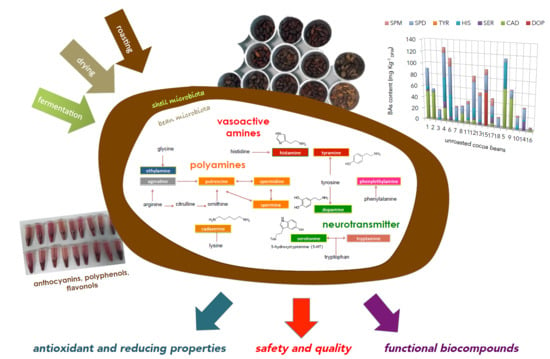
Effect of Fermentation, Drying and Roasting on Biogenic Amines and Other Biocompounds in Colombian Criollo Cocoa Beans and Shells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of the Samples
2.2. Samples Preparation and Defatting
2.3. Moisture and pH Determination
2.4. Microbiological Analyses
2.5. Biogenic Amines Determination
2.6. Colour Analysis
2.7. Anthocyanin Determination
2.8. Extraction of the Phenolic Fraction
2.8.1. Total Polyphenols Content (TPC)
2.8.2. Flavanols Identification and Quantification
2.8.3. ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfuric acid)) Assay
2.8.4. Ferric Reducing Antioxidant Power (FRAP)
2.9. Statistical Analyses
3. Results
3.1. Characterization of Fermented and Dried Cocoa Beans
3.1.1. Microbiota
3.1.2. pH, Moisture and Colour
3.2. Biogenic Amines Profile
3.3. Effect of Roasting on the BAs Content
3.4. Anthocyanins, Total Polyphenols and Flavanols Content
3.5. Trolox Equivalent Antioxidant Capacity (TEAC) and the Ferric Reducing Antioxidant Power (FRAP) Assays
3.6. Principal Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- International Cocoa Organization. Quarterly Bulletin of Cocoa Statistics; International Cocoa Organization: London, UK, 2019; Volume 45, p. 101. Available online: http:www.icco.org (accessed on 12 December 2019).
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, supply chain and processing of cocoa—A review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Afoakwa, E.O. The chemistry of flavour development during Cocoa processing and chocolate manufacture. In Chocolate Science and Technology, 1st ed.; Afoakwa, E.O., Ed.; Wiley online library: Hoboken, NJ, USA, 2016; p. 296. [Google Scholar]
- Di Mattia, C.; Martuscelli, M.; Sacchetti, G.; Scheirlinck, I.; Beheydt, B.; Mastrocola, D.; Pittia, P. Effect of fermentation and drying on procyanidins, antiradical activity and reducing properties of cocoa beans. Food Bioprocess. Technol. 2013, 6, 3420. [Google Scholar] [CrossRef]
- Coton, E.; Coton, M. Evidence of horizontal transfer as origin of strain to strain variation of the tyramine production trait in Lactobacillus brevis. Food Microbiol. 2009, 26, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.M.; Blancato, V.S.; Claisse, O.; Magni, C.; Lolkema, J.S.; Lonvaud-Funel, A. Agmatine deiminase pathway genes in Lactobacillus brevis are linked to the tyrosine decarboxylation operon in a putative acid resistance locus. Microbiology 2007, 153, 2221–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, P.M.; Wolken, W.A.M.; Claisse, O.; Lolkema, J.S.; Lonvaud-Funel, A. Histamine-producing pathway encoded on an unstable plasmid in Lactobacillus hilgardii 0006. Appl. Environ. Microbiol. 2005, 71, 1417–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcobal, Á.; De Las Rivas, B.; Moreno-Arribas, M.V.; Muñoz, R. Evidence for horizontal gene transfer as origin of putrescine production in Oenococcus oeni RM83. Appl. Environ. Microbiol. 2006, 72, 7954–7958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martuscelli, M.; Crudele, M.A.; Gardini, F.; Suzzi, G. Biogenic amine formation and oxidation by Staphylococcus xylosus strains from artisanal fermented sausages. Lett. Appl. Microbiol. 2000, 31, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Di Mattia, C.; Martuscelli, M.; Sacchetti, G.; Beheydt, B.; Mastrocola, D.; Pittia, P. Effect of different conching processes on procyanidin content and antioxidant properties of chocolate. Food Res. Int. 2014, 63, 367–372. [Google Scholar] [CrossRef]
- Do Carmo Brito, B.D.N.; Campos Chisté, R.; da Silva Pena, R.; Abreu Gloria, M.B.; Santos Lopes, A. Bioactive amines and phenolic compounds in cocoa beans are affected by fermentation. Food Chem. 2017, 228, 484–490. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E. Influence of roasting conditions on the biogenic amine content in cocoa beans of different Theobroma cacao cultivars. Food Res. Int. 2014, 55, 1–10. [Google Scholar] [CrossRef]
