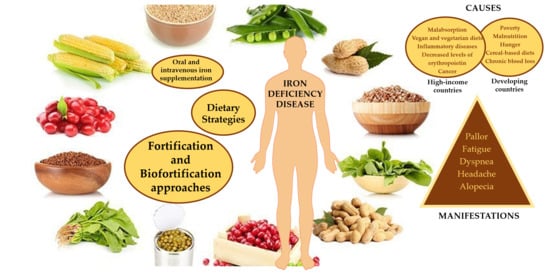
Fighting Iron-Deficiency Anemia: Innovations in Food Fortificants and Biofortification Strategies
Abstract
:1. Introduction
2. Iron: A Double-Edged Sword?
3. Iron Deficiency
4. Iron Absorption and Regulation Mechanisms
4.1. Effect of a Balanced Diet on the Iron Bioavailability and Absorption
Enhancers and Inhibitors of Iron Absorption
4.2. Recommended Dietary Allowances (RDA) of Iron
5. Dietary Strategies for Addressing Iron Deficiencies
5.1. Innovative Food Fortification and Biofortification Procedures
5.1.1. Fortification Approaches
| Plant Food | Typical Iron Content (µg/g) a | Biofortification Target (mg/g dw) | Fold Increase | Reference |
|---|---|---|---|---|
| Rice, brown | 15 | [52] | ||
| Rice, polished | 2 | 15 | 7.5× | [53] |
| Wheat, whole meal | 30 | 59 | 2× | [52] |
| Wheat, flour, white | 7 | [54] | ||
| Maize, whole | 30 | 60 | 2× | [52] |
| Common bean | 50 | 107 | 2.1× | [53] |
| Peas, dried | 50 | [53] | ||
| Pearl millet | 47 | 88 | 1.9× | [55] |
| Cassava root | 5 | 45 | 9× | [53] |
| Sweet potato | 6 | 85 | 14.2× | [56] |
| Irish potato | 3 | [57] | ||
| Cabbage, broccoli | 17 | |||
| Tomatoes | 5 | |||
| Beef steak | 35 |
| Fortification Approach | Food Vehicle | Key Outcomes | Reference |
|---|---|---|---|
| Ferrous sulfate and ferrous gluconate enhancement | Infant formula | Both ferrous sulfate and ferrous gluconate were successful when added as fortifiers in an infant formula, which showed positive effects on the iron status markers, namely in ferritin and sTfR levels and in body iron stores. | [63] |
| Microencapsulated iron pyrophosphate | Fruit juice | The daily consumption of a microencapsulated iron pyrophosphate-fortified fruit juice shows an increase in Fe status in a short period of time (4 weeks). This consumption was well-suited with the traditional diet, and the extra daily 18 mg of Fe provided was 100% of the RDA. | [66] |
| Dairy products | Iron-fortified seasoned skim milk does not expand the iron status in iron-deficient menstruating women due to the presence of calcium, a well-known iron absorption inhibitor. | [67] | |
| Treatment with cold plasma | Rice | Improved bioavailability of the free ferrous iron state (Fe2+) in the fortified rice next to cold plasma processing. Extended storage time without changing the rice characteristics. | [74] |
| Ferrous sulfate, ferrous fumarate, and NaFeEDTA enhancement | Curry powder | No hostile effects on the physical, chemical, and sensory attributes of curry powder. Bioavailability of NaFeEDTA were 1.05 times higher than that of ferrous sulfate. | [75] |
| Microencapsulation of ferrous sulfate | Liposomes | The approach applied to dairy products proved to be extremely effective, showing good oxidative stability and biocompatibility, and low cytotoxicity, once the encapsulation protects iron from the straight interaction with other components of the food vehicle. | [78] |
| Ferritin enhancement | Nanoparticles | Good iron supplier, since ferritin holds large amounts of iron. | [81] |
5.1.2. Biofortification Procedures
Conventional Biofortification
Agronomic Biofortification
Genetic Biofortification
5.2. Risks and Benefits of the Different Iron Supplementation Approaches: Balancing Efficacy and Safety
6. Conclusions and Future Trends
Author Contributions
Funding
Conflicts of Interest
References
- WHO; FAO. Handbook of Nutritionally Essential Mineral Elements, 1st ed.; O’Dell, B.L., Sunde, R.A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-367-40106-1. [Google Scholar]
- Guggenheim, K.Y. Chlorosis: The Rise and Disappearance of a Nutritional Disease. J. Nutr. 1995, 125, 1822–1825. [Google Scholar] [CrossRef]
- Allen, L.; de Benoist, B.; Dary, O.; Hurrell, R. Guidelines on Food Fortification with Micronutrients; World Health Organization and Food and Agricultural Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Brabin, B.J.; Premji, Z.; Verhoeff, F. An analysis of anemia and child mortality. J. Nutr. 2001, 131, 636S–648S. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Pettit, K.; Rowley, J.; Brown, N. Iron deficiency. Paediatr. Child Health 2011, 21, 339–343. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron deficiency across chronic inflammatory conditions: International expert opinion on definition, diagnosis, and management. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [Green Version]
- Camaschella, C. Iron deficiency: New insights into diagnosis and treatment. Hematology 2015, 2015, 8–13. [Google Scholar] [CrossRef] [Green Version]
- FAO; IFAD; WFP. The State of Food Insecurity in the World 2015. Meeting the 2015 International Hunger Targets: Taking Stock of Uneven Progress 2015. Available online: http://www.fao.org/3/a-i4646e.pdf (accessed on 10 August 2020).
