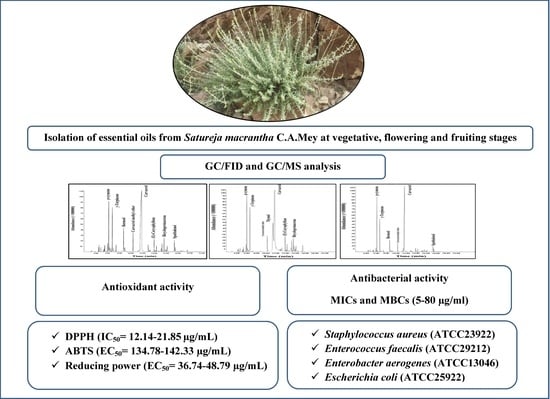
Chemical Composition, Antibacterial and Radical Scavenging Activity of Essential Oils from Satureja macrantha C.A.Mey. at Different Growth Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sampling
2.2. Isolation of the EOs
2.3. Gas Chromatography (GC-FID) and Gas Chromatography-Mass Spectrometry (GC-MS)
2.4. Antibacterial Screening
2.4.1. Bacterial Strains
2.4.2. Minimum Inhibitory Concentration (MIC)
2.4.3. Minimum Bactericidal Concentration (MBC)
2.5. Antioxidant Activity
2.6. Data Analysis
3. Results and Discussions
3.1. Essential Oil Analysis
3.2. Antibacterial Activity of the Satureja Macrantha EOs
3.3. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Faleiro, M.L. The Mode of Antibacterial Action of Essential Oils. In Science against Microbial Pathogens: Communicating Current Research and Technological Advances; Brown Walker Press: Boca Raton, USA, 2011. [Google Scholar]
- Fisher, K.; Phillips, C. Potential Antimicrobial Uses of Essential Oils in Food: Is Citrus the Answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 22, 201–206. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Adelakun, O.E.; Oyelade, O.J.; Olanipekun, B.F. Use of Essential Oils in Food Preservation. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Zahin, M.; Hasan, S.; Husain, F.M.; Ahmad, I. Inhibition of Quorum Sensing Regulated Bacterial Functions by Plant Essential Oils with Special Reference to Clove Oil. Lett. Appl. Microbiol. 2009, 49, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Jamzad, Z. Satureja Rechingeri (Labiatae)—A New Species from Iran. Flora 1996, 98, 75–77. [Google Scholar]
- Maroofi, H. Two New Plant Species from Kurdistan Province, West of Iran. Iran. J. Bot. 2010, 16, 76–80. [Google Scholar]
- Benelli, G.; Pavela, R.; Canale, A.; Cianfaglione, K.; Ciaschetti, G.; Conti, F.; Nicoletti, M.; Senthil-Nathan, S.; Mehlhorn, H.; Maggi, F. Acute Larvicidal Toxicity of Five Essential Oils (Pinus Nigra, Hyssopus Officinalis, Satureja Montana, Aloysia Citrodora and Pelargonium Graveolens) against the Filariasis Vector Culex Quinquefasciatus: Synergistic and Antagonistic Effects. Parasitol. Int. 2017, 66, 166–171. [Google Scholar] [CrossRef]
- Caprioli, G.; Lupidi, G.; Maggi, F. Comparison of Chemical Composition and Antioxidant Activities of Two Winter Savory Subspecies (Satureja Montana Subsp. Variegata and Satureja Montana Subsp. Montana) Cultivated in Northern Italy. Nat. Prod. Res. 2019, 33, 3143–3147. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Zomorodian, K.; Taban, A.; Pakshir, K.; Heshmati, K.; Rahimi, M.J. Chemical Composition and Antimicrobial Activities of Three Satureja Species Against Food-Borne Pathogens. J. Essent. Oil Bear. Plants 2016, 19, 1984–1992. [Google Scholar] [CrossRef]
- Rechinger, K.H. Satureja in Flora Iranica; Akad. Druck-u Verlagsanstalt: Graz, Austria, 1982; Volume 150, pp. 495–504. [Google Scholar]
- Behravan, J.; Ramezani, M.; Kasaian, J.; Sabeti, Z. Antimycotic Activity of the Essential Oil of Satureja Mutica Fisch & C.A. Mey from Iran. Flavour Fragr. J. 2004, 19, 421–423. [Google Scholar] [CrossRef]
- Sefidkon, F.; Jamzad, Z. Chemical Composition of the Essential Oil of Three Iranian Satureja Species (S. mutica, S. macrantha and S. intermedia). Food Chem. 2005, 91, 1–4. [Google Scholar] [CrossRef]
