Combination of Low Fluctuation of Temperature with TiO2 Photocatalytic/Ozone for the Quality Maintenance of Postharvest Peach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
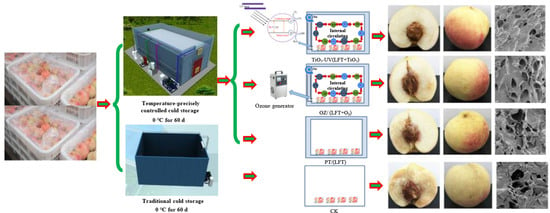
2.2. Specification of Cold Storage
2.3. Gaseous Ozone and TiO2 Treatment
2.4. Storage Condition
2.5. Analysis of Cold Storage Temperature Fluctuation
2.6. Analysis of Physicochemical Properties
2.6.1. Color and Firmness Analysis
2.6.2. Titratable Acidity (TA) and Total Soluble Solids (TSS) Analysis
2.6.3. Respiratory Rate and Ethylene Production
2.6.4. Decay Rate Analysis
2.6.5. Polyphenol Oxidase (PPO) Activity and Total Phenolics Content Analysis
2.6.6. Malondialdehyde (MDA) Content Analysis
2.6.7. Scanning Electron Microscope (SEM) Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Temperature Fluctuation
3.2. Physicochemical Properties
3.2.1. Color and Firmness
3.2.2. Titratable Acidity (TA) and Total Soluble Solids (TSS)
3.2.3. Respiratory Rate and Ethylene Production
3.2.4. PPO Activity and Total Phenolics Content
3.2.5. Malondialdehyde (MDA) Content
3.2.6. Decay Rate
3.2.7. Scanning Electron Microscopy (SEM) Observation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, J.; You, Y.; Chen, W.; Xu, Q.; Wang, J.; Liu, Y.; Song, L.; Wu, J. Optimal hypobaric treatment delays ripening of honey peach fruit via increasing endogenous energy status and enhancing antioxidant defence systems during storage. Postharvest Biol. Technol. 2015, 101, 1–9. [Google Scholar] [CrossRef]
- Hussain, P.R.; Meena, R.S.; Dar, M.A.; Wani, A.M. Studies on enhancing the keeping quality of peach (Prunus persica Bausch) Cv. Elberta by gamma-irradiation. Radiat. Phys. Chem. 2008, 77, 473–481. [Google Scholar] [CrossRef]
- Budde, C.O.; Polenta, G.; Lucangeli, C.D.; Murray, R.E. Air and immersion heat treatments affect ethylene production and organoleptic quality of ‘Dixiland’ peaches. Postharvest Biol. Technol. 2006, 41, 32–37. [Google Scholar] [CrossRef]
- Zhou, D.; Sun, Y.; Li, M.; Zhu, T.; Tu, K. Postharvest hot air and UV-C treatments enhance aroma-related volatiles by simulating the lipoxygenase pathway in peaches during cold storage. Food Chem. 2019, 292, 294–303. [Google Scholar] [CrossRef]
- Santana, L.R.; Benedetti, B.; Sigrist, J. Sensory characteristics of ‘Douradão’ peaches submitted to modified atmosphere packaging. Rev. Bras. Frutic. 2010, 32, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Artés, F.; Cano, A.; Fernández-Trujillo, J.P. Pectolytic Enzyme Activity during Intermittent Warming Storage of Peaches. J. Food Sci. 1996, 61, 311–314. [Google Scholar] [CrossRef]
- Aung, M.M.; Chang, Y.S. Temperature management for the quality assurance of a perishable food supply chain. Food Control 2014, 40, 198–207. [Google Scholar] [CrossRef]
- Urquiola, A.; Alvarez, G.; Flick, D. Frost formation modeling during the storage of frozen vegetables exposed to temperature fluctuations. J. Food Eng. 2017, 214, 16–28. [Google Scholar] [CrossRef]
