Optimization of the Effect of Pineapple By-Products Enhanced in Bromelain by Hydrostatic Pressure on the Texture and Overall Quality of Silverside Beef Cut
Abstract
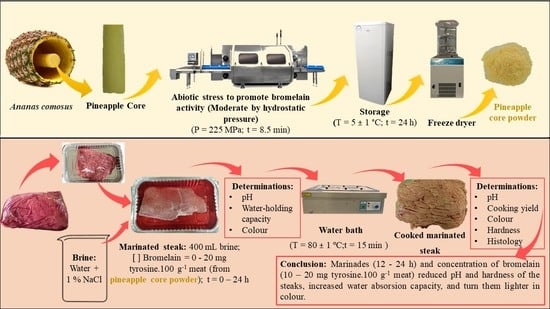
:1. Introduction
2. Materials and Methods
2.1. Meat Samples Collecting and Preparation
2.2. Production of Bromelain Powder from Pineapple By-Products
2.3. Marinating
2.3.1. Optimization of Pineapple By-Products Enhanced in Bromelain Application on Marinated Steaks
2.3.2. Brine Effect on Marinated Steaks
2.4. Cooking
2.5. Analytical Methods
2.5.1. pH Measurement
2.5.2. Measurement of Marination Yield
2.5.3. Measurement of Cooking Yield
2.5.4. Color Measurement
2.5.5. Mechanical Texture Measurement
2.5.6. Histological Technique
2.5.7. Statistical Analysis and Model Fitting
3. Results
3.1. Optimization of Pineapple By-Products Enhanced in Bromelain Application on Marinated Steaks
3.1.1. pH Value
3.1.2. Marination Yield
3.1.3. Cooking Yield
3.1.4. Color
3.1.5. Texture (Hardness)
3.1.6. Evaluation of Histological Technique
3.2. Brine Effect on Marinated Steaks
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Umesh Hebbar, H.; Sumana, B.; Raghavarao, K.S.M.S. Use of reverse micellar systems for the extraction and purification of bromelain from pineapple wastes. Bioresour. Technol. 2008, 99, 4896–4902. [Google Scholar] [CrossRef]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Extraction of bromelain from pineapple peels. Food Sci. Technol. Int. 2011, 17, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Pineapple wastes: A potential source for bromelain extraction. Food Bioprod. Process. 2012, 90, 385–391. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; del Rosario Cuéllar-Villarreal, M.; Welti-Chanes, J.; Cisneros-Zevallos, L.; Ramos-Parra, P.A.; Hernández-Brenes, C. Nonthermal processing technologies as elicitors to induce the biosynthesis and accumulation of nutraceuticals in plant foods. Trends Food Sci. Technol. 2017, 60, 80–87. [Google Scholar]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of fruit industrial by-products—a case study on circular economy approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [Green Version]
- Pathania, A.; McKee, S.R.; Bilgili, S.F.; Singh, M. Antimicrobial activity of commercial marinades against multiple strains of Salmonella spp. Int. J. Food Microbiol. 2010, 139, 214–217. [Google Scholar] [CrossRef]
- Burke, R.M.; Monahan, F.J. The tenderisation of shin beef using a citrus juice marinade. Meat Sci. 2003, 63, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Zochowska-Kujawska, J.; Lachowicz, K.; Sobczak, M. The tenderisation of wild boar meat using a calcium chloride, kefir, wine and pineapple marinade. Electron. J. Pol. Agric. Univ. Ser. Food Sci. Technol. 2010, 13, 2. [Google Scholar]
- Zochowska-Kujawska, J.; Lachowicz, K.; Sobczak, M. Effects of fibre type and kefir, wine lemon, and pineapple marinades on texture and sensory properties of wild boar and deer longissimus muscle. Meat Sci. 2012, 92, 675–680. [Google Scholar] [CrossRef]