- Azaz, A.D.; Kürkcüoglu, M.; Satil, F.; Baser, K.H.C.; Tümen, G. In Vitro Antimicrobial Activity and Chemical Composition of Some Satureja Essential Oils. Flavour Fragr. J. 2005, 20, 587–591. [Google Scholar] [CrossRef]
- Gohari, A.R.; Hadjiakhoondi, A.; Sadat-Ebrahimi, E.; Saeidnia, S.; Shafiee, A. Composition of the Volatile Oils of Satureja spicigera, C. Koch Boiss. and S. macrantha C.A. Mey from Iran. Flavour Fragr. J. 2006, 21, 510–512. [Google Scholar] [CrossRef]
- Ćavar, S.; Maksimović, M.; Šolić, M.E.; Jerković-Mujkić, A.; Bešta, R. Chemical Composition and Antioxidant and Antimicrobial Activity of Two Satureja Essential Oils. Food Chem. 2008, 111, 648–653. [Google Scholar] [CrossRef]
- Sefidkon, F.; Jamzad, Z.; Mirza, M. Chemical Variation in the Essential Oil of Satureja Sahendica from Iran. Food Chem. 2004, 88, 325–328. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Pirbalouti, A.G.; Neshat, S.H.; Rahimi, E.; Hamedi, B.; Malekpoor, F. Chemical Composition and Antibacterial Activity of Essential Oils of Iranian Herbs Against Staphylococcus Aureus Isolated from Milk. Int. J. Food Prop. 2014, 17, 2063–2071. [Google Scholar] [CrossRef] [Green Version]
- Falsafi, T.; Moradi, P.; Mahboubi, M.; Rahimi, E.; Momtaz, H.; Hamedi, B. Chemical Composition and Anti-Helicobacter Pylori Effect of Satureja Bachtiarica Bunge Essential Oil. Phytomedicine 2015, 22, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Cherrat, L.; Espina, L.; Bakkali, M.; Pagán, R.; Laglaoui, A. Chemical Composition, Antioxidant and Antimicrobial Properties of Mentha Pulegium, Lavandula Stoechas and Satureja Calamintha Scheele Essential Oils and an Evaluation of Their Bactericidal Effect in Combined Processes. Innov. Food Sci. Emerg. Technol. 2014, 22, 221–229. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadian, J.; Hatami, M.; Salehi-Arjomand, H.; Aliahmadi, A. Comparison of Chemical Compounds and Antioxidant and Antibacterial Properties of Various Satureja Species Growing Wild in Iran. J. Med. Plants 2016, 15, 58–72. [Google Scholar]
- Choulitoudi, E.; Bravou, K.; Bimpilas, A.; Tsironi, T.; Tsimogiannis, D.; Taoukis, P.; Oreopoulou, V. Antimicrobial and Antioxidant Activity of Satureja Thymbra in Gilthead Seabream Fillets Edible Coating. Food Bioprod. Process. 2016, 100, 570–577. [Google Scholar] [CrossRef]
- Ozkan, G.; Simsek, B.; Kuleasan, H. Antioxidant Activities of Satureja Cilicica Essential Oil in Butter and in Vitro. J. Food Eng. 2007, 79, 1391–1396. [Google Scholar] [CrossRef]
- Abdali, E.; Javadi, S.; Akhgari, M.; Hosseini, S.; Dastan, D. Chemical Composition and Biological Properties of Satureja Avromanica Maroofi. J. Food Sci. Technol. 2017, 54, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Ghani, A.; Saharkhiz, M.J.; Hassanzadeh, M.; Msaada, K. Changes in the Essential Oil Content and Chemical Compositions of Echinophora Platyloba Dc. during Three Different Growth and Developmental Stages. J. Essent. Oil Bear. Plants 2009, 12, 162–171. [Google Scholar] [CrossRef]
- Mohammadi, S.; Saharkhiz, M.J. Changes in Essential Oil Content and Composition of Catnip (Nepeta Cataria, L.) during Different Developmental Stages. J. Essent. Oil Bear. Plants 2011, 14, 396–400. [Google Scholar] [CrossRef]