- Ho, S.H.; Rosario, L.; Rahman, M.M. Numerical simulation of temperature and velocity in a refrigerated warehouse. Int. J. Refrig. 2010, 33, 1015–1025. [Google Scholar] [CrossRef]
- Brummell, D.A.; Dal Cin, V.; Lurie, S.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during the development of chilling injury in cold-stored peach fruit: Association of mealiness with arrested disassembly of cell wall pectins. J. Exp. Bot. 2004, 55, 2041–2052. [Google Scholar] [CrossRef] [Green Version]
- Lyons, J.M. Chilling injury in plants. Ann. Rev. Plant Phys. 1973, 20, 423–446. [Google Scholar] [CrossRef]
- Talasila, P.C.; Chau, K.V.; Brecht, J.K. Effects of gas concentrations and temperature on O2 consumption of strawberries. Trans. ASAE 1992, 35, 221–224. [Google Scholar] [CrossRef]
- Lehmann, D.C.; Ferguson, J.E. A modified jacketed cold storage design. Int. J. Refrig. 1984, 7, 186–189. [Google Scholar] [CrossRef]
- Peters, J.A.; McLane, D.T. Storage life of pink shrimp held in commercial and jacketed cold-storage rooms. Commer. Fish. Rev. 1959, 21, 1–7. [Google Scholar]
- Lentz, C.P.; Rooke, E.A. Use of the jacketed room system for cool storage. Food Technol. 1957, 11, 257–259. [Google Scholar]
- Lentz, C.P.; Van den Berg, L.; Jorgensen, E.G.; Sawler, R. The design and operation of a jacketed vegetable storage. Can. Inst. Food Sci. Technol. J. 1971, 4, 19–23. [Google Scholar] [CrossRef]
- Raghavan, G.S.V.; Bovell, R.; Chayet, M. Storability of fresh carrots in a simulated jacketed storage. Trans. Am. Soc. Agric. Eng. 1980, 23, 1521–1524. [Google Scholar] [CrossRef]
- Khadre, M.A.; Yousef, A.E.; Kim, J.G. Microbiological Aspects of Ozone Applications in Food: A Review. J. Food Sci. 2001, 66, 1242–1252. [Google Scholar] [CrossRef]
- Bodaghi, H.; Mostofi, Y.; Oromiehie, A.; Zamani, Z.; Ghanbarzadeh, B.; Costa, C.; Conte, A.; Del Nobile, M.A. Evaluation of the photocatalytic antimicrobial effects of a TiO2 nanocomposite food packaging film by in vitro and in vivo tests. LWT Food Sci. Technol. 2013, 50, 702–706. [Google Scholar] [CrossRef]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Linear correlation between inactivation of E. coli and OH radical concentration in TiO2 photocatalytic disinfection. Water Res. 2004, 38, 1069–1077. [Google Scholar] [CrossRef]
- Huang, Z.; Maness, P.-C.; Blake, D.M.; Wolfrum, E.J.; Smolinski, S.L.; Jacoby, W.A. Bactericidal mode of titanium dioxide photocatalysis. J. Photochem. Photobiol. A 2000, 130, 163–170. [Google Scholar] [CrossRef]
- Shahbaz, H.M.; Kim, S.; Hong, J.; Kim, J.U.; Lee, D.U.; Ghafoor, K.; Park, J. Effects of TiO2–UVC photocatalysis and thermal pasteurisation on microbial inactivation and quality characteristics of the Korean rice-and-malt drink sikhye. Int. J. Food Sci. Tech. 2016, 51, 123–132. [Google Scholar] [CrossRef]
- Maneerat, C.; Hayata, Y. Efficiency of TiO2 photocatalytic reaction on delay of fruit ripening and removal of off-flavors from the fruit storage atmosphere. Trans. ASAE 2006, 49, 833–837. [Google Scholar] [CrossRef]