- Botinestean, C.; Gomez, C.; Nian, Y.; Auty, M.A.E.; Kerry, J.P.; Hamill, R.M. Possibilities for developing texture-modified beef steaks suitable for older consumers using fruit-derived proteolytic enzymes. J. Texture Stud. 2018, 49, 256–261. [Google Scholar] [CrossRef]
- Zainal, S.; Nadzirah, K.Z.; Noriham, A.; Normah, I. Optimisation of beef tenderisation treated with bromelain using response surface methodology (RSM). Agric. Sci. 2013, 04, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Calkins, C.R.; Sullivan, G. Adding Enzymes to Improve Beef Tenderness; National Cattlemen’s Beef Association: Centennial, CO, USA, 2007; pp. 1–5. [Google Scholar]
- Bekhit, A.A.; Hopkins, D.L.; Geesink, G.; Bekhit, A.A.; Franks, P. Exogenous Proteases for Meat Tenderization. Crit. Rev. Food Sci. Nutr. 2014, 54, 1012–1031. [Google Scholar] [CrossRef] [PubMed]
- Buyukyavuz, A. Effect of Bromelain on Duck. In All Theses; Clemson University: Clemson, SC, USA, 2014; 68p. [Google Scholar]
- Nadzirah, K.Z.; Zainal, S.; Noriham, A.; Normah, I. Application of bromelain powder produced from pineapple crowns in tenderising beef round cuts. Int. Food Res. J. 2016, 23, 1590–1599. [Google Scholar]
- Gerelt, B.; Ikeuchi, Y.; Suzuki, A. Meat tenderization by proteolytic enzymes after osmotic dehydration. Meat Sci. 2000, 56, 311–318. [Google Scholar] [CrossRef]
- Manzoor, Z.; Nawaz, A.; Mukhtar, H.; Haq, I. Bromelain: Methods of Extraction, Purification and Therapeutic Applications. Braz. Arch. Biol. Technol. 2016, 59. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, G.A.; Calkins, C.R. Application of exogenous enzymes to beef muscle of high and low-connective tissue. Meat Sci. 2010, 85, 730–734. [Google Scholar] [CrossRef]
- Qihe, C.; Guoqing, H.; Yingchun, J.; Hui, N. Effects of elastase from a Bacillus strain on the tenderization of beef meat. Food Chem. 2006, 98, 624–629. [Google Scholar] [CrossRef]
- Santos, D.I.; Pinto, C.A.; Corrêa-Filho, L.C.; Saraiva, J.A.; Vicente, A.A.; Moldão-Martins, M. Effect of moderate hydrostatic pressures on the enzymatic activity and bioactive composition of pineapple by-products. J. Food Process Eng. 2020. [Google Scholar] [CrossRef]
- Chakraborty, S.; Rao, P.S.; Mishra, H.N. Effect of pH on Enzyme inactivation kinetics in high-pressure processed pineapple (Ananas comosus L.) puree using response surface methodology. Food Bioprocess Technol. 2014, 7, 3629–3645. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 9th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; ISBN 9781119113478. [Google Scholar]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- ISO 2917:1999 ISO 2917:1999(en) Meat and Meat Products — Measurement of pH (Reference Method); International Organization for Standardization: Geneva, Switzerland, 1999.
- James, B.J.; Yang, S.W. Effect of cooking method on the toughness of bovine M. Semitendinosus. Int. J. Food Eng. 2012, 8. [Google Scholar] [CrossRef]
- Barekat, S.; Soltanizadeh, N. Effects of Ultrasound on Microstructure and Enzyme Penetration in Beef Longissimus lumborum Muscle. Food Bioprocess Technol. 2018, 11, 680–693. [Google Scholar] [CrossRef]
- StatSoft STATISTICA, Data Analysis Software System; TIBCO Software: Palo Alto, CA, USA, 2007.