- Ioannone, F.; Di Mattia, C.D.; De Gregorio, M.; Sergi, M.; Serafini, M.; Sacchetti, G. Flavanols, proanthocyanidins and antioxidant activity changes during cocoa (Theobroma cacao L.) roasting as affected by temperature and time of processing. Food Chem. 2015, 174, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, G.; Ioannone, F.; De Gregorio, M.; Di Mattia, C.; Serafini, M.; Mastrocola, D. Non enzymatic browning during cocoa roasting as affected by processing time and temperature. J. Food Eng. 2016, 169, 44–52. [Google Scholar] [CrossRef]
- Hinneh, M.; Abotsi, E.E.; Van De Walle, D.; Tzompa-Sosa, D.A.; De Winne, A.; Simonis, J.; Messens, K.; Van Durme, J.; Afoakwa, E.O.; De Cooman, L.; et al. Pod storage with roasting: A tool to diversifying the flavor profiles of dark chocolates produced from ‘bulk’ cocoa beans? (part I: Aroma profiling of chocolates). Food Res. Int. 2019, 119, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, T.; Capuano, E.; Cämmerer, B.; Fogliano, V. Influence of roasting on the antioxidant activity and HMF formation of a cocoa bean model systems. J. Agric. Food Chem. 2009, 57, 147–152. [Google Scholar] [CrossRef]
- Djikeng, F.T.; Teyomnou, W.T.; Tenyang, N.; Tiencheu, B.; Morfor, A.T.; Touko, B.A.H.; Houketchang, S.N.; Boungo, G.T.; Karuna, M.S.L.; Ngoufack, F.Z.; et al. Effect of traditional and oven roasting on the physicochemical properties of fermented cocoa beans. Heliyon 2018, 4, e00533. [Google Scholar] [CrossRef] [Green Version]
- International agency for research on cancer (IARC). Monographs on the evaluation of carcinogenic risks to humans. In Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals; IARC Publications: Lyon, France, 1995; Volume 63, pp. 3194–3407. [Google Scholar]
- Codex Alimentarius Commission. Standard for Cocoa (Cacao) Mass (Cocoa/Chocolate Liquor) and Cocoa Cake Codex Stan 141-1983; 2014. Available online: http://www.fao.org/input/download/standards/69/CXS_141e.pdf (accessed on 1 March 2020).
- Okiyama, D.C.G.; Navarro, S.L.B.; Rodrigues, C.E.C. Cocoa shell and its compounds: Applications in the food industry. Trends Food Sci. Technol. 2017, 63, 103–112. [Google Scholar] [CrossRef]
- Quelal-Vásconez, M.A.; Lerma-García, M.J.; Pérez-Esteve, É.; Arnau-Bonachera, A.; Barat, J.M.; Talens, P. Fast detection of cocoa shell in cocoa powders by near infrared spectroscopy and multivariate analysis. Food Control 2019, 99, 68–72. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 17th ed.; AOAC: Gaithersburg, MD, USA, 2002. [Google Scholar]
- Chaves-López, C.; Serio, A.; Montalvo, C.; Ramirez, C.; Peréz-Álvarez, J.A.; Paparella, A.; Mastrocola, D.; Martuscelli, M. Effect of nisin on biogenic amines and shelf life of vacuum packaged rainbow trout (Oncorhynchus mykiss) fillets. J. Food Sci. Technol. 2017, 54, 3268–3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Kongor, J.E.; Hinneh, M.; Van de Walle, D.; Afoakwa, E.O.; Boeckx, P.; Dewettinck, K. Factors influencing quality variation in cocoa Theobroma cacao bean flavour profile: A review. Food Res. Int. 2016, 82, 44–52. [Google Scholar] [CrossRef]
- Schwan, R.F.; Pereira, G.D.M.; Fleet, G.H. Microbial activities during cocoa fermentation. In Cocoa and Coffee Fermentations, 1st ed.; Shwan, R.F., Fleet, G.H., Eds.; Taylor & Francis: Boca Raton, FL, USA, 2014; pp. 129–192. [Google Scholar]
- Schwan, R.F.; Wheals, A.E. The microbiology of cocoa fermentation and its role in chocolate quality. Crit. Rev. Food Sci. Nutr. 2004, 44, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.J.R.; van der Velpen, V.; Wolkers-Rooijackers, J.; Kamphuis, H.J.; Zwietering, M.H.; Rob Nout, M.J. Microbiota dynamics and diversity at different stages of industrial processing of cocoa beans into cocoa powder. Appl. Environ. Microbiol. 2012, 78, 2904–2913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baraggio, N.G.; Velázquez, N.S.; Simonetta, A.C. Aminas biógenas generadas por cepas bacterianas provenientes de alimentos lácteos y cárnicos. Rev. Cienc. Tecnol. 2010, 13. Available online: http://www.scielo.org.ar/scielo.php?script=sci_arttext&pid=S1851-75872010000100012 (accessed on 20 March 2020).