- Miller, F.A.; Silva, C.L.; Brandão, T.R. A review on ozone-based treatments for fruit and vegetables preservation. Food Eng. Rev. 2013, 5, 77–106. [Google Scholar] [CrossRef]
- Kying, M.; Ali, A.; Alderson, P.; Forney, C.; Malaysia, S.D.E.; Tunku, A.; Rahman, J.; Barat Kampar, P. Effect of different concentrations of ozone on physiological changes associated to gas exchange, fruit ripening, fruit surface quality and defence-related enzymes levels in papaya fruit during ambient storage. Sci. Hortic. 2014, 179, 163–169. [Google Scholar]
- Santana, L.R.R.D.; Benedetti, B.C.; Sigrist, J.M.M.; Sato, H.H.; Anjos, V.D.D.A. Effect of controlled atmosphere on postharvest quality of ‘Douradão’ peaches. Food Sci. Technol. 2011, 31, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Lu, Y.; Yang, Q.; Yang, H.; Li, Y.; Zhou, B.; Li, T.; Gao, Y.; Qiao, L. Cod peptides inhibit browning in fresh-cut potato slices: A potential anti-browning agent of random peptides for regulating food properties. Postharvest Biol. Technol. 2018, 146, 36–42. [Google Scholar] [CrossRef]
- Wang, Q.; Cao, Y.; Zhou, L.; Jiang, C.-Z.; Feng, Y.; Wei, S. Effects of postharvest curing treatment on flesh colour and phenolic metabolism in fresh-cut potato products. Food Chem. 2015, 169, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Gong, Y.; Chen, L.; Peng, Y.; Wang, Q.; Shi, J. Hypotaurine delays senescence of peach fruit by regulating reactive oxygen species metabolism. Sci. Hortic. 2019, 253, 295–302. [Google Scholar] [CrossRef]
- Zhong, Q.; Xia, W. Effect of 1-methylcyclopropene and/or chitosan coating treatments on storage life and quality maintenance of Indian jujube fruit. LWT Food Sci. Technol. 2007, 40, 404–411. [Google Scholar]
- Li, X.; Li, W.; Jiang, Y.; Ding, Y.; Yun, J.; Tang, Y.; Zhang, P. Effect of nano-ZnO-coated active packaging on quality of fresh-cut ‘Fuji’ apple. Int. J. Food Sci. Technol. 2011, 46, 1947–1955. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, S.; Su, X.; Jiang, Y. Respiratory activity and mitochondrial membrane associated with fruit senescence in postharvest peaches in response to UV-C treatment. Food Chem. 2014, 161, 16–21. [Google Scholar] [CrossRef]
- Huan, C.; An, X.J.; Yu, M.L.; Jiang Li, J.; Ma, R.J.; Tu, M.G.; Yu, Z.F. Effect of combined heat and 1-MCP treatment on the quality and antioxidant level of peach fruit during storage. Postharvest Biol. Technol. 2018, 145, 193–202. [Google Scholar] [CrossRef]
- Han, Q.; Gao, H.; Chen, H.; Fang, X.; Wu, W. Precooling and ozone treatments affects postharvest quality of black mulberry (Morus nigra) fruit. Food Chem. 2017, 221, 1947–1953. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Fiorentino, A.; Pacifico, S.; D’Abrosca, B.; Uzzo, P.; Monaco, P. Antioxidant Properties of Sour Cherries (Prunus cerasus L.): Role of Colorless Phytochemicals from the Methanolic Extract of Ripe Fruit. J. Agric. Food Chem. 2008, 56, 1928–1935. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumbdhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Nicola, B.M.; Verlinden, B.; Beuselinck, A.; Jancsok, P.; Valéry, Q.; Scheerlinck, N.; Verboven, P.; De Baerdemaeker, J. Propagation of stochastic temperature fluctuations in refrigerated fruit: Propagation des fluctuations de température stochastiques dans des fruit réfrigérés. Int. J. Refrig. 1999, 22, 81–90. [Google Scholar] [CrossRef]