- Black, R.E.; Allen, L.H.; Bhutta, Z.A.; Caulfield, L.E.; de Onis, M.; Ezzati, M.; Mathers, C.; Rivera, J. Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet 2008, 371, 243–260. [Google Scholar] [CrossRef]
- Meenakshi, J.V.; Johnson, N.L.; Manyong, V.M.; De Groote, H.; Javelosa, J.; Yanggen, D.R.; Naher, F.; Gonzalez, C.; García, J.; Meng, E. How cost-effective is biofortification in combating micronutrient malnutrition? An ex ante assessment. World Dev. 2010, 38, 64–75. [Google Scholar] [CrossRef] [Green Version]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Aisen, P.; Enns, C.; Wessling-Resnick, M. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 2001, 33, 940–959. [Google Scholar] [CrossRef]
- Muñoz, M.; Villar, I.; García-Erce, J.A. An update on iron physiology. World J. Gastroenterol. 2009, 15, 4617–4626. [Google Scholar] [CrossRef] [PubMed]
- IOM (Institute of Medicine). Dietary Reference Intakes for Vitamin a, Vitamin k, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Chua, A.C.G.; Graham, R.M.; Trinder, D.; Olynyk, J.K. The regulation of cellular iron metabolism. Crit. Rev. Clin. Lab. Sci. 2007, 44, 413–459. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J. Mechanisms of iron loading and toxicity. Am. J. Hematol. 2007, 82, 1128–1131. [Google Scholar] [CrossRef]
- Cairo, G.; Recalcati, S. Iron-regulatory proteins: Molecular biology and pathophysiological implications. Expert Rev. Mol. Med. 2007, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rouault, T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Jasrasaria, R.; Naghavi, M.; Wulf, S.K.; Johns, N.; Lozano, R.; Regan, M.; Weatherall, D.; Chou, D.P.; Eisele, T.P.; et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood 2014, 123, 615–624. [Google Scholar] [CrossRef]
- Bach, V.; Schruckmayer, G.; Sam, I.; Kemmler, G.; Stauder, R. Prevalence and possible causes of anemia in the elderly: A cross-sectional analysis of a large European university hospital cohort. Clin. Interv. Aging 2014, 9, 1187–1196. [Google Scholar]
- Aigner, E.; Feldman, A.; Datz, C. Obesity as an emerging risk factor for iron deficiency. Nutrients 2014, 6, 3587–3600. [Google Scholar] [CrossRef]
- Cohen-Solal, A.; Damy, T.; Terbah, M.; Kerebel, S.; Baguet, J.-P.; Hanon, O.; Zannad, F.; Laperche, T.; Leclercq, C.; Concas, V.; et al. High prevalence of iron deficiency in patients with acute decompensated heart failure. Eur. J. Heart Fail. 2014, 16, 984–991. [Google Scholar] [CrossRef] [Green Version]
- Camaschella, C. Iron-deficiency anemia. N. Engl. J. Med. 2015, 372, 1832–1843. [Google Scholar] [CrossRef] [Green Version]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Nickol, A.H.; Frise, M.C.; Cheng, H.Y.; McGahey, A.; McFadyen, B.M.; Harris-Wright, T.; Bart, N.K.; Curtis, M.K.; Khandwala, S.; O’Neill, D.P.; et al. A cross-sectional study of the prevalence and associations of iron deficiency in a cohort of patients with chronic obstructive pulmonary disease. Bmj Open 2015, 5, e007911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auerbach, M.; Adamson, J.W. How we diagnose and treat iron deficiency anemia. Am. J. Hematol. 2016, 91, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Grotto, H.Z.W. Iron metabolism: An overview on the main mechanisms involved in its homeostasis. Rev. Bras. Hematol. E Hemoter. 2008, 30, 390–397. [Google Scholar]
- Hunt, J.R. Dietary and physiological factors that affect the absorption and bioavailability of iron. Int. J. Vitam. Nutr. Res. 2005, 75, 375–384. [Google Scholar] [CrossRef]