- Ke, S.; Huang, Y.; Decker, E.A.; Hultin, H.O. Impact of citric acid on the tenderness, microstructure and oxidative stability of beef muscle. Meat Sci. 2009, 82, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Sai-Ut, S.; Theppakorn, T.; Chaiwut, P.; Rawdkuen, S. Partitioning of bromelain from pineapple peel (Nang Lae cultv.) by aqueous two phase system. Asian J. Food Agro-Ind. 2009, 2, 457–468. [Google Scholar]
- Ketnawa, S.; Rawdkuen, S. Application of Bromelain Extract for Muscle Foods Tenderization. Food Nutr. Sci. 2011, 2, 393–401. [Google Scholar] [CrossRef] [Green Version]
- Chaurasiya, R.S.; Sakhare, P.Z.; Bhaskar, N.; Hebbar, H.U. Efficacy of reverse micellar extracted fruit bromelain in meat tenderization. J. Food Sci. Technol. 2015, 52, 3870–3880. [Google Scholar] [CrossRef] [Green Version]
- Gokoglu, N.; Yerlikaya, P.; Ucak, I.; Yatmaz, H.A. Effect of bromelain and papain enzymes addition on physicochemical and textural properties of squid (Loligo vulgaris). J. Food Meas. Charact. 2017, 11, 347–353. [Google Scholar] [CrossRef]
- Honikel, K.O.; Hamm, R. Measurement of water-holding capacity and juiciness. In Quality Attributes and their Measurement in Meat, Poultry and Fish Products; Springer: Berlin/Heidelberg, Germany, 1994; pp. 125–161. [Google Scholar]
- Honikel, K.O.; Kim, C.J.; Hamm, R.; Roncales, P. Sarcomere shortening of prerigor muscles and its influence on drip loss. Meat Sci. 1986, 16, 267–282. [Google Scholar] [CrossRef]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Hamm, R. Biochemistry Of Meat Hydration. Adv. Food Res. 1961, 10, 355–463. [Google Scholar]
- Hamm, R. The influence of pH on the protein net charge in the myofibrillar system. Reciprocal Meat Conf. Proc. 1994, 47, 5–9. [Google Scholar]
- Medyński, A.; Pospiech, E.; Kniat, R. Effect of various concentrations of lactic acid and sodium chloride on selected physico-chemical meat traits. Meat Sci. 2000, 55, 285–290. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. In Proceedings of the Meat Science; Elsevier: Amsterdam, The Netherlands, 2005; Volume 71, pp. 194–204. [Google Scholar]
- Puolanne, E.; Halonen, M. Theoretical aspects of water-holding in meat. Meat Sci. 2010, 86, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Grigsby, J.J.; Blanch, H.W.; Prausnitz, J.M. Cloud-point temperatures for lysozyme in electrolyte solutions: Effect of salt type, salt concentration and pH. Biophys. Chem. 2001, 91, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Murphy, R.Y.; Marks, B.P. Effect of meat temperature on proteins, texture, and cook loss for ground chicken breast patties. Poult. Sci. 2000, 79, 99–104. [Google Scholar] [CrossRef]
- Naveena, B.M.; Mendiratta, S.K.; Anjaneyulu, A.S.R. Tenderization of buffalo meat using plant proteases from Cucumis trigonus Roxb (Kachri) and Zingiber officinale roscoe (Ginger rhizome). Meat Sci. 2004, 68, 363–369. [Google Scholar] [CrossRef]
- Karakaya, M.; Ockerman, W.H. The effect of NaCl-K2HPO4, some plant enzymes and oils on the emulsion and water holding capacities in beef. GIDA 2002. [Google Scholar]