- Cameron, A.C.; Talasila, P.C.; Joles, D.J. Predicting the film permeability needs for modified-atmosphere packaging of lightly processed fruit and vegetables. HortScience 1995, 30, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Forney, C.F.; Song, J.; Hildebrand, P.D.; Fan, L.; McRae, K.B. Interactive effects of ozone and 1-methylcyclopropene on decay resistance and quality of stored carrots. Postharvest Biol. Technol. 2007, 45, 341–348. [Google Scholar] [CrossRef]
- Bridges, D.F.; Rane, B.; Wu, V.C.H. The effectiveness of closed-circulation gaseous chlorine dioxide or ozone treatment against bacterial pathogens on produce. Food Control 2018, 91, 261–267. [Google Scholar] [CrossRef]
- Ali, A.; Ong, M.K.; Forney, C.F. Effect of ozone pre-conditioning on quality and antioxidant capacity of papaya fruit during ambient storage. Food Chem. 2014, 142, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Ye, Q.; Jiang, L.; Luo, Z. Effects of nano-TiO2 -LDPE packaging on postharvest quality and antioxidant capacity of strawberry (Fragaria ananassa Duch.) stored at refrigeration temperature. J. Sci. Food Agric. 2016, 97, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Chen, Y.; Li, C.; Wei, M.; Li, X.; Li, S.; Lu, S.; Li, J. Effect of trisodium phosphate dipping treatment on the quality and energy metabolism of apples. Food Chem. 2019, 274, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Yang, H.; Guo, X.; Bi, X.; Liu, X.; Xu, Q.; Wang, Q.; Li, W.; Li, X.; Shui, Y.; et al. Effect of chitosan/Nano-TiO2 composite coatings on the postharvest quality and physicochemical characteristics of mango fruits. Sci. Hortic. 2020, 263, 109–135. [Google Scholar] [CrossRef]
- Lee, T.T. Inhibition of oxidative phosphorylation and respiration by ozone in Tobacco Mitochondria. Plant Physiol. 1967, 42, 691–696. [Google Scholar] [CrossRef] [Green Version]
- Chun, J.P.; Seo, J.S.; Kim, M.S.; Lim, B.S.; Ahn, Y.J.; Hwang, Y.S. Effects of 1-MCP and storage condition on shelf life and quality of ‘Janghowon Hwangdo’ peach (Prunus persica Batsch). Kor. J. Hort. Sci. Technol. 2010, 28, 585–592. [Google Scholar]
- Tao, X.; Wang, M. Study on the Optimal Composition of Chitosan/Nano- TiO2 Composite Film and Its Application in the Preservation of Jinqiu Pear. Agric. Biotechnol. 2015, 4, 20–23. [Google Scholar]
- Huang, D.; Hu, S.; Zhu, S.; Feng, J. Regulation by nitric oxide on mitochondrial permeability transition of peaches during storage. Plant Physiol. Biochem. 2019, 138, 17–25. [Google Scholar] [CrossRef]
