154, 1016–1024. [Google Scholar] [CrossRef] [Green Version]
- Xi, L.; Qian, Z.; Xu, G.; Zhou, C.; Sun, S. Crocetin attenuates palmitate-induced insulin insensitivity and disordered tumor necrosis factor-alpha and adiponectin expression in rat adipocytes. Br. J. Pharmacol. 2007, 151, 610–617. [Google Scholar] [CrossRef] [Green Version]
- Valle García-Rodríguez, M.; Serrano-Díaz, J.; Tarantilis, P.A.; López-Córcoles, H.; Carmona, M.; Alonso, G.L. Determination of saffron quality by high-performance liquid chromatography. J. Agric. Food Chem. 2014, 62, 8068–8074. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.M.; Moore, L.B.; Smith-Oliver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An Antidiabetic Thiazolidinedione Is a High Affinity Ligand for Peroxisome Proliferator-activated Receptor γ (PPARγ). J. Biol. Chem. 1995, 270, 12953–12956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Haile, A.; Jones, L.C. Rosiglitazone-induced adipogenesis in a bone marrow mesenchymal stem cell line. In Proceedings of the 48th Annual Rocky Mountain Bioengineering Symposium and 48th International ISA Biomedical Sciences Instrumentation Symposium 2011, Denver, CO, USA, 15–17 April 2011; pp. 202–210. [Google Scholar]
- Harmon, A.W.; Patel, Y.M.; Harp, J.B. Genistein inhibits CCAAT/enhancer-binding protein β (C/EBPβ) activity and 3T3-L1 adipogenesis by increasing C/EBP homologous protein expression. Biochem. J. 2002, 367, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Della-Fera, M.A.; Hausman, D.B.; Rayalam, S.; Ambati, S.; Baile, C.A. Genistein inhibits differentiation of primary human adipocytes. J. Nutr. Biochem. 2009, 20, 140–148. [Google Scholar] [CrossRef]
- Chryssanthi, D.G.; Lamari, F.N.; Georgakopoulos, C.D.; Cordopatis, P. A new validated SPE-HPLC method for monitoring crocetin in human plasma--application after saffron tea consumption. J. Pharm. Biomed. Anal. 2011, 55, 563–568. [Google Scholar] [CrossRef]
- Dludla, P.V.; Jack, B.; Viraragavan, A.; Pheiffer, C.; Johnson, R.; Louw, J.; Muller, C.J.F. A dose-dependent effect of dimethyl sulfoxide on lipid content, cell viability and oxidative stress in 3T3-L1 adipocytes. Toxicol. Rep. 2018, 5, 1014–1020. [Google Scholar] [CrossRef]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res 1987, 47, 936–942. [Google Scholar]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Bernlohr, D.A.; Bolanowski, M.A.; Kelly, T.J., Jr.; Lane, M.D. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J. Biol. Chem. 1985, 260, 5563–5567. [Google Scholar]
- Feng, S.; Reuss, L.; Wang, Y. Potential of Natural Products in the Inhibition of Adipogenesis through Regulation of PPARγ Expression and/or Its Transcriptional Activity. Molecules 2016, 21, 1278. [Google Scholar] [CrossRef]
- Cao, Z.; Umek, R.M.; McKnight, S.L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991, 5, 1538–1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.W.; Tang, Q.Q.; Vinson, C.; Lane, M.D. Dominant-negative C/EBP disrupts mitotic clonal expansion and differentiation of 3T3-L1 preadipocytes. Proc. Natl. Acad. Sci. USA 2004, 101, 43–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, M.N.; Zhou, L.; Smale, S.T. C/EBPbeta regulation in lipopolysaccharide-stimulated macrophages. Mol. Cell Biol. 2003, 23, 4841–4858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, M.; Tanabe, H.; Matsumoto, A.; Takagi, M.; Umegaki, K.; Amagaya, S.; Takahashi, J. Astaxanthin functions differently as a selective peroxisome proliferator-activated receptor γ modulator in adipocytes and macrophages. Biochem. Pharmacol. 2012, 84, 692–700. [Google Scholar] [CrossRef]