- Rodriguez-Campos, J.; Escalona-Buendía, H.B.; Orozco-Avila, I.; Lugo-Cervantes, E.; Jaramillo-Flores, M.E. Dynamics of volatile and non-volatile compounds in cocoa (Theobroma cacao L.) during fermentation and drying processes using principal components analysis. Food Res. Int. 2011, 44, 250–258. [Google Scholar] [CrossRef]
- García-Alamilla, P.; Lagunes-Gálvez, L.M.; Barajas-Fernández, J.; García-Alamilla, R. Physicochemical changes of cocoa beans during roasting process. J. Food Qual. 2017, 12, 1–11. [Google Scholar] [CrossRef]
- Gloria, M.B.A.; Tavares-Neto, J.; Labanca, R.A.; Carvalho, M.S. Influence of cultivar and germination on bioactive amines in soybeans (Glycine max L. Merril). J. Agric. Food Chem. 2005, 53, 7480–7485. [Google Scholar] [CrossRef]
- Bandeira, C.M.; Evangelista, W.P.; Gloria, M.B.A. Bioactive amines in fresh, canned and dried sweet corn, embryo and endosperm and germinated corn. Food Chem. 2012, 131, 1355–1359. [Google Scholar] [CrossRef] [Green Version]
- Albertini, B.; Schoubben, A.; Guarnaccia, D.; Pinelli, F.; Della Vecchia, M.; Ricci, M.; Di Renzo, G.C.; Blasi, P. Effect of fermentation and drying on cocoa polyphenols. J. Agric. Food Chem. 2015, 63, 9948–9953. [Google Scholar] [CrossRef]
- Granvogl, M.; Bugan, S.; Schieberle, P. Formation of amines and aldehydes from parent amino acids during thermal processing of cocoa and model systems: New insights into pathways of the Strecker reaction. J. Agric. Food Chem. 2006, 54, 1730–1739. [Google Scholar] [CrossRef]
- Zamora, R.; Delgado, R.M.; Hidalgo, F.J. Formation of β-phenylethylamine as a consequence of lipid oxidation. Food Res. Int. 2012, 46, 321–325. [Google Scholar] [CrossRef]
- Martins, A.C.C.L.; Gloria, M.B.A. Changes on the levels of serotonin precursors - tryptophan and 5-hydroxytryptophan-during roasting of Arabica and Robusta coffee. Food Chem. 2010, 118, 529–533. [Google Scholar] [CrossRef]
- Luten, J.B.; Bouquet, W.; Seuren, L.A.J.; Burggraaf, M.M.; Riekwel-Booy, G.; Durand, P.; Etienne, M.; Gouyou, J.P.; Landrein, A.; Ritchie, A.; et al. Biogenic amines in fishery products: Standardization methods within E.C. In Quality Assurance in the Fish Industry; Elsevier Science Publishers, B.V.: Amsterdam, The Netherlands, 1992; pp. 427–439. [Google Scholar]
- Wendakoon, C.N.; Sakaguchi, M. Combined effect of sodium chloride and clove on growth and biogenic amine formation of Enterobacter aerogenes in Mackerel muscle extract. J. Food Prot. 1993, 56, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Park, S.Y.; Byun, Y.S.; Son, J.H.; Choi, Y.W.; Cho, H.S.; Kim, H.O.; Park, C.W. Effect of different cooking methods on Histamine levels in selected foods. Ann. Dermatol. 2017, 296, 706–714. [Google Scholar] [CrossRef] [Green Version]
