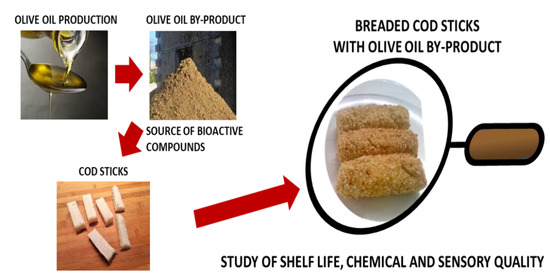
Food By-Products to Extend Shelf Life: The Case of Cod Sticks Breaded with Dried Olive Paste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Breaded Cod Sticks Preparation
2.3. Chemicals
2.4. Extraction of Bioactive Compounds
2.5. Determination of Total Phenols Content, Total Flavonoids, and Antioxidant Activity
2.6. Microbiological Analyses and pH Determination
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Total Phenols, Total Flavonoids, and Antioxidant Activity of Breaded Cod Sticks
3.2. Microbial Quality of Breaded Cod Sticks
3.3. Sensory Quality of Breaded Cod Sticks
3.4. Breaded Cod Sticks Shelf Life
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hosomi, R.; Yoshida, M.; Fukunaga, K. Seafood Consumption and Components for Health. GJHS 2012, 4, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heredia, A.; Andrés, A.; Betoret, N.; Fito, P. Application of the SAFES (systematic approach of food engineering systems) methodology to salting, drying and desalting of cod. J. Food Eng. 2007, 83, 267–276. [Google Scholar] [CrossRef]
- Bjørkevoll, I.; Olsen, L.R.; Skjerdal, O.T. Origin and spoilage potential of the microbiota dominating genus Psychrobacter in sterile rehydrated salt-cured and dried salt-cured cod (Gadus morhua). Int. J. Food Microbiol. 2003, 84, 175–187. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Ho, P.; Lopez-Caballero, M.E.; Vaz-Pires, P.; Nunes, M.L. Characterization and identification of microflora from soaked cod and respective salted raw materials. Food Microbiol. 2003, 20, 471–481. [Google Scholar] [CrossRef]
- Geeroms, N.; Verbeke, W.; Van Kenhove, P. Consumers’ health-related motive orientations and ready meal consumption behaviour. Appetite 2008, 51, 704–712. [Google Scholar] [CrossRef]
- Smaldone, G.; Marrone, R.; Zottola, T.; Vollano, L.; Grossi, G.; Cortesi, M.L. Formulation and shelf-life of fish burgers served to preschool children. Ital. J. Food Saf. 2017, 6, 6373. [Google Scholar] [CrossRef] [Green Version]
- Olatunde, O.O.; Benjakul, S. Natural Preservatives for Extending the Shelf-Life of Seafood: A Revisit: Natural preservatives for seafood. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef] [Green Version]
- Alves, V.L.C.D.; Rico, B.P.M.; Cruz, R.M.S.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Preparation and characterization of a chitosan film with grape seed extract-carvacrol microcapsules and its effect on the shelf-life of refrigerated Salmon (Salmo salar). LWT 2018, 89, 525–534. [Google Scholar] [CrossRef] [Green Version]
- Perez-Won, M.; Lemus-Mondaca, R.; Herrera-Lavados, C.; Reyes, J.E.; Roco, T.; Palma-Acevedo, A.; Tabilo-Munizaga, G.; Aubourg, S.P. Combined Treatments of High Hydrostatic Pressure and CO2 in Coho Salmon (Oncorhynchus kisutch): Effects on Enzyme Inactivation, Physicochemical Properties, and Microbial Shelf Life. Foods 2020, 9, 273. [Google Scholar] [CrossRef] [Green Version]
- Senturk, T.; Alpas, H. Effect of High Hydrostatic Pressure Treatment (HHPT) on Quality and Shelf Life of Atlantic Mackerel (Scomber scombrus). Food Bioprocess Technol. 2013, 6, 2306–2318. [Google Scholar] [CrossRef]
- Rode, T.M.; Rotabakk, B.T. Extending shelf life of desalted cod by high pressure processing. Innov. Food Sci. Emerg. Technol. 2020, 102476. [Google Scholar] [CrossRef]