- Doneva, M.; Miteva, D.; Dyankova, S.; Nacheva, I.; Metodieva, P.; Dimov, K. Efficiency of plant proteases bromelain and papain on turkey meat tenderness. Biotechnol. Anim. Husb. 2015, 31, 407–413. [Google Scholar] [CrossRef]
- Kadıoğlu, P.; Karakaya, M.; Unal, K.; Babaoğlu, A.S. Technological and textural properties of spent chicken breast, drumstick and thigh meats as affected by marinating with pineapple fruit juice. Br. Poult. Sci. 2019, 60, 381–387. [Google Scholar] [CrossRef]
- Aktaş, N.; Aksu, M.I.; Kaya, M. The Influence of Marination with Different Salt Concentrations on the Tenderness, Water Holding Capacity and Bound Water Content of Beef. Turk. J. Vet. Anim. Sci. 2003, 27, 1207–1211. [Google Scholar]
- Vaudagna, S.R.; Pazos, A.A.; Guidi, S.M.; Sanchez, G.; Carp, D.J.; Gonzalez, C.B. Effect of salt addition on sous vide cooked whole beef muscles from Argentina. Meat Sci. 2008, 79, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Sánchez del Pulgar, J.; Gázquez, A.; Ruiz-Carrascal, J. Physico-chemical, textural and structural characteristics of sous-vide cooked pork cheeks as affected by vacuum, cooking temperature, and cooking time. Meat Sci. 2012, 90, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Sharedeh, D.; Gatellier, P.; Astruc, T.; Daudin, J.D. Effects of pH and NaCl levels in a beef marinade on physicochemical states of lipids and proteins and on tissue microstructure. Meat Sci. 2015, 110, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Janz, J.A.M.; Pietrasik, Z.; Aalhus, J.L.; Shand, P.J. The effects of enzyme and phosphate injections on the quality of beef semitendinosus. Can. J. Anim. Sci. 2005, 85, 327–334. [Google Scholar] [CrossRef]
- Fletcher, D.L.; Qiao, M.; Smith, D.P. The relationship of raw broiler breast meat color and pH to cooked meat color and pH. Poult. Sci. 2000, 79, 784–788. [Google Scholar] [CrossRef]
- Sen, A.R.; Naveena, B.M.; Muthukumar, M.; Vaithiyanathan, S. Colour, myoglobin denaturation and storage stability of raw and cooked mutton chops at different end point cooking temperature. J. Food Sci. Technol. 2014, 51, 970–975. [Google Scholar] [CrossRef] [Green Version]
- Bille, P.G.; Taapopi, M.S. Effects of two commercial meat tenderizers on different cuts of goat’s meat in Namibia. Afr. J. Food, Agric. Nutr. Dev. 2008, 8, 417–426. [Google Scholar]
- Ieowsakulrat, S.; Theerasamran, P.; Chokpitinun, C.; Pinitglang, S. Tenderization of pork steak with fruit bromelain from pineapple monitored by sensory evaluation and texturometer. Warasan Witthayasat Kaset 2011. [Google Scholar]
- Kemp, C.M.; Sensky, P.L.; Bardsley, R.G.; Buttery, P.J.; Parr, T. Tenderness—An enzymatic view. Meat Sci. 2010, 84, 248–256. [Google Scholar] [CrossRef] [Green Version]
- Archile-Contreras, A.C.; Cha, M.C.; Mandell, I.B.; Miller, S.P.; Purslow, P.P. Vitamins e and C May increase collagen turnover by intramuscular fibroblasts. Potential for improved meat quality. J. Agric. Food Chem. 2011, 59, 608–614. [Google Scholar] [CrossRef]
- Regulamento (CE), n.o 853/2004 REGULAMENTO (CE) n.o 853/2004 do PARLAMENTO EUROPEU E DO CONSELHO de 29 de Abril de 2004 que estabelece regras específicas de higiene aplicáveis aos géneros alimentícios de origem animal. J. Of. União Eur. 2004, 61.