- Beaumont, C.; Canonne-Hergaux, F. Erythrophagocytosis and recycling of heme iron in normal and pathological conditions; regulation by hepcidin. Transfus. Clin. Biol. 2005, 12, 123–130. [Google Scholar] [CrossRef]
- Gibson, R.S.; Heath, A.L.M.; Szymlek-Gay, E.A. Is iron and zinc nutrition a concern for vegetarian infants and young children in industrialized countries? Am. J. Clin. Nutr. 2014, 100, 459S–468S. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Anukiruthika, T.; Dutta, S.; Kashyap, A.; Moses, J.; Anandharamakrishnan, C. Iron deficiency anemia: A comprehensive review on iron absorption, bioavailability and emerging food fortification approaches. Trends Food Sci. 2020, 99, 58–75. [Google Scholar]
- Hurrell, R.; Egli, I. Iron bioavailability and dietary reference values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Akibode, S.; Maredia, M. Global and Regional Trends in Production, Trade and Consumption of Food Legume Crops; 2012. Available online: https://ageconsearch.umn.edu/record/136293/ (accessed on 10 July 2020).
- Magalhaes, S.; Taveira, M.; Cabrita, A.; Fonseca, A.; Valentão, P.; Andrade, P. European marketable grain legume seeds: Further insight into phenolic compounds profiles. Food Chem. 2017, 215, 177–184. [Google Scholar] [CrossRef]
- Mann, J.; Truswell, A. Essentials in Human Nutrition; Mann, J., Truswell, A., Eds.; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Pavord, S.; Daru, J.; Prasannan, N.; Robinson, S.; Stanworth, S.; Girling, J. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 2019, 188, 819–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson, R.; Raboy, V.; King, J. Implications of phytate in plant-based foods for iron and zinc bioavailability, setting dietary requirements, and formulating programs and policies. Nutr. Rev. 2018, 76, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Hoppe, M.; Andersson, M.; Pediatrics, L.H. The role of meat to improve the critical iron balance during weaning. Am Acad Pediatr. 2003, 111, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.A. Intestinal iron absorption: Regulation by dietary & systemic factors. Int. J. Vitam. Nutr. Res. 2010, 80, 231–242. [Google Scholar]
- Ma, Q.; Kim, E.Y.; Lindsay, E.A.; Han, O. Bioactive dietary polyphenols inhibit heme iron absorption in a dose-dependent manner in human intestinal Caco-2 cells. J. Food Sci. 2011, 76, H143–H150. [Google Scholar] [CrossRef] [Green Version]
- Serna-Saldivar, S.; Corn, E.C. Food Uses of Whole Corn and Dry-Milled Fractions; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Bjørklund, G.; Aaseth, J.; Skalny, A.; Suliburska, J.; Skalnaya, M.; Nikonorov, A.; Tinkov, A. Interactions of iron with manganese, zinc, chromium, and selenium as related to prophylaxis and treatment of iron deficiency. J. Trace Elem. Med. Biol. 2017, 41, 41–53. [Google Scholar] [CrossRef]
- Roth, J.; Garrick, M. Iron interactions and other biological reactions mediating the physiological and toxic actions of manganese. Biochem. Pharm. 2003, 66, 1–13. [Google Scholar] [CrossRef]
- Erikson, K.; Syversen, T.; Aschner, J.; Aschener, M. Interactions between excessive manganese exposures and dietary iron-deficiency in neurodegeneration. Environ. Toxicol. Pharm. 2005, 19, 415–421. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M.; McLaren, G.D. Iron absorption and metabolism. Curr. Opin. Gastroenterol. 2009, 25, 129–135. [Google Scholar] [CrossRef]
- World Health Organization. The Global Prevalence of Anaemia in 2011; Geneva World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Huma, N.; Salim-Ur-Rehman; Anjum, F.M.; Murtaza, M.A.; Sheikh, M.A. Food fortification strategy—Preventing iron deficiency anemia: A review. Crit. Rev. Food Sci. Nutr. 2007, 47, 259–265. [Google Scholar] [CrossRef]