- Thadhani, V.M.; Jansz, E.R.; Peiris, H. Destruction of histamine by cooking ingredients-an artifact of analysis. J. Natl. Sci. Found. Sri Lanka 2001, 29, 129–135. [Google Scholar] [CrossRef] [Green Version]
- Zapata-Bustamante, S.; Tamayo-Tenorio, A.; Rojano, B.A. Effect of roasting on the secondary metabolites and antioxidant activity of Colombian cocoa clones. Rev. Fac. Nac. Agron. Medellín 2015, 68, 7497–7507. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Ofosu-Ansah, E.; Budu, A.S.; Mensah-Brown, H.; Takrama, J.F. Roasting effects on phenolic content and free-radical scavenging activities of pulp preconditioned and fermented cocoa (Theobroma cacao) beans. Afr. J. Food Agric. Nutr. Dev. 2015, 15, 9635–9650. [Google Scholar]
- Carrillo, L.C.; Londoño-Londoño, J.; Gil, A. Comparison of polyphenol, methylxanthines and antioxidant activity in Theobroma cacao beans from different cocoa-growing areas in Colombia. Food Res. Int. 2014, 60, 273–280. [Google Scholar] [CrossRef]
- Porras Barrientos, L.D.; Torres Oquendo, J.D.; Gil Garzón, M.A.; Martínez Álvarez, O.L. Effect of the solar drying process on the sensory and chemical quality of cocoa (Theobroma cacao L.) cultivated in Antioquia, Colombia. Food Res. Int. 2019, 115, 259–267. [Google Scholar] [CrossRef]
- Loureiro, G.A.H.A. Qualidade de solo e Qualidade de Cacau. Master’s Thesis, Universidade Estadual de Santa Cruz, Ilhéus, Bahia, Brazil, 2014. [Google Scholar]
- Janszky, I.; Mukamai, K.J.; Ljung, R.; Ahnve, S.; Ahlbom, A.; Hallqvist, J. Chocolate consumption and mortality following a first acute myocardial infarction: The Stockholm Heart Epidemiology Study. J. Intern. Med. 2009, 266, 248–257. [Google Scholar] [CrossRef]
- Payne, M.J.; Hurst, W.J.; Miller, K.B.; Rank, C.; Stuart, D.A. Impact of fermentation, drying, roasting, and dutch processing on epicatechin and catechin content of cacao beans and cocoa ingredients. J. Agric. Food Chem. 2010, 58, 10518–10527. [Google Scholar] [CrossRef] [PubMed]
- Caligiani, A.; Cirlini, M.; Palla, G.; Ravaglia, R.; Arlorio, M. GC-MS detection of chiral markers of cocoa beans of different quality and geographic origin. Chirality 2007, 19, 329–334. [Google Scholar] [CrossRef]