- Bajalan, I.; Rouzbahani, R.; Pirbalouti, A.G.; Maggi, F. Variation in Chemical Composition and Antibacterial Activity of the Essential Oil of Wild Populations of Phlomis Olivieri. Chem. Biodivers. 2017, 14, e1600444. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas. Chromatography/Mass Spectroscopy; Allured Pub. Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- NCCLS (National Committee for Clinical Laboratory Standards). Performance Standards for Antimicrobial Susceptibility Testing CLSI Supplement M100S; NCCLS: Wayne, NJ, USA, 2002. [Google Scholar]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential Oil Composition, Total Phenolic, Flavonoid Contents, and Antioxidant Activity of Thymus Species Collected from Different Regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Ray, A.; Jena, S.; Dash, B.; Kar, B.; Halder, T.; Chatterjee, T.; Ghosh, B.; Panda, P.C.; Nayak, S.; Mahapatra, N. Chemical Diversity, Antioxidant and Antimicrobial Activities of the Essential Oils from Indian Populations of Hedychium Coronarium Koen. Ind. Crops Prod. 2018, 112, 353–362. [Google Scholar] [CrossRef]
- Jamali, C.A.; Kasrati, A.; Bekkouche, K.; Hassani, L.; Wohlmuth, H.; Leach, D.; Abbad, A. Phenological Changes to the Chemical Composition and Biological Activity of the Essential Oil from Moroccan Endemic Thyme (Thymus Maroccanus Ball). Ind. Crops Prod. 2013, 49, 366–372. [Google Scholar] [CrossRef]
- Kizil, S.; Ipek, A.; Arslan, N.; Khawar, K.M. Effect of Different Developing Stages on Some Agronomical Characteristics and Essential Oil Composition of Oregano (Origanum Onites). N. Z. J. Crop Hortic. Sci. 2008, 36, 71–76. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Frezza, C.; Conti, F.; Bini, L.M.; Giuliani, C.; Bramucci, M.; Quassinti, L.; Damiano, S.; Lupidi, G.; et al. Essential Oil Composition, Polar Compounds, Glandular Trichomes and Biological Activity of Hyssopus Officinalis Subsp. Aristatus (Godr.) Nyman from Central Italy. Ind. Crops Prod. 2015, 77, 353–363. [Google Scholar] [CrossRef]
- Esmaeili, H.; Karami, A.; Maggi, F. Essential Oil Composition, Total Phenolic and Flavonoids Contents, and Antioxidant Activity of Oliveria Decumbens Vent. (Apiaceae) at Different Phenological Stages. J. Clean. Prod. 2018, 198, 91–95. [Google Scholar] [CrossRef]
- Tabari, M.A.; Youssefi, M.R.; Maggi, F.; Benelli, G. Toxic and Repellent Activity of Selected Monoterpenoids (Thymol, Carvacrol and Linalool) against the Castor Bean Tick, Ixodes Ricinus (Acari: Ixodidae). Vet. Parasitol. 2017, 245, 86–91. [Google Scholar] [CrossRef]
- Youssefi, M.R.; Moghaddas, E.; Tabari, M.A.; Moghadamnia, A.A.; Hosseini, S.M.; Hosseini Farash, B.R.; Ebrahimi, M.A.; Mousavi, N.N.; Fata, A.; Maggi, F.; et al. In Vitro and in Vivo Effectiveness of Carvacrol, Thymol and Linalool against Leishmania Infantum. Molecules 2019, 24, 2072. [Google Scholar] [CrossRef] [Green Version]
- Youssefi, M.R.; Tabari, M.A.; Esfandiari, A.; Kazemi, S.; Moghadamnia, A.A.; Sut, S.; Dall’Acqua, S.; Benelli, G.; Maggi, F. Efficacy of Two Monoterpenoids, Carvacrol and Thymol, and Their Combinations against Eggs and Larvae of the West Nile Vector Culex Pipiens. Molecules 2019, 24, 1867. [Google Scholar] [CrossRef] [Green Version]