- Xia, S.; Xu, S. Ferrous sulfate liposomes: Preparation, stability and application in fluid milk. Food Res. Int. 2005, 38, 289–296. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Lynch, S.; Bothwell, T.; Cori, H.; Glahn, R.; Hertrampf, E.; Kratky, Z.; Miller, D.; Rodenstein, M.; Streekstra, H.; et al. Enhancing the absorption of fortification iron: A SUSTAIN Task Force report. Int. J. Vitam. Nutr. Res. 2004, 74, 387–401. [Google Scholar] [CrossRef]
- Connorton, J.; Balk, J. Iron biofortification of staple crops: Lessons and challenges in plant genetics. Plant Cell Physiol. 2019, 60, 1447–1456. [Google Scholar] [CrossRef] [Green Version]
- Daoura Goudia, B.; Hash, C.T. Breeding for High Grain Fe and Zn Levels in Cereals. Int. J. Innov. Appl. Stud. 2015, 12, 342–354. [Google Scholar]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets - Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Tang, J.; Zou, C.; He, Z.; Shi, R.; Ortiz-Monasterio, I.; Qu, Y.; Zhang, Y. Mineral element distributions in milling fractions of Chinese wheats. J. Cereal Sci. 2008, 48, 821–828. [Google Scholar] [CrossRef]
- Bouis, H.E.; Hotz, C.; McClafferty, B.; Meenakshi, J.V.; Pfeiffer, W.H. Biofortification: A new tool to reduce micronutrient malnutrition. Food Nutr. Bull. 2011, 32, S31–S40. [Google Scholar] [CrossRef]
- Laurie, S.; Van Jaarsveld, P.; Faber, M.; Philpott, M.; Labuschangne, M. Trans-β-carotene, selected mineral content and potential nutritional contribution of 12 sweetpotato varieties. J. Food Compos. Anal. 2012, 27, 151–159. [Google Scholar] [CrossRef]
- De Haan, S.; Burgos, G.; Ccanto, R.; Arcos, J.; Scurrah, M.; Salas, E.; Bonierbale, M. Effect of production environment, genotype and process on the mineral content of native bitter potato cultivars converted into white chuño. J. Sci. Food Agric. 2012, 92, 2098–2105. [Google Scholar] [CrossRef]
- Khan, A.; Singh, J.; Upadhayay, V.K.; Singh, A.V.; Shah, S. Microbial biofortification: A green technology through plant growth promoting microorganisms. In Sustainable Green Technologies for Environmental Management; Springer: Singapore, 2019; pp. 255–269. ISBN 9789811327728. [Google Scholar]
- EFSA. Panel on Food Additives and Nutrient Sources added to Food (ANS) Scientific Opinion on the use of ferric sodium EDTA as a source of iron added for nutritional purposes to foods for the general population (including food supplements) and to foods for particular nutritional uses. EFSA J. 2010, 8, 141414. [Google Scholar]
- EFSA. Iron (II) taurate, magnesium taurate and magnesium acetyl taurate as sources of iron or magnesium added for nutritional purposes in food supplements. EFSA J. 2009, 7, 947. [Google Scholar] [CrossRef]
- EFSA. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to Calcium, iron, magnesium, potassium and zinc L-pidolate as sources for calcium, iron, magnesium, potassium and zinc added for nutritional purposes to food supplements and to foods intended for particular nutritional uses. EFSA J. 2007, 5, 495. [Google Scholar]
- Diego Quintaes, K.; Barberá, R.; Cilla, A. Iron bioavailability in iron-fortified cereal foods: The contribution of in vitro studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 2028–2041. [Google Scholar] [CrossRef] [PubMed]
- Shamah-Levy, T.; Villalpando, S.; Rivera-Dommarco, J.A.; Mundo-Rosas, V.; Cuevas-Nasu, L.; Jiménez-Aguilar, A. Ferrous gluconate and ferrous sulfate added to a complementary food distributed by the Mexican Nutrition program oportunidades have a comparable efficacy to reduce iron deficiency in toddlers. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 660–666. [Google Scholar] [CrossRef]