- Hurst, W.J.; Krake, S.H.; Bergmeier, S.C.; Payne, M.J.; Miller, K.B.; Stuart, D.A. Impact of fermentation, drying, roasting and Dutch processing on flavan-3-ol stereochemistry in cacao beans and cocoa ingredients. Chem. Cent. J. 2011, 5, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauer, D.; de Abreu, J.P.; Oliveira, H.S.S.; Goes-Neto, A.; Koblitz, M.G.B.; Teodoro, A.J. Antioxidant activity and cytotoxicity effect of cocoa beans subjected to different processing conditions in human lung carcinoma cells. Oxid. Med. Cell. Longev. 2016, 2016, 7428515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabanelli, G.; Montanari, C.; Gardini, F. Biogenic amines in food: A review of factors affecting their formation. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: New York, NY, USA, 2019; pp. 337–343. [Google Scholar]
- Carta, F.; Temperini, C.; Innocenti, A.; Scozzafava, A.; Kaila, K.; Supuran, C.T. Polyamines inhibit carbonic anhydrases by anchoring to the zinc-coordinated water molecule. J. Med. Chem. 2010, 53, 511–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Farm Location | Altitude (masl *) | Fermentation | Drying | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tday (°C) | Tnight (°C) | Time (d) | Box | Tday (°C) | Tnight (°C) | Time (d) | Drying Surface | |||
| 1 | Valle del Cauca | 1000 | 27–31 | 18–20 | 4 | plastic | 28–30 | 17–18 | 4 | wooden trays |
| 2 | Valle del Cauca | 1000 | 27–31 | 18–20 | 3 | plastic | 28–30 | 17–18 | 5 | wooden trays |
| 3 | Valle del Cauca | 1000 | 25–27 | 17–18 | 6 | wooden | 25–27 | 18–19 | 3 | wooden trays |
| 4 | Valle del Cauca | 1000 | 27–31 | 18–20 | 6 | plastic | 28–30 | 17–18 | 3 | wooden trays |
| 5 | Cauca | 990 | 29–30 | 19–20 | 4 | wooden | 29–30 | 18–19 | 5 | wooden trays |
| 6 | Valle del Cauca | 1000 | 27–31 | 18–20 | 6 | wooden | 28–30 | 17–18 | 6 | floors |
| 7 | Valle del Cauca | 1000 | 25–27 | 17–18 | 6 | plastic | 25–27 | 18–19 | 4 | wooden trays |
| 8 | Valle del Cauca | 1000 | 25–27 | 17–18 | 6 | plastic | 25–27 | 18–19 | 4 | metal trays |
| 9 | Cauca | 990 | 29–30 | 19–20 | 4 | wooden | 29–30 | 18–19 | 6 | floors |
| 10 | Cauca | 990 | 28–33 | 19–20 | 4 | wooden | 28–33 | 17–20 | 7 | floors |
| 11 | Valle del Cauca | 1000 | 27–31 | 18–20 | 6 | wooden | 28–30 | 17–18 | 4 | floors |
| 12 | Valle del Cauca | 1000 | 27–31 | 18–20 | 6 | plastic | 28–30 | 17–18 | 3 | floors |
| 13 | Valle del Cauca | 1000 | 25–27 | 17–18 | 6 | plastic | 25–27 | 18–19 | 3 | wooden trays |
| 14 | Cauca | 990 | 29–30 | 19–20 | 4 | wooden | 29–30 | 18–19 | 5 | wooden trays |
| 15 | Valle del Cauca | 1000 | 27–31 | 18–20 | 6 | plastic | 28–30 | 17–18 | 5 | wooden trays |