- Hawkins, M.; Kohlmiller, C.K.; Andrews, L. Matrix infrared spectra and photolysis and pyrolysis of isotopic secondary ozonides of ethylene. J. Phys. Chem. 1982, 86, 3154–3166. [Google Scholar] [CrossRef]
- Coseteng, M.Y.; Lee, C.Y. Changes in apple polyphenoloxidase and polyphenol concentrations in relation to degree of browning. J. Food Sci. 2010, 52, 985–989. [Google Scholar] [CrossRef]
- Gao, H.; Chai, H.; Cheng, N.; Cao, W. Effects of 24-epibrassinolide on enzymatic browning and antioxidant activity of fresh-cut lotus root slices. Food Chem. 2017, 217, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.K.; Ali, A.; Alderson, P.G.; Forney, C.F. Effect of different concentrations of ozone on physiological changes associated to gas exchange, fruit ripening, fruit surface quality and defence-related enzymes levels in papaya fruit during ambient storage. Sci. Hortic. 2014, 179, 163–169. [Google Scholar] [CrossRef]
- Jin, P.; Zhu, H.; Wang, L.; Shan, T.; Zheng, Y. Oxalic acid alleviates chilling injury in peach fruit by regulating energy metabolism and fatty acid contents. Food Chem. 2014, 161, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, A.; Barreno, E. Chlorophyll a fluorescence, antioxidant enzymes and lipid peroxidation in tomato in response to ozone and benomyl. Environ. Pollut. 2001, 115, 283–289. [Google Scholar] [CrossRef]
- Lurie, S.; Crisosto, C.H. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Tian, F.; Chen, W.; Wu, C.E.; Kou, X.; Fan, G.; Li, T.; Wu, Z. Preservation of Ginkgo biloba seeds by coating with chitosan/nano-TiO2 and chitosan/nano-SiO2 films. Int. J. Biol. Macromol. 2019, 126, 917–925. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Victorin, K. Review of genotoxicity of ozone. Mutat. Res. 1992, 277, 221–238. [Google Scholar] [CrossRef]
- Barth, M.; Zhou, M.; Mercier, C.; Payne, J. Ozone storage effects on anthocyanin content and fungal growth in blackberries. J. Food Sci. 1994, 60, 1286–1288. [Google Scholar] [CrossRef]






| Physicochemical Properties | Storage Period (d) | Treatments | |||
|---|---|---|---|---|---|
| CK | LFT | LFT + O3 | LFT + TiO2 | ||
| Color (L* value) | 0 | 78.75 ± 0.12 a | 78.75 ± 0.09 a | 78.82 ± 0.08 a | 78.85 ± 0.05 a |
| 10 | 76.47 ± 0.10 a | 76.49 ± 0.11 a | 76.53 ± 0.07 a | 76.81 ± 0.06 a | |
| 20 | 73.44 ± 0.13 c | 74.81 ± 0.10 b | 76.35 ± 0.05 a | 76.23 ± 0.06 a | |
| 30 | 72.56 ± 0.11 c | 74.20 ± 0.12 b | 75.60 ± 0.04 a | 74.86 ± 0.05 ab | |
| 40 | 68.54 ± 0.09 c | 72.35 ± 0.10 b | 73.25 ± 0.13 b | 74.56 ± 0.12 a | |
| 50 | 65.52 ± 0.13 d | 69.01 ± 0.09 c | 71.52 ± 0.08 b | 73.67 ± 0.12 a | |
| 60 | 59.95 ± 0.10 d | 63.79 ± 0.14 c | 70.46 ± 0.10 b | 72.43 ± 0.09 a | |
| Color (b* value) | 0 | 5.25 ± 0.02 a | 5.16 ± 0.01 a | 5.21 ± 0.01 a | 5.23 ± 0.02 a |
| 10 | 5.66 ± 0.01 a | 5.56 ± 0.02 a | 5.53 ± 0.03 a | 5.51 ± 0.04 a | |
| 20 | 6.01 ± 0.04 a | 5.92 ± 0.05 a | 5.96 ± 0.03 a | 5.94 ± 0.03 a | |
| 30 | 6.32 ± 0.05 a | 6.22 ± 0.02 a | 6.28 ± 0.03 a | 6.19 ± 0.02 a | |
| 40 | 7.82 ± 0.01 a | 6.86 ± 0.01 b | 6.57 ± 0.03 b | 6.35 ± 0.03 c | |