- Nabti, L.Z.; Sahli, F.; Laouar, H.; Olowo-Okere, A.; Wandjou, J.G.N.; Maggi, F. Chemical Composition and Antibacterial Activity of Essential Oils from the Algerian Endemic Origanum Glandulosum Desf. against Multidrug-Resistant Uropathogenic, E. Coli Isolates. Antibiotics 2020, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Nejad Ebrahimi, S.; Hadian, J.; Mirjalili, M.H.; Sonboli, A.; Yousefzadi, M. Essential Oil Composition and Antibacterial Activity of Thymus Caramanicus at Different Phenological Stages. Food Chem. 2008, 110, 927–931. [Google Scholar] [CrossRef]
- Nezhadali, A.; Nabavi, M.; Rajabian, M.; Akbarpour, M.; Pourali, P.; Amini, F. Chemical Variation of Leaf Essential Oil at Different Stages of Plant Growth and in Vitro Antibacterial Activity of Thymus Vulgaris Lamiaceae, from Iran. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, R.; Lo Verde, G.; Sinacori, M.; Maggi, F.; Cappellacci, L.; Petrelli, R.; Vittori, S.; Morshedloo, M.R.; Fofie, N.G.B.Y.; Benelli, G. Developing Green Insecticides to Manage Olive Fruit Flies? Ingestion Toxicity of Four Essential Oils in Protein Baits on Bactrocera Oleae. Ind. Crops Prod. 2020, 143, 111884. [Google Scholar] [CrossRef]
- Flamini, G.; Smaili, T.; Zellagui, A.; Gherraf, N.; Cioni, P.L. Effect of Growth Stage on Essential-Oil Yield and Composition of Daucus Sahariensis. Chem. Biodivers. 2013, 10, 2014–2020. [Google Scholar] [CrossRef]
- Nejadhabibvash, F. Phytochemical Composition of the Essential Oil of Anthemis Wiedemanniana Fisch. and C.A. Mey. (Asteraceae) from Iran during Different Growth Stages. J. Essent. Oil Bear. Plants 2017, 20, 1349–1359. [Google Scholar] [CrossRef]
- Şanli, A.; Karadoğan, T.; Tosun, B.; Tonguç, M.; Erbaş, S. Growth Stage and Drying Methods Affect Essential Oil Content and Composition of Pickling Herb (Echinophora Tenuifolia Subsp. Sibthorpiana Tutin). SDÜ Fen Bilim. Enstitüsü Derg. 2016, 20, 43–49. [Google Scholar] [CrossRef]
- Serrano, C.; Matos, O.; Teixeira, B.; Ramos, C.; Neng, N.; Nogueira, J.; Nunes, M.L.; Marques, A. Antioxidant and Antimicrobial Activity of Satureja Montana, L. Extracts. J. Sci. Food Agric. 2011, 91, 1554–1560. [Google Scholar] [CrossRef]
- Jamali, C.A.; El Bouzidi, L.; Bekkouche, K.; Lahcen, H.; Markouk, M.; Wohlmuth, H.; Leach, D.; Abbad, A. Chemical Composition and Antioxidant and Anticandidal Activities of Essential Oils from Different Wild Moroccan Thymus Species. Chem. Biodivers. 2012, 9, 1188–1197. [Google Scholar] [CrossRef]
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Effect of Growth Stage on the Content and Composition of the Essential Oil and Phenolic Fraction of Sweet Marjoram (Origanum Majorana, L.). Ind. Crops Prod. 2009, 30, 395–402. [Google Scholar] [CrossRef]
- Schelz, Z.; Molnar, J.; Hohmann, J. Antimicrobial and Antiplasmid Activities of Essential Oils. Fitoterapia 2006, 77, 279–285. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential Oils as Antimicrobials in Food Systems—A Review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Vitali, L.A.; Beghelli, D.; Biapa Nya, P.C.; Bistoni, O.; Cappellacci, L.; Damiano, S.; Lupidi, G.; Maggi, F.; Orsomando, G.; Papa, F.; et al. Diverse Biological Effects of the Essential Oil from Iranian Trachyspermum Ammi. Arab. J. Chem. 2016, 9, 775–786. [Google Scholar] [CrossRef]