| 16 | Nariño | 30 | 21–25 | 12–22 | 4 | wooden | 20–25 | 11–15 | 4 | wooden trays |
| 17 | Valle del Cauca | 1000 | 27–31 | 18–20 | 6 | plastic | 28–30 | 17–18 | 4 | floors |
| 18 | Valle del Cauca | 1000 | 25–27 | 17–18 | 6 | plastic | 25–27 | 18–19 | 3 | wooden trays |
| Samples | Origin | pH | Moisture (%) | Colour | |||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | h° | ||||
| 1 | Valle de Cauca | 5.01 ± 0.02 | 3.4 ± 0.3 | 40.83 ± 0.80 | 7.06 ± 0.08 | 11.17 ± 0.35 | 57.67± 0.01 |
| 2 | Valle de Cauca | 4.79 ± 0.11 | 3.9 ± 0.2 | 48.92 ± 0.27 | 3.64 ± 0.08 | 9.38 ± 0.10 | 68.77 ± 0.02 |
| 3 | Valle de Cauca | 4.43 ± 0.08 | 4.2 ± 0.4 | 41.78 ± 0.43 | 9.87 ± 0.12 | 12.35 ± 0.22 | 51.35 ± 0.06 |
| 4 | Valle de Cauca | 4.49 ± 0.03 | 3.8 ± 0.3 | 38.40 ± 0.16 | 9.49 ± 0.12 | 9.57 ± 0.13 | 45.24 ± 0.02 |
| 5 | Cauca | 5.85 ± 0.06 | 5.0 ± 0.2 | 41.42 ± 0.30 | 9.21 ± 0.05 | 10.25 ± 0.16 | 48.07 ± 0.01 |
| 6 | Valle de Cauca | 4.54 ± 0.10 | 1.2 ± 0.1 | 38.76 ± 0.67 | 9.05 ± 0.32 | 6.70 ± 0.54 | 36.49 ± 0.02 |
| 7 | Valle de Cauca | 4.99 ± 0.07 | 3.5 ± 0.2 | 41.71 ± 0.65 | 8.29 ± 0.15 | 10.85 ± 0.16 | 52.62 ± 0.01 |
| 8 | Valle de Cauca | 5.05 ± 0.05 | 4.8 ± 0.4 | 43.26 ± 0.37 | 8.25 ± 0.27 | 13.43 ± 0.20 | 58.45 ± 0.01 |
| 9 | Cauca | 5.11 ± 0.18 | 2.5 ± 0.1 | 36.84 ± 0.30 | 7.30 ± 0.16 | 11.79 ± 0.41 | 58.22 ± 0.01 |
| 10 | Cauca | 5.52 ± 0.15 | 1.7 ± 0.1 | 38.89 ± 0.68 | 4.37 ± 0.02 | 8.30 ± 0.09 | 62.22 ± 0.01 |
| 11 | Valle de Cauca | 4.41 ± 0.08 | 2.5 ± 0.2 | 39.85 ± 0.34 | 9.60 ± 0.24 | 10.27 ± 0.38 | 46.90 ± 0.01 |
| 12 | Valle de Cauca | 4.62 ± 0.03 | 2.2 ± 0.0 | 44.50 ± 0.38 | 7.31 ± 0.16 | 11.34 ± 0.18 | 57.19 ± 0.00 |
| 13 | Valle de Cauca | 4.67 ± 0.33 | 4.9 ± 0.4 | 37.54 ± 0.18 | 10.04 ± 0.05 | 15.77 ± 0.03 | 57.53 ± 0.00 |
| 14 | Cauca | 5.45 ± 0.09 | 6.2 ± 0.3 | 38.44 ± 1.02 | 8.18 ± 0.09 | 12.07 ± 0.26 | 55.87 ± 0.01 |
| 15 | Valle de Cauca | 5.07 ± 0.12 | 2.5 ± 0.2 | 38.23 ± 0.91 | 8.33 ± 0.14 | 9.30 ± 0.08 | 48.15 ± 0.00 |
| 16 | Nariño | 4.68 ± 0.13 | 3.9 ± 0.1 | 43.78 ± 0.07 | 5.34 ± 0.09 | 12.25 ± 0.25 | 66.43 ± 0.01 |
| 17 | Valle de Cauca | 4.44 ± 0.10 | 2.8 ± 0.2 | 48.89 ± 0.73 | 5.34 ± 0.09 | 12.25 ± 0.25 | 66.43 ± 0.01 |
| 18 | Valle de Cauca | 6.17 ± 0.27 | 4.7 ± 0.7 | 32.96 ± 0.89 | 9.39 ± 0.32 | 12.93 ± 0.46 | 54.00 ± 0.00 |
| Sample | Biogenic Amines Content (mg kg−1DFW) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ETH * | DOP | PHE | PUT | CAD | SER | HIS | TYR | SPD | SPM | ||