| 50 | 9.02 ± 0.04 a | 8.52 ± 0.01 b | 8.01 ± 0.02 c | 6.52 ± 0.03 d | |
| 60 | 10.53 ± 0.02 a | 9.54 ± 0.04 b | 8.85 ± 0.03 c | 6.68 ± 0.05 d | |
| Fruit Firmness (N) | 0 | 58.72 ± 0.02 a | 58.62 ± 0.03 a | 58.71 ± 0.01 a | 58.63 ± 0.04 a |
| 10 | 52.51 ± 0.03 a | 52.62 ± 0.03 a | 52.54 ± 0.04 a | 52.53 ± 0.03 a | |
| 20 | 46.03 ± 0.04 a | 47.41 ± 0.03 a | 48.32 ± 0.06 a | 47.44 ± 0.03 a | |
| 30 | 40.72 ± 0.03 b | 42.01 ± 0.04 b | 45.23 ± 0.05 a | 46.22 ± 0.06 a | |
| 40 | 36.31 ± 0.05 b | 38.81 ± 0.06 b | 43.12 ± 0.03 a | 44.13 ± 0.02 a | |
| 50 | 30.92 ± 0.04 c | 31.43 ± 0.03 c | 37.92 ± 0.02 b | 42.01 ± 0.03 a | |
| 60 | 25.62 ± 0.06 d | 29.31 ± 0.02 c | 35.91 ± 0.05 b | 41.82 ± 0.04 a | |
| Titratable Acidity (%) | 0 | 0.42 ± 0.01 a | 0.42 ± 0.02 a | 0.42 ± 0.01 a | 0.42 ± 0.01 a |
| 10 | 0.36 ± 0.02 a | 0.37 ± 0.00 a | 0.38 ± 0.01 a | 0.36 ± 0.01 a | |
| 20 | 0.28 ± 0.00 a | 0.29 ± 0.02 a | 0.36 ± 0.03 a | 0.34 ± 0.01 a | |
| 30 | 0.18 ± 0.01 d | 0.26 ± 0.02 b | 0.25 ± 0.03 c | 0.27 ± 0.02 a | |
| 40 | 0.12 ± 0.02 d | 0.20 ± 0.02 c | 0.22 ± 0.03 b | 0.21 ± 0.01 a | |
| 50 | 0.09 ± 0.01 d | 0.15 ± 0.02 c | 0.18 ± 0.00 b | 0.16 ± 0.01 a | |
| 60 | 0.06 ± 0.00 d | 0.12 ± 0.01 c | 0.15 ± 0.02 b | 0.14 ± 0.03 a | |
| Total Soluble Solids (%) | 0 | 15.12 ±0.16 a | 15.12 ± 0.12 a | 15.12 ± 0.20 a | 15.12 ± 0.11 a |
| 10 | 15.53 ± 0.14 a | 15.41 ± 0.12 a | 15.62 ± 0.18 a | 15.85 ± 0.19 a | |
| 20 | 15.83 ± 0.15 a | 15.65 ± 0.16 a | 15.85 ± 0.12 a | 15.92 ± 0.11 a | |
| 30 | 12.23 ± 0.10 b | 13.97 ± 0.11 a | 14.47 ± 0.14 a | 14.32 ± 0.10 a | |
| 40 | 11.36 ± 0.15 c | 13.05 ± 0.22 b | 14.32 ± 0.10 ab | 13.85 ± 0.08 a | |
| 50 | 10.85 ± 0.12 c | 12.51 ± 0.11 b | 13.22 ± 0.10 ab | 13.55 ± 0.21 a | |
| 60 | 9.81 ± 0.19 c | 11.32 ± 0.22 b | 12.23 ± 0.08 b | 13.20 ± 0.10 a | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Li, J.; Du, M.; Zhao, Z.; Song, J.; Yang, W.; Zheng, Y.; Chen, L.; Li, X. Combination of Low Fluctuation of Temperature with TiO2 Photocatalytic/Ozone for the Quality Maintenance of Postharvest Peach. Foods 2020, 9, 234. https://doi.org/10.3390/foods9020234
Jia X, Li J, Du M, Zhao Z, Song J, Yang W, Zheng Y, Chen L, Li X. Combination of Low Fluctuation of Temperature with TiO2 Photocatalytic/Ozone for the Quality Maintenance of Postharvest Peach. Foods. 2020; 9(2):234. https://doi.org/10.3390/foods9020234
Chicago/Turabian StyleJia, Xiaoyu, Jiangkuo Li, Meijun Du, Zhiyong Zhao, Jianxin Song, Weiqiao Yang, Yanli Zheng, Lan Chen, and Xihong Li. 2020. "Combination of Low Fluctuation of Temperature with TiO2 Photocatalytic/Ozone for the Quality Maintenance of Postharvest Peach" Foods 9, no. 2: 234. https://doi.org/10.3390/foods9020234
APA StyleJia, X., Li, J., Du, M., Zhao, Z., Song, J., Yang, W., Zheng, Y., Chen, L., & Li, X. (2020). Combination of Low Fluctuation of Temperature with TiO2 Photocatalytic/Ozone for the Quality Maintenance of Postharvest Peach. Foods, 9(2), 234. https://doi.org/10.3390/foods9020234