- Magnússon, H.; Sveinsdóttir, K.; Lauzon, E.H.; Thorkelsdóttir, A.; Martinsdóttir, E. Keeping Quality of Desalted Cod Fillets in Consumer Packs. J. Food Sci. 2006, 71, 69–76. [Google Scholar] [CrossRef]
- Wang, T.; Sveinsdóttir, K.; Magnússon, H.; Martinsdóttir, E. Combined Application of Modified Atmosphere Packaging and Superchilled Storage to Extend the Shelf Life of Fresh Cod (Gadus morhua) Loins. J. Food Sci. 2007, 73, S11–S19. [Google Scholar] [CrossRef]
- Demartini, E.; Gaviglio, A.; La Sala, P.; Fiore, M. Impact of information and Food Technology Neophobia in consumers’ acceptance of shelf-life extension in packaged fresh fish fillets. Sustain. Prod. Consump. 2019, 17, 116–125. [Google Scholar] [CrossRef]
- Seto, K.; Fiorella, K.J. From Sea to Plate: The Role of Fish in a Sustainable Diet. Front. Mar. Sci. 2017, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Alongi, M.; Melchior, S.; Anese, M. Reducing the glycemic index of short dough biscuits by using apple pomace as a functional ingredient. LWT 2019, 100, 300–305. [Google Scholar] [CrossRef]
- Licciardello, F.; Kharchoufi, S.; Muratore, G.; Restuccia, C. Effect of edible coating combined with pomegranate peel extract on the quality maintenance of white shrimps (Parapenaeus longirostris) during refrigerated storage. Food Packag. Shelf Life 2018, 17, 114–119. [Google Scholar] [CrossRef]
- Spinelli, S.; Conte, A.; Del Nobile, M.A. Microencapsulation of extracted bioactive compounds from brewer’s spent grain to enrich fish-burgers. Food Bioprod. Process. 2016, 100, 450–456. [Google Scholar] [CrossRef]
- Martínez, L.; Castillo, J.; Ros, G.; Nieto, G. Antioxidant and Antimicrobial Activity of Rosemary, Pomegranate and Olive Extracts in Fish Patties. Antioxidants 2019, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Cedola, A.; Cardinali, A.; Del Nobile, M.A.; Conte, A. Fish burger enriched by olive oil industrial by-product. Food Sci. Nutr. 2017, 5, 837–844. [Google Scholar] [CrossRef]
- Tufariello, M.; Durante, M.; Veneziani, G.; Taticchi, A.; Servili, M.; Bleve, G.; Mita, G. Patè Olive Cake: Possible Exploitation of a By-Product for Food Applications. Front. Nutr. 2019, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive Mill Waste Extracts: Polyphenols Content, Antioxidant, and Antimicrobial Activities. Adv. Pharmacol. Sci. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.; Santos, S.A.O.; Guerra, Â.R.; Guerreiro, O.; Felício, L.; Jerónimo, E.; Silvestre, A.J.D.; Neto, C.P.; Duarte, M. Valorization of olive mill residues: Antioxidant and breast cancer antiproliferative activities of hydroxytyrosol-rich extracts derived from olive oil by-products. Ind. Crops Prod. 2013, 46, 359–368. [Google Scholar] [CrossRef]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [Green Version]
- Yener, M.E. Supercritical Fluid Processing for the Recovery of Bioactive Compounds from Food Industry By-Products. In High Pressure Fluid Technology for Green Food Processing; Fornari, T., Stateva, R.P., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 305–355. ISBN 978-3-319-10610-6. [Google Scholar]