- Morshedloo, M.R.; Craker, L.E.; Salami, A.; Nazeri, V.; Sang, H.; Maggi, F. Effect of Prolonged Water Stress on Essential Oil Content, Compositions and Gene Expression Patterns of Mono- and Sesquiterpene Synthesis in Two Oregano (Origanum Vulgare, L.) Subspecies. Plant. Physiol. Biochem. 2017, 111, 119–128. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Bartolucci, F.; Canale, A.; Maggi, F. Origanum Syriacum Subsp. Syriacum: From an Ingredient of Lebanese ‘Manoushe’ to a Source of Effective and Eco-Friendly Botanical Insecticides. Ind. Crops Prod. 2019, 134, 26–32. [Google Scholar] [CrossRef]
- Bouhdid, S.; Skali, S.; Idaomar, M.; Zhiri, A.; Baudoux, D.; Amensour, M.; Abrini, J. Antibacterial & Antioxidant Activities of Origanum Compactum Essential Oil. Afr. J. Biotechnol. 2008, 7, 1563–1570. [Google Scholar] [CrossRef]
- Mirjana, S.; Nada, B.; Valerija, D. Variability of Satureja Cuneifolia Ten. Essential Oils and Their Antimicrobial Activity Depending on the Stage of Development. Eur. Food Res. Technol. 2004, 218, 367–371. [Google Scholar] [CrossRef]
- Gill, A.O.; Delaquis, P.; Russo, P.; Holley, R.A. Evaluation of Antilisterial Action of Cilantro Oil on Vacuum Packed Ham. Int. J. Food Microbiol. 2002, 73, 83–92. [Google Scholar] [CrossRef]
- Chavan, P.S.; Tupe, S.G. Antifungal Activity and Mechanism of Action of Carvacrol and Thymol against Vineyard and Wine Spoilage Yeasts. Food Control 2014, 46, 115–120. [Google Scholar] [CrossRef]
- Pavoni, L.; Maggi, F.; Mancianti, F.; Nardoni, S.; Ebani, V.V.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. Microemulsions: An Effective Encapsulation Tool to Enhance the Antimicrobial Activity of Selected EOs. J. Drug Deliv. Sci. Technol. 2019, 53, 101101. [Google Scholar] [CrossRef]
- Sabzi Nojadeh, M.; Pouresmaeil, M.; Younessi-Hamzekhanlu, M.; Venditt, A. Phytochemical Profile of Fennel Essential Oils and Possible Applications for Natural Antioxidant and Controlling Convolvulus Arvensis L. Nat. Prod. Res. 2020, 34, 1–5. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- De Oliveira, T.M.; De Carvalho, R.B.F.; Da Costa, I.H.F.; De Oliveira, G.A.L.; De Souza, A.A.; De Lima, S.G.; De Freitas, R.M. Evaluation of P-Cymene, a Natural Antioxidant. Pharm. Biol. 2015, 53, 423–428. [Google Scholar] [CrossRef]
- Singh, G.; Marimuthu, P.; Murali, H.S.; Bawa, A.S. Antioxidative and Antibacterial Potentials of Essential Oils and Extracts Isolated from Various Spice Materials. J. Food Saf. 2005, 25, 130–145. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Tepe, B.; Daferera, D.; Polissiou, M.; Harmandar, M. Studies on the Antioxidant Activity of the Essential Oil and Methanol Extract of Marrubium Globosum Subsp. Globosum (Lamiaceae) by Three Different Chemical Assays. Bioresour. Technol. 2008. [Google Scholar] [CrossRef]
- Boukhris, M.; Hadrich, F.; Chtourou, H.; Dhouib, A.; Bouaziz, M.; Sayadi, S. Chemical Composition, Biological Activities and DNA Damage Protective Effect of Pelargonium Graveolens L’Hér. Essential Oils at Different Phenological Stages. Ind. Crops Prod. 2015, 99, 4239–4246. [Google Scholar] [CrossRef]