| 1 | T1 | nd | nd | nd | nd | 49.75 ± 1.4 | 3.06 ± 0.0 | 5.96 ± 1.2 | nd | 30.85 ± 3.6 | nd |
| T2 | 14.72 ± 2.3 | nd | 12.2 ± 0.1 | 1.32 ± 0.2 | nd | 17.18 ± 4.2 | 0.21 ± 3.1 | 17.02 ± 0.2 | nd | 14.30 ± 2.2 | |
| T3 | 15.56 ± 5.2 | nd | 26.23 ± 0.3 | 59.02 ± 3.5 | 5.38 ± 0.6 | nd | 13.25 ± 1.1 | 11.11 ± 0.0 | nd | 36.84 ± 5.2 | |
| 2 | T1 | nd | nd | nd | nd | 48.62 ± 0.2 | 0.60 ± 0.0 | nd | nd | 4.67 ± 0.3 | nd |
| T2 | 24.77 ± 0.7 | 33.27 ± 0.2 | 11.18 ± 0.1 | nd | nd | 17.70 ± 0.0 | nd | 26.51 ± 4.2 | nd | 46.42 ± 8.4 | |
| T3 | 19.22 ± 0.4 | 1.99 ± 0.0 | 25.98 ± 1.2 | 62.58 ± 6.2 | nd | 2.95 ± 0.2 | 17.13 ± 0.9 | 11.27 ± 0.8 | 2.50 ± 0.0 | 52.82 ± 3.9 | |
| 3 | T1 | nd | nd | nd | nd | nd | nd | 3.56 ± 1.1 | nd | 13.91 ± 0.2 | 0.37 ± 0.0 |
| T2 | 0.89 ± 0.3 | nd | 12.55 ± 0.5 | nd | nd | 9.11 ± 0.6 | 0.44 ± 0.5 | 25.22 ± 1.7 | nd | nd | |
| T3 | 1.08 ± 0.4 | nd | 9.69 ± 136 | nd | nd | nd | 0.80 ± 1.2 | 8.29 ± 0.3 | nd | nd | |
| 4 | T1 | nd | nd | nd | nd | 22.60 ± 0.6 | 11.65 ± 1.1 | 41.9 ± 2.2 | nd | 42.06 ± 2.8 | 8.86 ± 3.2 |
| T2 | 2.74 ± 0.1 | nd | 11.77 ± 0.1 | nd | 2.22 ± 0.1 | 3.07 ± 0.0 | 0.82 ± 0.5 | 14.96 ± 1.1 | nd | 2.03 ± 1.8 | |
| T3 | 1.68 ± 0.2 | nd | 17.22 ± 0.0 | nd | nd | 1.01 ± 0.0 | 1.01 ± 1.2 | 15.98 ± 0.4 | nd | nd | |
| 5 | T1 | nd | nd | nd | nd | 66.57 ± 0.7 | 0.36 ± 0.0 | 39.8 ± 7.1 | nd | 5.99 ± 0.0 | nd |
| T2 | 29.17 ± 0.3 | 65.12 ± 0.1 | 10.36 ± 0.5 | 6.46 ± 0.3 | 3.28 ± 0.0 | 25.63 ± 2.3 | 0.46 ± 0.7 | 20.80 ± 0.5 | nd | 60.04 ± 8.4 | |
| T3 | 17.06 ± 1.2 | 95.03 ± 0.1 | 10.67 ± 0.1 | 2.51 ± 0.1 | 3.87 ± 0.0 | nd | 0.38 ± 0.2 | 9.87 ± 0.0 | 1.15 ± 0.0 | nd | |
| 6 | T1 | nd | nd | nd | nd | 1.50 ± 0.0 | 6.98 ± 0.1 | 38.90 ± 5.3 | nd | 48.66 ± 2.1 | 13.61 ± 2.2 |
| T2 | 2.95 ± 1.2 | nd | 11.36 ± 0.1 | nd | 3.14 ± 0.0 | nd | 17.13 ± 2.2 | 17.03 ± 0.7 | nd | 5.38 ± 0.4 | |
| T3 | 2.17 ± 0.7 | nd | 14.75 ± 0.1 | nd | nd | nd | nd | 13.50 ± 0.4 | nd | nd | |
| 7 | T1 | nd | nd | nd | nd | 1.80 ± 0.0 | 0.11 ± 0.0 | 5.96 ± 1.2 | nd | 18.53 ± 6.2 | 1.12 ± 0.0 |
| T2 | 0.98 ± 0.1 | nd | 13.85 ± 0.0 | nd | nd | nd | 13.55 ± 3.0 | 20.51 ± 0.2 | nd | nd | |
| T3 | 1.24 ± 0.1 | nd | 15.72 ± 0.2 | nd | nd | nd | nd | 13.12 ± 0.8 | nd | nd | |
| 8 | T1 | nd | nd | nd | nd | 15.86 ± 0.1 | nd | nd | nd | 12.81 ± 2.5 | 0.12 ± 0.1 |
| T2 | 3.61 ± 0.4 | nd | 12.70 ± 0.1 | nd | nd | nd | 48.18 ± 1.9 | 14.88 ± 0.1 | nd | 1.05 ± | |
| T3 | 3.36 ± 0.3 | nd | 12.81 ± 0.1 | nd | nd | nd | nd | 10.82 ± 0.0 | nd | nd | |
| 9 | T1 | nd | nd | nd | nd | 51.77 ± 0.5 | 0.85 ± 0.1 | nd | nd | 11.93 ± 1.1 | 0.37 ± 0.3 |