- Ghisleni, G.; Stella, S.; Radaelli, E.; Mattiello, S.; Scanziani, E. Qualitative evaluation of tortellini meat filling by histology and image analysis. Int. J. Food Sci. Technol. 2010, 45, 265–270. [Google Scholar] [CrossRef]
- Liu, Z.; Xiong, Y.L.; Chen, J. Morphological examinations of oxidatively stressed pork muscle and myofibrils upon salt marination and cooking to elucidate the water-binding potential. J. Agric. Food Chem. 2011, 59, 13026–13034. [Google Scholar] [CrossRef] [PubMed]
- Vasanthi, C.; Venkataramanujam, V.; Dushyanthan, K. Effect of cooking temperature and time on the physico-chemical, histological and sensory properties of female carabeef (buffalo) meat. Meat Sci. 2007, 76, 274–280. [Google Scholar] [CrossRef]
- Palka, K. The influence of post-mortem ageing and roasting on the microstructure, texture and collagen solubility of bovine semitendinosus muscle. Meat Sci. 2003, 64, 191–198. [Google Scholar] [CrossRef]






| Run | Coded Factors | Decoded Factors | pH | Marination Yield (%) | Cooking Yield (%) | Colour | Hardness Steak (N) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Concentration | Time (h) | Concentration (mg Tyrosine. 100 g−1 Meat) | Brine | Marinated Steak | Cooked Steak | Initial | Marinated Steak | Cooked Steak | ||||||||||
| L* | a* | b* | L* | a* | b* | L* | a* | b* | |||||||||||
| 8 | 0.00000 | 1.41421 | 12 | 20 | 4.69 ± 0.18 | 5.05 ± 0.01 | 5.83 ± 0.03 | 2.38 ± 0.37 | 69.31 ± 0.76 | 38.48 ± 1.12 | 23.57 ± 1.08 | 7.23 ± 0.76 | 51.24 ± 1.99 | 11.72 ± 1.35 | 4.27 ± 1.43 | 56.67 ± 1.48 | 7.72 ± 0.69 | 8.75 ± 0.68 | 32.54 ± 3.91 |
| 9 (C) | 0.00000 | 0.00000 | 12 | 10 | 4.81 ± 0.04 | 5.11 ± 0.02 | 5.84 ± 0.03 | 2.68 ± 0.29 | 67.70 ± 0.25 | 38.20 ± 1.20 | 24.50 ± 1.28 | 7.48 ± 1.15 | 48.89 ± 2.23 | 12.92 ± 1.35 | 3.73 ± 0.60 | 56.80 ± 1.53 | 7.33 ± 0.47 | 8.83 ± 0.72 | 35.50 ± 4.70 |
| 2 | −1.00000 | 1.00000 | 3.5 | 17 | 4.31 ± 0.03 | 5.29 ± 0.10 | 5.65 ± 0.03 | 1.48 ± 0.08 | 68.08 ± 0.49 | 38.27 ± 1.92 | 25.05 ± 1.58 | 7.17 ± 1.10 | 45.08 ± 1.41 | 15.01 ± 0.76 | 3.75 ± 0.54 | 54.51 ± 0.90 | 7.85 ± 0.29 | 8.82 ± 0.51 | 38.36 ± 3.19 |
| 4 | 1.00000 | 1.00000 | 20.5 | 17 | 4.60 ± 0.06 | 4.99 ± 0.05 | 5.73 ± 0.01 | 1.97 ± 0.46 | 68.23 ± 1.95 | 38.40 ± 0.82 | 24.37 ± 1.19 | 7.34 ± 0.72 | 49.90 ± 3.13 | 11.94 ± 1.58 | 5.93 ± 1.12 | 58.53 ± 0.78 | 6.83 ± 0.41 | 8.77 ± 0.32 | 30.35 ± 2.50 |
| 10 (C) | 0.00000 | 0.00000 | 12 | 10 | 4.93 ± 0.01 | 5.18 ± 0.01 | 5.84 ± 0.03 | 3.53 ± 0.87 | 66.27 ± 0.05 | 38.91 ± 1.63 | 24.27 ± 2.18 | 7.33 ± 1.59 | 54.11 ± 4.02 | 11.27 ± 0.78 | 4.35 ± 1.91 | 55.90 ± 4.86 | 8.20 ± 1.84 | 10.01 ± 0.42 | 35.47 ± 4.40 |
| 7 | 0.00000 | −1.41421 | 12 | 0 | 5.61 ± 0.05 | 5.39 ± 0.07 | 5.99 ± 0.03 | 3.58 ± 0.16 | 69.64 ± 0.84 | 39.23 ± 1.28 | 24.88 ± 1.37 | 7.86 ± 1.22 | 51.22 ± 2.30 | 12.72 ± 1.29 | 4.26 ± 0.40 | 55.98 ± 1.27 | 8.44 ± 0.69 | 10.73 ± 0.44 | 48.14 ± 4.99 |