- Salem, N.; Kefi, S.; Tabben, O.; Ayed, A.; Jallouli, S.; Feres, N.; Hammami, M.; Khammassi, S.; Hrigua, I.; Nefisi, S.; et al. Variation in Chemical Composition of Eucalyptus Globulus Essential Oil under Phenological Stages and Evidence Synergism with Antimicrobial Standards. Ind. Crops Prod. 2018, 124, 115–125. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Z.; Wei, J.; Su, P.; Pan, W.; Zheng, X.; Zhang, K.; Lin, L.; Tang, J.; Fang, Y.; et al. Essential Oil Composition and Bioactivity Variation in Wild-Growing Populations of Curcuma Phaeocaulis Valeton Collected from China. Ind. Crops Prod. 2017, 103, 274–282. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical Profile, Antioxidant and Antibacterial Activity of Thyme and Oregano Essential Oils, Thymol and Carvacrol and Their Possible Synergism. J. Essent. Oil-Bear. Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sökmen, M.; Polissiou, M.; Sökmen, A. The in Vitro Antioxidant and Antimicrobial Activities of the Essential Oil and Various Extracts of Origanum Syriacum L. Var Bevanii. J. Sci. Food Agric. 2004, 84, 1389–1396. [Google Scholar] [CrossRef]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant Activity in Meat Treated with Oregano and Sage Essential Oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Chivandi, E.; Dangarembizi, R.; Nyakudya, T.T.; Erlwanger, K.H. Use of Essential Oils as a Preservative of Meat. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Karami, A.; Kavoosi, G.; Maggi, F. The Emulsion Made with Essential Oil and Aromatic Water from Oliveria Decumbens Protects Murine Macrophages from LPS-Induced Oxidation and Exerts Relevant Radical Scavenging Activities. Biocatal. Agric. Biotechnol. 2019, 17, 538–544. [Google Scholar] [CrossRef]

| No. | Compound | RI a | RI b | Area (%) c | ||
|---|---|---|---|---|---|---|
| Vegetative | Flowering | Fruiting | ||||
| 1 | α-Thujene | 928 | 929 | 0.6 | 0.3 | - d |
| 2 | α-Pinene | 935 | 935 | 0.8 | 0.4 | 0.4 |
| 3 | Camphene | 938 | 937 | 0.6 | 0.4 | 0.5 |
| 4 | 3-Octanone | 959 | 960 | 0.5 | 0.3 | 0.3 |
| 5 | Sabinene | 969 | 968 | 0.5 | - | 0.3 |
| 6 | β-Pinene | 980 | 979 | 0.3 | 0.2 | 0.1 |
| 7 | Myrcene | 994 | 992 | 0.7 | 0.5 | 0.5 |
| 8 | α-Phellandrene | 1002 | 1003 | - | 1.0 | 0.8 |
| 9 | δ-3-Carene | 1009 | 1011 | - | 0.2 | 0.1 |
| 10 | α-Terpinene | 1015 | 1017 | 1.2 | 0.9 | 0.9 |
| 11 | p-Cymene | 1021 | 1021 | 10.1 | 11.1 | 14.7 |
| 12 | (E)-β-Ocimene | 1025 | 1026 | 1.1 | 0.3 | 0.3 |
| 13 | γ-Terpinene | 1054 | 1058 | 9.1 | 7.9 | 8.0 |
| 14 | α-Terpinolene | 1188 | 1189 | 0.2 | 0.1 | 0.2 |
| 15 | Linalool | 1094 | 1095 | 1.0 | - | 0.6 |
| 16 | cis-β-Terpineol | 1139 | 1140 | - | - | 0.1 |
| 17 | Camphor | 1140 | 1141 | - | - | 0.2 |
| 18 | Borneol | 1160 | 1166 | 4.9 | - | 3.8 |
| 19 | cis-3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl) | 1195 | 1195 | 0.6 | 0.6 | 0.6 |
| 20 | Methyl chavicol | 1196 | 1196 | - | - | 0.4 |
| 21 | Carvacrol methyl ether | 1240 | 1244 | 3.1 | 2.9 | 2.8 |
| 22 | (E)-Cinnamaldehyde | 1260 | 1266 | - | - | 0.2 |
| 23 | Thymol | 1290 | 1291 | 0.2 | 16.5 | 2.0 |
| 24 | Carvacrol | 1295 | 1297 | 48.2 | 42.7 | 45.6 |
| 25 | Isoledene | 1372 | 1373 | - | - | 0.1 |
| 26 | α-Copaene | 1375 | 1374 | - | 0.3 | 0.2 |
| 27 | β-Bourbonene | 1387 | 1388 | 0.2 | 0.3 | 0.2 |