- Villalpando, S.; Shamah, T.; Rivera, J.A.; Lara, Y.; Monterrubio, E. Fortifying milk with ferrous gluconate and zinc oxide in a public nutrition program reduced the prevalence of anemia in toddlers. J. Nutr. 2006, 136, 2633–2637. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Rojo, R.; Vaquero, M.P. Iron bioavailability from food fortification to precision nutrition. A review. Innov. Food Sci. Emerg. Technol. 2019, 51, 126–138. [Google Scholar] [CrossRef]
- Blanco-Rojo, R.; Pérez-Granados, A.M.; Toxqui, L.; González-Vizcayno, C.; Delgado, M.A.; Vaquero, M.P. Efficacy of a microencapsulated iron pyrophosphate-fortified fruit juice: 2 a randomised, double-blind, placebo-controlled study in Spanish iron-deficient women. Br. J. Nutr. 2011, 105, 1652–1659. [Google Scholar] [CrossRef] [Green Version]
- Toxqui, L.; Pérez-Granados, A.M.; Blanco-Rojo, R.; Wright, I.; González-Vizcayno, C.; Vaquero, M.P. Effects of an Iron or Iron and Vitamin D-Fortified Flavored Skim Milk on Iron Metabolism: A Randomized Controlled Double-Blind Trial in Iron-Deficient Women. J. Am. Coll. Nutr. 2013, 32, 312–320. [Google Scholar] [CrossRef] [Green Version]
- Shilpashree, B.G.; Arora, S.; Sharma, V.; Singh, A.K. Preparation of succinylated sodium caseinate-iron complex by adopting ultrafiltration technology: A novel food fortificant. Innov. Food Sci. Emerg. Technol. 2015, 32, 165–171. [Google Scholar] [CrossRef]
- Podder, R.; Hassan Al Imam, M.; Jahan, I.; Yunus, F.M.; Muhit, M.; Vandenberg, A. Sensory Acceptability of Dual-Fortified Milled Red and Yellow Lentil (Lens culinaris Medik.) Dal in Bangladesh. Foods 2020, 9, 992. [Google Scholar] [CrossRef]
- Podder, R.; M DellaValle, D.; Tyler, R.T.; Glahn, R.P.; Tako, E.; Vandenberg, A. Relative Bioavailability of Iron in Bangladeshi Traditional Meals Prepared with Iron-Fortified Lentil Dal. Nutrients 2018, 10, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martınez-Navarrete, N. Camacho MM Iron deficiency and iron fortified foods—A review. Food Res. Int. 2002, 35, 225–231. [Google Scholar] [CrossRef]
- FAO. The State of Food and Agriculture 2001; FAO: Rome, Italy, 2001. [Google Scholar]
- Thirumdas, R.; Saragapani, C.; Ajinkya, M.; Deshmukh, R.; Annapure, U. Influence of low pressure cold plasma on cooking and textural properties of brown rice. Innov. Food Sci. Emerg. Technol. 2016, 37, 53–60. [Google Scholar] [CrossRef]
- Akasapu, K.; Ojah, N.; Gupta, A.K.; Choudhury, A.J.; Mishra, P. An innovative approach for iron fortification of rice using cold plasma. Food Res. Int. 2020, 136, 109599. [Google Scholar] [CrossRef] [PubMed]
- Karn, S.K.; Chavasit, V.; Kongkachuichai, R.; Tangsuphoom, N. Shelf stability, sensory qualities, and bioavailability of iron-fortified Nepalese curry powder. Food Nutr. Bull. 2011, 32, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharibzahedi, S.; Jafari, S. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Fodd Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Mehansho, H. Iron fortification technology development: New approaches. J. Nutr. 2006, 136, 1059–1063. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Kenaan, A.; Zhao, D.; Qi, D.; Song, J. Photo-polymerizable ferrous sulfate liposomes as vehicles for iron fortification of food. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102286. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; Tran, C.; Lawrence, A. Liposomal delivery systems for encapsulation of ferrous sulfate: Preparation and characterization. J. Liposome Res. 2006, 16, 347–358. [Google Scholar] [CrossRef]