| 5 | −1.41421 | 0.00000 | 0 | 10 | 4.14 ± 0.03 | 5.34 ± 0.06 | 5.77 ± 0.03 | 0.07 ± 0.09 | 71.18 ± 1.98 | 37.52 ± 1.08 | 23.57 ± 0.68 | 5.87 ± 0.96 | 39.24 ± 0.51 | 21.21 ± 1.47 | 5.75 ± 0.72 | 50.65 ± 1.68 | 10.32 ± 1.33 | 9.57 ± 0.39 | 41.30 ± 8.58 |
| 6 | 1.41421 | 0.00000 | 24 | 10 | 4.86 ± 0.05 | 5.07 ± 0.06 | 5.81 ± 0.05 | 4.19 ± 0.42 | 63.13 ± 1.92 | 38.54 ± 1.66 | 25.71 ± 2.97 | 8.26 ± 2.55 | 52.11 ± 3.80 | 11.47 ± 0.68 | 5.18 ± 1.52 | 59.63 ± 0.93 | 6.42 ± 0.37 | 9.18 ± 0.32 | 36.16 ± 6.81 |
| 11 (C) | 0.00000 | 0.00000 | 12 | 10 | 4.88 ± 0.01 | 5.12 ± 0.09 | 5.80 ± 0.04 | 2.95 ± 0.63 | 64.33 ± 0.95 | 39.30 ± 2.40 | 25.42 ± 2.59 | 8.46 ± 1.61 | 49.70 ± 6.45 | 13.55 ± 1.72 | 5.31 ± 1.51 | 59.45 ± 0.85 | 6.72 ± 0.26 | 9.42 ± 0.46 | 37.14 ± 11.02 |
| 12 (C) | 0.00000 | 0.00000 | 12 | 10 | 4.89 ± 0.16 | 5.14 ± 0.02 | 5.81 ± 0.06 | 3.54 ± 0.74 | 66.44 ± 0.46 | 39.93 ± 0.90 | 25.25 ± 2.88 | 8.75 ± 1.63 | 53.60 ± 0.39 | 11.05 ± 1.92 | 4.60 ± 1.10 | 59.45 ± 1.93 | 6.54 ± 0.91 | 8.59 ± 0.65 | 37.03 ± 8.04 |
| 1 | −1.00000 | −1.00000 | 3.5 | 3 | 5.16 ± 0.03 | 5.34 ± 0.04 | 5.77 ± 0.08 | 2.01 ± 0.12 | 67.21 ± 0.29 | 39.61 ± 1.66 | 24.34 ± 1.63 | 7.22 ± 1.72 | 46.45 ± 0.51 | 16.03 ± 0.91 | 4.34 ± 0.89 | 55.56 ± 1.00 | 7.72 ± 0.44 | 9.80 ± 0.56 | 43.10 ± 7.60 |
| 3 | 1.00000 | −1.00000 | 20.5 | 3 | 5.34 ± 0.06 | 5.21 ± 0.03 | 5.86 ± 0.01 | 3.97 ± 0.51 | 67.47 ± 0.76 | 38.64 ± 1.17 | 25.31 ± 1.46 | 7.73 ± 1.33 | 50.34 ± 3.80 | 11.93 ± 1.13 | 4.74 ± 0.97 | 58.60 ± 0.73 | 7.41 ± 0.94 | 10.25 ± 0.54 | 38.02 ± 7.83 |
| Parameter | Equation | R2 | R2 adj | Lack of Fit | |
|---|---|---|---|---|---|
| pH | Brine | pH = 5.111* + 0.077 t* − 0.002 t2* − 0.115 C* + 0.003 C2* + 0.0005 tC | 0.97 | 0.94 | Not significant |
| Marinated steak | pH = 5.476* − 0.016 t* + 0.0004 t2 − 0.021 C* + 0.0008 C2* − 0.0007 tC | 0.95 | 0.92 | Not significant | |
| Cooked steak | pH = 5.820* + 0.017 t − 0.0005 t2 − 0.016 C + 0.0004 C2 − 0.00006 tC | 0.68 | 0.42 | Significant | |
| Marination yield (MY) | MY = 0.275 + 0.375 t* − 0.008 t2 + 0.059 C − 0.003 C2 − 0.006 tC | 0.86 | 0.75 | Not significant | |
| Cooking yield (CY) | CY = 66.180* − 2.746 t + 0.706 t2 + 0.290 C + 2.969 C2 − 0.053 tC | 0.55 | 0.18 | Not significant | |
| Color | Marinated steak | ΔL = 1.858 + 1.267 t* − 0.036 t2* + 0.125 C − 0.005 C2 − 0.0007 tC | 0.84 | 0.71 | Not significant |
| Δa = −2.836 − 1.123 t* + 0.027 t2* − 0.185 C + 0.002 C2 + 0.011 tC | 0.86 | 0.74 | Not significant | ||
| Δb = −0.484 − 0.421 t* + 0.012 t2 − 0.094 C + 0.001 C2 + 0.009 tC | 0.57 | 0.21 | Not significant | ||
| Cooked steak | ΔL = 12.630* + 0.507 t* − 0.009 t2 + 0.246 C − 0.009 C2 − 0.0006 tC | 0.85 | 0.73 | Not significant | |