- Roselló-Soto, E.; Koubaa, M.; Moubarik, A.; Lopes, R.P.; Saraiva, J.A.; Boussetta, N.; Grimi, N.; Barba, F.J. Emerging opportunities for the effective valorization of wastes and by-products generated during olive oil production process: Non-conventional methods for the recovery of high-added value compounds. Trends Food Sci. Technol. 2015, 45, 296–310. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Danza, A.; Conte, A.; Del Nobile, M.A. Technological options to control quality of fish burgers. J. Food Sci. Technol. 2017, 54, 1802–1808. [Google Scholar] [CrossRef]
- NMKL Method No. 184. Aerobic Count and Specific Spoilage Organisms in Fish and Fish Products; Nordic Committee on Food Analysis: Espoo, Finland, 2006; pp. 1–6. [Google Scholar]
- Corbo, M.R.; Speranza, B.; Filippone, A.; Conte, A.; Sinigaglia, M.; Del Nobile, M.A. Natural compounds to preserve fresh fish burgers. Int. J. Food Sci. Technol. 2009, 44, 2021–2027. [Google Scholar] [CrossRef]
- Del Nobile, M.A.; Corbo, M.R.; Speranza, B.; Sinigaglia, M.; Conte, A.; Caroprese, M. Combined effect of MAP and active compounds on fresh blue fish burger. Int. J. Food Microbiol. 2009, 135, 281–287. [Google Scholar] [CrossRef]
- Angiolillo, L.; Conte, A.; Del Nobile, M.A. A new method to bio-preserve sea bass fillets. Int. J. Food Microbiol. 2018, 271, 60–66. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Casazza, A.A.; Perego, P. Valorization of olive oil solid waste using high pressure–High temperature reactor. Food Chem. 2011, 128, 704–710. [Google Scholar] [CrossRef]
- Moudache, M.; Colon, M.; Nerín, C.; Zaidi, F. Phenolic content and antioxidant activity of olive by-products and antioxidant film containing olive leaf extract. Food Chem. 2016, 212, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Murador, D.; Braga, A.R.; Da Cunha, D.; De Rosso, V. Alterations in phenolic compound levels and antioxidant activity in response to cooking technique effects: A meta-analytic investigation. Crit. Rev. Food Sci. Nutr. 2018, 58, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, M.; Agostinho, C.; Ribeiro, D.; Rocha, G.D.S.; Barroso, S.G.; Ferreira, D.; Polinati, R.; Ciarelli, G.; Fialho, E. Effect of different cooking methods on the polyphenol concentration and antioxidant capacity of selected vegetables. J. Culin. Sci. Technol. 2016, 14, 1–12. [Google Scholar] [CrossRef]
- Ng, Z.-X.; Chai, J.-W.; Kuppusamy, U.R. Customized cooking method improves total antioxidant activity in selected vegetables. Int. J. Food Sci. Nutr. 2011, 62, 158–163. [Google Scholar] [CrossRef]
- Lodolini, E.M.; Cabrera-Bañegil, M.; Fernández, A.; Delgado-Adámez, J.; Ramírez, R.; Martín-Vertedor, D. Monitoring of acrylamide and phenolic compounds in table olive after high hydrostatic pressure and cooking treatments. Food Chem. 2019, 286, 250–259. [Google Scholar] [CrossRef]
- Suárez, M.; Romero, M.-P.; Ramo, T.; Macià, A.; Motilva, M.-J. Methods for Preparing Phenolic Extracts from Olive Cake for Potential Application as Food Antioxidants. J. Agric. Food Chem. 2009, 57, 1463–1472. [Google Scholar] [CrossRef]
- Kuley, E.; Durmus, M.; Balikci, E.; Ucar, Y.; Regenstein, J.M.; Özoğul, F. Fish spoilage bacterial growth and their biogenic amine accumulation: Inhibitory effects of olive by-products. Int. J. Food Prop. 2017, 20, 1029–1043. [Google Scholar] [CrossRef] [Green Version]