- Chamorro, S.; Gutiérrez, L.; Vaquero, M.; Verdoy, D.; Salas, G.; Luengo, Y.; Teran, F. Safety assessment of chronic oral exposure to iron oxide nanoparticles Related content. Nanotechnology 2015, 26, 205101. [Google Scholar] [CrossRef]
- Latunde-Dada, G.; Pereira, D.; Tempest, B.; Ilyas, H.; Flynn, A.; Aslam, M.; Powell, J. A nanoparticulate ferritin-core mimetic is well taken up by HuTu 80 duodenal cells and its absorption in mice is regulated by body iron. J. Nutr. 2014, 144, 1896–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiran, K. Advanced Approaches for Biofortification. In Advances in Agri-Food Biotechnology; Springer: Singapore, 2020; pp. 29–55. [Google Scholar]
- Finkelstein, J.; Haas, J.; Mehta, S. Iron-biofortified staple food crops for improving iron status: A review of the current evidence. Curr. Opin. Biotechnol. 2017, 44, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, A.; Birol, E.; Oparinde, A. Availability, production, and consumption of crops biofortified by plant breeding: Current evidence and future potential. Ann. N. Y. Acad. Sci. 2017, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef]
- Shariatipour, N.; Heidari, B. Genetic-Based Biofortification of Staple Food Crops to Meet Zinc and Iron Deficiency-Related Challenges. In Plant Micronutrients; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; pp. 173–223. [Google Scholar]
- Garcia-Oliveira, A.L.; Chander, S.; Ortiz, R.; Menkir, A.; Gedil, M. Genetic basis and breeding perspectives of grain iron and zinc enrichment in cereals. Front. Nutr. 2018, 9, 937. [Google Scholar] [CrossRef]
- Cakmak, I.; Torun, A.; Millet, E.; Feldman, M.; Fahima, T.; Korol, A.; Nevo, E.; Braun, H.J.; Özkan, H. Triticum dicoccoides: An important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci. Plant Nutr. 2004, 50, 1047–1054. [Google Scholar] [CrossRef] [Green Version]
- Heidari, B.; Padash, S.; Dadkhodaie, A. Variations in micronutrients, bread quality and agronomic traits of wheat landrace varieties and commercial cultivars. Aust. J. Crop Sci. 2016, 10, 377–384. [Google Scholar] [CrossRef]
- Monasterio, I.; Graham, R.D. Breeding for trace minerals in wheat. Food Nutr. Bull. 2000, 21, 392–396. [Google Scholar] [CrossRef]
- Velu, G.; Singh, R.; Crespo-Herrera, L.; Juliana, P.; Dreisigacker, S.; Valluru, R.; Balasubramaniam, A. Genetic dissection of grain zinc concentration in spring wheat for mainstreaming biofortification in CIMMYT wheat breeding. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Zingore, S.; Delve, R.J.; Nyamangara, J.; Giller, K.E. Multiple benefits of manure: The key to maintenance of soil fertility and restoration of depleted sandy soils on African smallholder farms. Nutr. Cycl. Agroecosystems 2008, 80, 267–282. [Google Scholar] [CrossRef]
- Marschner, H.; Romheld, V.; Kissel, M. Different strategies in higher plants in mobilization and uptake of iron. J. Plant Nutr. 1986, 9, 695–713. [Google Scholar] [CrossRef]
- Murata, Y.; Ma, J.F.; Yamaji, N.; Ueno, D.; Nomoto, K.; Iwashita, T. A specific transporter for iron(III)-phytosiderophore in barley roots. Plant J. 2006, 46, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Zuchi, S.; Cesco, S.; Astolfi, S. High S supply improves Fe accumulation in durum wheat plants grown under Fe limitation. Environ. Exp. Bot. 2012, 77, 25–32. [Google Scholar] [CrossRef]
- Astolfi, S.; Pii, Y.; Terzano, R.; Mimmo, T.; Celletti, S.; Allegretta, I.; Lafiandra, D.; Cesco, S. Does Fe accumulation in durum wheat seeds benefit from improved whole-plant sulfur nutrition? J. Cereal Sci. 2018, 83, 74–82. [Google Scholar] [CrossRef]