- Shirakura, Y.; Takayanagi, K.; Mukai, K.; Tanabe, H.; Inoue, M. β-Cryptoxanthin Suppresses the Adipogenesis of 3T3-L1 Cells via RAR Activation. J. Nutr. Sci. Vitaminol. 2011, 57, 426–431. [Google Scholar] [CrossRef] [Green Version]
- Carmona, M.; Zalacain, A.; Sánchez, A.M.; Novella, J.L.; Alonso, G.L. Crocetin esters, picrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J. Agric. Food Chem. 2006, 54, 973–979. [Google Scholar] [CrossRef]
- Lobo, G.P.; Amengual, J.; Li, H.N.M.; Golczak, M.; Bonet, M.L.; Palczewski, K.; von Lintig, J. Beta,beta-carotene decreases peroxisome proliferator receptor gamma activity and reduces lipid storage capacity of adipocytes in a beta,beta-carotene oxygenase 1-dependent manner. J. Biol. Chem. 2010, 285, 27891–27899. [Google Scholar] [CrossRef] [Green Version]
- Gul, T.; Balkhi, H.M.; Haq, E. Inhibition of Adipocyte Differentiation by Crocin in vitro Model of Obesity. Saudi J. Life Sci. 2017, 2, 306–311. [Google Scholar]
- Rosen, E.D.; Walkey, C.J.; Puigserver, P.; Spiegelman, B.M. Transcriptional regulation of adipogenesis. Genes Dev. 2000, 14, 1293–1307. [Google Scholar]
- Yamauchi, T.; Kamon, J.; Waki, H.; Murakami, K.; Motojima, K.; Komeda, K.; Ide, T.; Kubota, N.; Terauchi, Y.; Tobe, K.; et al. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J. Biol. Chem. 2001, 276, 41245–41254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.D.; Finegold, M.J.; Bradley, A.; Ou, C.N.; Abdelsayed, S.V.; Wilde, M.D.; Taylor, L.R.; Wilson, D.R.; Darlington, G.J. Impaired energy homeostasis in C/EBP alpha knockout mice. Science 1995, 269, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Torres-Villarreal, D.; Camacho, A.; Castro, H.; Ortiz-Lopez, R.; de la Garza, A.L. Anti-obesity effects of kaempferol by inhibiting adipogenesis and increasing lipolysis in 3T3-L1 cells. Am. J. Physiol. Biochem. Pharmacol. 2019, 75, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.S.; Naaz, A. Effects of estrogens and the phytoestrogen genistein on adipogenesis and lipogenesis in males and females. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ikeda, K.; Xu, J.-W.; Yamori, Y.; Gao, X.-M.; Zhang, B.-L. Genistein suppresses adipogenesis of 3T3-L1 cells via multiple signal pathways. Phytother. Res. 2009, 23, 713–718. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Ortega, E.; Braza-Boïls, A.; Burgos, M.; Moratalla-López, N.; Vicente, M.; Alonso, G.L.; Nava, E.; Llorens, S. Crocetin Isolated from the Natural Food Colorant Saffron Reduces Intracellular Fat in 3T3-L1 Adipocytes. Foods 2020, 9, 1648. https://doi.org/10.3390/foods9111648
Jiménez-Ortega E, Braza-Boïls A, Burgos M, Moratalla-López N, Vicente M, Alonso GL, Nava E, Llorens S. Crocetin Isolated from the Natural Food Colorant Saffron Reduces Intracellular Fat in 3T3-L1 Adipocytes. Foods. 2020; 9(11):1648. https://doi.org/10.3390/foods9111648
Chicago/Turabian StyleJiménez-Ortega, Elena, Aitana Braza-Boïls, Miguel Burgos, Natalia Moratalla-López, Manuel Vicente, Gonzalo L. Alonso, Eduardo Nava, and Sílvia Llorens. 2020. "Crocetin Isolated from the Natural Food Colorant Saffron Reduces Intracellular Fat in 3T3-L1 Adipocytes" Foods 9, no. 11: 1648. https://doi.org/10.3390/foods9111648
APA StyleJiménez-Ortega, E., Braza-Boïls, A., Burgos, M., Moratalla-López, N., Vicente, M., Alonso, G. L., Nava, E., & Llorens, S. (2020). Crocetin Isolated from the Natural Food Colorant Saffron Reduces Intracellular Fat in 3T3-L1 Adipocytes. Foods, 9(11), 1648. https://doi.org/10.3390/foods9111648