| 28 | Carvacrol acetate | 1390 | 1391 | 0.8 | 0.3 | - |
| 29 | α-Cubebene | 1340 | 1346 | 0.2 | - | - |
| 30 | (E)-Caryophyllene | 1418 | 1420 | 1.9 | 2.3 | 1.2 |
| 31 | Aromadendrene | 1438 | 1440 | 0.9 | 1.3 | 1.2 |
| 32 | α-humulene | 1450 | 1452 | - | 0.2 | 0.2 |
| 33 | α-Amorphene | 1478 | 1480 | 1.0 | 1.1 | - |
| 34 | Germacrene D | 1483 | 1485 | 1.5 | 2.0 | 1.1 |
| 35 | 7-epi-α-Selinene | 1496 | 1495 | - | - | 0.2 |
| 36 | Bicyclogermacrene | 1497 | 1499 | 3.3 | 2.9 | 1.5 |
| 37 | β-Bisabolene | 1500 | 1504 | 0.4 | 0.8 | 0.3 |
| 38 | (E,E)-α-Farnesene | 1506 | 1507 | - | - | 0.1 |
| 39 | cis-α-Bisabolene | 1508 | 1511 | - | 0.2 | 0.1 |
| 40 | α-Calacorene | 1540 | 1542 | - | - | 0.1 |
| 41 | Spathulenol | 1579 | 1577 | 2.1 | - | 1.9 |
| 42 | Caryophyllene oxide | 1580 | 1581 | 0.9 | 1.0 | 1.1 |
| Total identified (%) | 96.7 | 99.1 | 92.5 | |||
| Yield (%, w/w) | 1.5 | 1.8 | 1.4 | |||
| Grouped compounds (%) | ||||||
| Monoterpene hydrocarbons | 25.0 | 23.4 | 27.0 | |||
| Oxygenated monoterpenes | 58.7 | 63.0 | 55.6 | |||
| Sesquiterpene hydrocarbons | 9.4 | 11.4 | 6.2 | |||
| Oxygenated sesquiterpenes | 3.0 | 1.0 | 3.0 | |||
| Others | 0.5 | 0.3 | 0.9 | |||
| Bacteria Strains | Vegetative Stage | Flowering Stage | Fruiting Stage | ||||
|---|---|---|---|---|---|---|---|
| MIC a | MBC b | MIC | MBC | MIC | MBC | ||
| Gram-positive | Staphylococcus aureus | 10 | 10 | 5 | 7.5 | 10 | 10 |
| Enterococcus faecalis | 60 | 80 | 20 | 20 | 40 | 40 | |
| Gram-negative | Enterobacter aerogenes | 40 | 40 | 7.5 | 10 | 20 | 20 |
| Escherichia coli | 20 | 20 | 10 | 10 | 20 | 20 | |
| Phenological Stage | DPPH (IC50, µg/mL) | ABTS (EC50, µg/mL) | Reducing Power (EC50, µg/mL) |
|---|---|---|---|
| Vegetative | 20.69 ± 1.05 b | 142.33 ± 6.47 a | 42.55 ± 1.84 c |
| Flowering | 12.14 ± 0.63 c | 135.25 ± 5.63 b | 36.74 ± 1.56 d |
| Fruiting | 21.85 ± 1.02 b | 134.78 ± 4.83 b | 48.79 ± 2.47 a |
| Positive control (BHT) | 25.76 ± 1.98 a | 110.17 ± 4.12 c | 40.32 ± 1.69 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghbash, B.N.; Pouresmaeil, M.; Dehghan, G.; Nojadeh, M.S.; Mobaiyen, H.; Maggi, F. Chemical Composition, Antibacterial and Radical Scavenging Activity of Essential Oils from Satureja macrantha C.A.Mey. at Different Growth Stages. Foods 2020, 9, 494. https://doi.org/10.3390/foods9040494
Aghbash BN, Pouresmaeil M, Dehghan G, Nojadeh MS, Mobaiyen H, Maggi F. Chemical Composition, Antibacterial and Radical Scavenging Activity of Essential Oils from Satureja macrantha C.A.Mey. at Different Growth Stages. Foods. 2020; 9(4):494. https://doi.org/10.3390/foods9040494
Chicago/Turabian StyleAghbash, Behzad Nezhadasad, Mohammad Pouresmaeil, Gholamreza Dehghan, Mohsen Sabzi Nojadeh, Haedeh Mobaiyen, and Filippo Maggi. 2020. "Chemical Composition, Antibacterial and Radical Scavenging Activity of Essential Oils from Satureja macrantha C.A.Mey. at Different Growth Stages" Foods 9, no. 4: 494. https://doi.org/10.3390/foods9040494
APA StyleAghbash, B. N., Pouresmaeil, M., Dehghan, G., Nojadeh, M. S., Mobaiyen, H., & Maggi, F. (2020). Chemical Composition, Antibacterial and Radical Scavenging Activity of Essential Oils from Satureja macrantha C.A.Mey. at Different Growth Stages. Foods, 9(4), 494. https://doi.org/10.3390/foods9040494