- Kutman, U.; Yildiz, B.; Cakmak, I. Improved nitrogen status enhances zinc and iron concentrations both in the whole grain and the endosperm fraction of wheat. J. Cereal Sci. 2011, 53, 118–125. [Google Scholar] [CrossRef]
- Li, D.; Wu, Z. Impact of chemical fertilizers application on soil ecological environment. J. Appl. Ecol. 2008, 19, 1158–1165. [Google Scholar]
- Fan, Y.; Zhu, T.; Li, M.; He, J.; Huang, R. Heavy metal contamination in soil and brown rice and human health risk assessment near three mining areas in central China. J. Healthc. Eng. 2017, 2017, 4124302. [Google Scholar] [CrossRef]
- Fakharzadeh, S.; Hafizi, M.; Baghaei, M.; Etesami, M.; Khayamzadeh, M.; Kalanaky, S.; Nazaran, M. Using nanochelating technology for Biofortification and Yield increase in Rice. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Yin, X.; Biswal, A.K.; Dionora, J.; Perdigon, K.M.; Balahadia, C.P.; Mazumdar, S.; Chater, C.; Lin, H.C.; Coe, R.A.; Kretzschmar, T.; et al. CRISPR-Cas9 and CRISPR-Cpf1 mediated targeting of a stomatal developmental gene EPFL9 in rice. Plant Cell Rep. 2017, 36, 745–757. [Google Scholar] [CrossRef]
- Carvalho, S.M.P.; Vasconcelos, M.W. Producing more with less: Strategies and novel technologies for plant-based food biofortification. Food Res. Int. 2013, 54, 961–971. [Google Scholar] [CrossRef]
- Beasley, J.T.; Bonneau, J.P.; Sánchez-Palacios, J.T.; Moreno-Moyano, L.T.; Callahan, D.L.; Tako, E.; Glahn, R.P.; Lombi, E.; Johnson, A.A.T. Metabolic engineering of bread wheat improves grain iron concentration and bioavailability. Plant Biotechnol. J. 2019, 17, 1514–1526. [Google Scholar] [CrossRef]
- Boonyaves, K.; Wu, T.Y.; Gruissem, W.; Bhullar, N.K. Enhanced grain iron levels in iron-regulated metal transporter, nicotianamine synthase, and ferritin gene cassette. Front. Plant Sci. 2017, 8, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Massot, E.; Banakar, R.; Gómez-Galera, S.; Zorrilla-López, U.; Sanahuja, G.; Arjó, G.; Miralpeix, B.; Vamvaka, E.; Farré, G.; Rivera, S.M.; et al. The contribution of transgenic plants to better health through improved nutrition: Opportunities and constraints. Genes Nutr. 2013, 8, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mushtaq, M.; Mukhtar, S.; Sakina, A.; Dar, A.; Dar, A.A.; Bhat, R.; Deshmukh, R.; Molla, K.; Kundoo, A.A.; Dar, S. Tweaking genome-editing approaches for virus interference in crop plants Identification of core set from large germplasm collection in various crops. View project salinity View project Tweaking genome-editing approaches for virus interference in crop plants. Plant Physiol. Biochem. 2020, 147, 242–250. [Google Scholar] [PubMed]
- Ricroch, A.E.; Hénard-Damave, M.-C. Next biotech plants: New traits, crops, developers and technologies for addressing global challenges Next biotech plants: New traits, crops, developers and technologies for addressing global challenges. Crit. Rev. Biotechnol. 2016, 36, 675–690. [Google Scholar] [CrossRef]
- Tolay, I.; Erenoglu, B.; Römheld, V.; Braun, H.; Cakmak, I. Phytosiderophore release in Aegilops tauschii and Triticum species under zinc and iron deficiencies. J. Exp. Bot. 2001, 52, 1093–1099. [Google Scholar] [CrossRef] [Green Version]
- Prasanna, R.; Nain, L.; Rana, A.; Shivay, Y.S. Biofortification with microorganisms: Present status and future challenges. In Biofortification of Food Crops; Singh, U., Praharaj, C.S., Singh, S.S., Singh, N.P., Eds.; Springer: New Delhi, India, 2016; pp. 249–262. ISBN 978-81-322-2716-8. [Google Scholar]