- Yangui, T.; Sayadi, S.; Gargoubi, A.; Dhouib, A. Fungicidal effect of hydroxytyrosol-rich preparations from olive mill wastewater against Verticillium dahliae. Crop Prot. 2010, 29, 1208–1213. [Google Scholar] [CrossRef]
- Romero-García, J.M.; Niño, L.; Martínez-Patiño, C.; Álvarez, C.; Castro, E.; Negro, M.J. Biorefinery based on olive biomass. State of the art and future trends. Bioresour. Technol. 2014, 159, 421–432. [Google Scholar] [CrossRef]
- Haouache, N.; Bouchaleta, A. Olive mill wastewater: Effect on the survival and reproduction of the ecological indicator “Gammarus gauthieri”. J. Mat. Environ. Sci. 2016, 7, 2288–2294. [Google Scholar]




| Samples | Total Phenols (mg GAE/g dw) ± SD | Total Flavonoids (mg QE/g dw) ± SD | Antioxidant Activity (mg Trolox/g dw) ± SD |
|---|---|---|---|
| R-Ctrl | 2.70 ± 0.15 a,A | 1.69 ± 0.09 a,A | 5.88 ± 0.18 a,A |
| R-Active | 12.63 ± 0.18 b,A | 13.68 ± 0.90 b,A | 20.02 ± 0.43 b,A |
| C-Ctrl | 2.82 ± 0.13 a,A | 1.38 ± 0.13 a,B | 4.40 ± 0.15 a,B |
| C-Active | 12.46 ± 0.26 b,A | 10.61 ± 0.53 b,B | 12.55 ± 0.75 b,B |
| Samples | Microbiological Acceptability Limit (Day) | Sensory Acceptability Limit (Day) | Shelf Life (Day) | ||||
|---|---|---|---|---|---|---|---|
| MALTPB | MALTMB | MALPse. | MALShew. | MALPhot. | SAL | ||
| Ctrl | 9.05 ± 0.87 a | >15 | 9.62 ± 0.55 a | 12.66 ± 0.57 a | >15 | 13.67 ± 0.38 a | 9.05 ± 0.87 a |
| Active | >15 | >15 | 12.23 ± 0.72 b | 13.01 ± 0.68 a | >15 | 14.24 ± 0.45 a | 12.23 ± 0.72 b |
| Sensory Attributes | Samples | Storage Time (Day) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | 12 | 15 | ||
| Appearance | Ctrl Active | 8.0 ± 0.0 a 8.0 ± 0.0 a | 8.0 ± 0.0 a 8.0 ± 0.0 a | 8.0 ± 0.0 a 8.0 ± 0.0 a | 7.50 ± 0.0 a 7.50 ± 0.0 a | 6.17 ± 0.29 a 7.0 ± 0.0 b | 4.33 ± 0.29 a 6.17 ± 0.29 b |
| Color | Ctrl Active | 8.0 ± 0.0 a 8.0 ± 0.0 a | 8.0 ± 0.0 a 8.0 ± 0.0 a | 8.0 ± 0.0 a 8.0 ± 0.0 a | 7.33 ± 0.29 a 7.33 ± 0.29 a | 6.33 ± 0.29 a 7.0 ± 0.0 b | 4.83 ± 0.29 a 5.17 ± 0.29 a |
| Odor | Ctrl Active | 8.17 ± 0.29 a 8.50 ± 0.0 a | 7.50 ± 0.0 a 8.33 ± 0.29 b | 7.33 ± 0.29 a 7.83 ± 0.29 a | 7.0 ± 0.50 a 7.0 ± 0.50 a | 5.33 ± 0.29 a 6.17 ± 0.29 b | 3.67 ± 0.29 a 4.50 ± 0.0 b |
| Texture | Ctrl Active | 8.50 ± 0.0 a 8.50 ± 0.0 a | 8.33 ± 0.29 a 8.33 ± 0.29 a | 8.0 ± 0.0 a 8.0 ± 0.0 a | 7.83 ± 0.29 a 8.00 ± 0.0 a | 6.33 ± 0.29 a 6.83 ± 0.29 a | 5.0 ± 0.0 a 6.0 ± 0.50 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panza, O.; Lacivita, V.; Palermo, C.; Conte, A.; Del Nobile, M.A. Food By-Products to Extend Shelf Life: The Case of Cod Sticks Breaded with Dried Olive Paste. Foods 2020, 9, 1902. https://doi.org/10.3390/foods9121902
Panza O, Lacivita V, Palermo C, Conte A, Del Nobile MA. Food By-Products to Extend Shelf Life: The Case of Cod Sticks Breaded with Dried Olive Paste. Foods. 2020; 9(12):1902. https://doi.org/10.3390/foods9121902
Chicago/Turabian StylePanza, Olimpia, Valentina Lacivita, Carmen Palermo, Amalia Conte, and Matteo Alessandro Del Nobile. 2020. "Food By-Products to Extend Shelf Life: The Case of Cod Sticks Breaded with Dried Olive Paste" Foods 9, no. 12: 1902. https://doi.org/10.3390/foods9121902
APA StylePanza, O., Lacivita, V., Palermo, C., Conte, A., & Del Nobile, M. A. (2020). Food By-Products to Extend Shelf Life: The Case of Cod Sticks Breaded with Dried Olive Paste. Foods, 9(12), 1902. https://doi.org/10.3390/foods9121902