| Δa = −13.691* − 0.330 t + 0.006 t2 − 0.232 C + 0.010 C2 + 0.004 tC | 0.53 | 0.14 | Not significant | ||
| Δb = 4.468* − 0.223 t + 0.007 t2 − 0.240 C + 0.009 C2 − 0.0007 tC | 0.53 | 0.13 | Not significant | ||
| Hardness steak (H) | H = 48.916* - 0.398 t + 0.009 t2 − 1.064 C* + 0.030 C2 − 0.012 tC | 0.89 | 0.80 | Not significant | |
| Sample Processing | Concentration (mg Tyrosine. 100 g−1 Meat) | Time (h) | Microphotography | Qualitative Description |
|---|---|---|---|---|
| Raw | 10 | 12 |  | On this microphotograph is visible a moderate degree of disorganization of skeletal muscle fibers and a moderate degree of disorganization of the inter-muscular collagen fibers (H&E. Barr 200 µm). |
| 17 | 3.5 |  | On this microphotograph is visible a slight degree of disorganization of skeletal muscle fibers. (H&E. Barr 200 µm). | |
| 20.5 |  | On this microphotography is visible a moderate degree of disorganization of the inter-muscular collagen fibers and muscle fibers are generally preserved (H&E. Barr 200 µm). | ||
| 20 | 12 |  | On this microphotography is visible a moderate degree of disorganization of the inter-muscular collagen fibers and muscle fibers are swollen (H&E. Barr 200 µm). | |
| Cooked | 12 |  | On this microphotograph is visible a severe degree of disorganization of skeletal muscle fibers and a complete disorganization of the inter-muscular collagen fibers (H&E. Barr 200 µm). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, D.I.; Fraqueza, M.J.; Pissarra, H.; Saraiva, J.A.; Vicente, A.A.; Moldão-Martins, M. Optimization of the Effect of Pineapple By-Products Enhanced in Bromelain by Hydrostatic Pressure on the Texture and Overall Quality of Silverside Beef Cut. Foods 2020, 9, 1752. https://doi.org/10.3390/foods9121752
Santos DI, Fraqueza MJ, Pissarra H, Saraiva JA, Vicente AA, Moldão-Martins M. Optimization of the Effect of Pineapple By-Products Enhanced in Bromelain by Hydrostatic Pressure on the Texture and Overall Quality of Silverside Beef Cut. Foods. 2020; 9(12):1752. https://doi.org/10.3390/foods9121752
Chicago/Turabian StyleSantos, Diana I., Maria João Fraqueza, Hugo Pissarra, Jorge A. Saraiva, António A. Vicente, and Margarida Moldão-Martins. 2020. "Optimization of the Effect of Pineapple By-Products Enhanced in Bromelain by Hydrostatic Pressure on the Texture and Overall Quality of Silverside Beef Cut" Foods 9, no. 12: 1752. https://doi.org/10.3390/foods9121752
APA StyleSantos, D. I., Fraqueza, M. J., Pissarra, H., Saraiva, J. A., Vicente, A. A., & Moldão-Martins, M. (2020). Optimization of the Effect of Pineapple By-Products Enhanced in Bromelain by Hydrostatic Pressure on the Texture and Overall Quality of Silverside Beef Cut. Foods, 9(12), 1752. https://doi.org/10.3390/foods9121752