- Barret, M.; Morrissey, J.P.; O’Gara, F. Functional genomics analysis of plant growth-promoting rhizobacterial traits involved in rhizosphere competence. Biol. Fertil. Soils 2011, 47, 729–743. [Google Scholar] [CrossRef]
- Hardoim, P.R.; Andreote, F.D.; Reinhold-Hurek, B.; Sessitsch, A.; van Overbeek, L.S.; van Elsas, J.D. Rice root-associated bacteria: Insights into community structures across10 cultivars. Fems Microbiol. Ecol. 2011, 77, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Van der Heijden, M.G.A.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Antoun, H.; Prévost, D. Ecology of plant growth promoting rhizobacteria. In PGPR: Biocontrol and Biofertilization; Springer: Dordrecht, The Netherlands, 2006; pp. 1–38. ISBN 1-4020-4002-4. [Google Scholar]
- Rana, A.; Joshi, M.; Prasanna, R.; Shivay, Y.S.; Nain, L. Biofortification of wheat through inoculation of plant growth promoting rhizobacteria and cyanobacteria. Eur. J. Soil Biol. 2012, 50, 118–126. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations and CAB International. Combating Micronutrient Deficiencies: Food-Based Approaches; Thompson, B., Amoroso, L., Eds.; Food and Agriculture Organization of the United Nations and CAB International: Rome, Italy, 2011. [Google Scholar]
- World Health Organization. Food and Agricultura Organization of the United Nations. Guidelines on Food Fortification with Micronutrients; Allen, L., Benoist, B., Dary, O., Hurrel, R., Eds.; World Health Organization: Geneva, Switzerland, 2006; ISBN 92-4-159401. [Google Scholar]
- FAO; WHO Codex Alimentarius. Guidelines on Nutrition Labelling. 1985. Available online: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B2-1985%252FCXG_002e.pdf (accessed on 18 October 2020).
- Mannar, V.; Hurrell, R. Food Fortification in a Globalized World. In Food Fortification in a Globalized World; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Eichler, K.; Wieser, S.; Rüthemann, I.; Brügger, U. Effects of Micronutrient Fortified Milk and Cereal Food for Infants and Children: A Systematic Review. BMC Public Health 2012, 12, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meenakshi, J.V.; Banerji, A.; Manyong, V.; Tomlins, K.; Mittal, N.; Hamukwala, P. Using a discrete choice experiment to elicit the demand for a nutritious food: Willingness-to-pay for orange maize in rural Zambia. J. Health Econ. 2012, 31, 62–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saltzman, A.; Birol, E.; Bouis, H.E.; Boy, E.; De Moura, F.F.; Islam, Y.; Pfeiffer, W.H. Biofortification: Progress toward a more nourishing future. Global Food Secur. 2013, 2, 9–17. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liberal, Â.; Pinela, J.; Vívar-Quintana, A.M.; Ferreira, I.C.F.R.; Barros, L. Fighting Iron-Deficiency Anemia: Innovations in Food Fortificants and Biofortification Strategies. Foods 2020, 9, 1871. https://doi.org/10.3390/foods9121871
Liberal Â, Pinela J, Vívar-Quintana AM, Ferreira ICFR, Barros L. Fighting Iron-Deficiency Anemia: Innovations in Food Fortificants and Biofortification Strategies. Foods. 2020; 9(12):1871. https://doi.org/10.3390/foods9121871
Chicago/Turabian StyleLiberal, Ângela, José Pinela, Ana Maria Vívar-Quintana, Isabel C. F. R. Ferreira, and Lillian Barros. 2020. "Fighting Iron-Deficiency Anemia: Innovations in Food Fortificants and Biofortification Strategies" Foods 9, no. 12: 1871. https://doi.org/10.3390/foods9121871
APA StyleLiberal, Â., Pinela, J., Vívar-Quintana, A. M., Ferreira, I. C. F. R., & Barros, L. (2020). Fighting Iron-Deficiency Anemia: Innovations in Food Fortificants and Biofortification Strategies. Foods, 9(12), 1871. https://doi.org/10.3390/foods9121871