| T2 | 13.67 ± 0.5 | nd | 14.14 ± 0.1 | nd | nd | nd | 37.73 ± 2.8 | 18.16 ± 7.7 | 0.51 ± 0.0 | nd | |
| T3 | 11.3 ± 0.1 | nd | 14.98 ± 0.1 | 1.50 ± | 2.31 ± 0.0 | nd | nd | 15.85 ± 1.6 | 0.03 ± 0.0 | nd | |
| 10 | T1 | nd | nd | nd | nd | nd | nd | 7.76 ± 4.1 | nd | 12.37 ± 0.0 | 2.37 ± 0.2 |
| T2 | 21.65 ± 0.1 | 76.92 ± 0.1 | 12.04 ± 0.1 | 3.99 ± 0.4 | 2.22 ± 0.0 | 3.71 ± 0.1 | 0.27 ± 0.7 | 14.97 ± 1.1 | 1.87 ± 0.0 | nd | |
| T3 | 12.55 ± 0.2 | 56.68 ± 0.1 | 8.42 ± 0.5 | nd | nd | nd | 1.25 ± 0.2 | 11.5 ± 2.7 | nd | nd | |
| 11 | T1 | nd | nd | nd | nd | 5.54 ± 0.1 | 0.24 ± 0.0 | 5.04 ± 0.1 | nd | 23.15 ± 1.2 | 4.37 ± 0.6 |
| T2 | 2.63 ± 0.1 | nd | 13.35 ± 0.0 | nd | nd | nd | 21.53 ± 3.1 | 14.20 ± 0.7 | nd | nd | |
| T3 | 2.89 ± 0.1 | nd | 14.83 ± 0.6 | nd | nd | nd | 0.27 ± 0.5 | 14.49 ± 5.7 | nd | 0.37 ± 0.2 | |
| 12 | T1 | nd | nd | nd | nd | 7.56 ± 0.1 | 4.28 ± 0.1 | 16.74 ± 3.1 | nd | 44.48 ± 4.2 | 9.61 ± 1.1 |
| T2 | 4.55 ± 0.4 | nd | 13.94 ± 3.2 | nd | nd | nd | 59.78 ± 6.4 | 19.68 ± 0.3 | nd | nd | |
| T3 | 3.19 ± 0.2 | nd | 16.16 ± 4.1 | nd | nd | nd | 1.24 ± 0.2 | 8.54 ± 0.6 | nd | nd | |
| 13 | T1 | nd | nd | nd | nd | 0.61 ± 0.0 | 4.41 ± 1.1 | 4.76 ± 2.6 | nd | 40.75 ± 2.7 | 4.86 ± 0.2 |
| T2 | 8.38 ± 0.1 | nd | 11.81 ± 2.9 | nd | nd | nd | 0.60 ± 0.3 | 16.24 ± 0.5 | nd | nd | |
| T3 | 7.46 ± 0.1 | nd | 13.43 ± 1.6 | nd | nd | nd | 0.17 ± 0.4 | 14.42 ± 0.8 | nd | nd | |
| 14 | T1 | nd | nd | nd | nd | nd | 15.33 ± 1.2 | 2.96 ± 0.3 | nd | 18.31 ± 3.1 | 0.62 ± 0.1 |
| T2 | 6.47 ± 0.2 | nd | 14.65 ± 2.8 | nd | nd | 5.62 ± 0.1 | 0.79 ± 0.7 | 18.51 ± 1.0 | nd | nd | |
| T3 | 6.33 ± 0.3 | nd | 12.50 ± 3.1 | nd | 2.87 ± 0.0 | nd | 2.70 ± 0.5 | 12.41 ± 3.4 | nd | nd | |
| 15 | T1 | nd | 57.35 ± 01 | nd | nd | 0.83 ± 0.0 | 3.55 ± 0.0 | nd | 0.19 ± 0.0 | 24.47 ± 2.2 | 8.86 ± 0.8 |
| T2 | 11.92 ± 0.0 | nd | 16.27 ± 2.1 | nd | nd | 4.90 ± 0.0 | nd | 13.65 ± 0.3 | nd | nd | |
| T3 | 6.102 ± 0.2 | nd | 10.81 ± 1.0 | nd | nd | nd | nd | 12.14 ± 0.6 | nd | nd | |
| 16 | T1 | nd | nd | nd | nd | 2.85 ± 0.0 | 1.34 ± 0.0 | nd | nd | 0.28 ± 0.0 | 0.42 ± 0.0 |
| T2 | 27.00 ± 0.2 | 92.17 ± 0.1 | 18.78 ± 1.2 | nd | nd | nd | 29.87 ± 5.6 | 9.64 ± 0.0 | 2.62 ± 0.2 | 3.35 ± 0.7 | |
| T3 | 9.41 ± 0.8 | 55.76 ± 0.1 | 6.02 ± 0.0 | nd | nd | nd | 1.18 ± 9.4 | 0.79 ± 0.0 | nd | nd | |
| 17 | T1 | nd | nd | nd | nd | 5.77 ± 0.3 | 9.68 ± 0.4 | 5.36 ± 0.7 | nd | 14.79 ± 2.1 | 1.19 ± 0.5 |
| T2 | 23.52 ± 0.4 | 77.52 ± 0.1 | 11.81 ± 0.8 | nd | 5.45 ± 0.2 | nd | 27.27 ± 4.9 | 11.37 ± 0.4 | 2.30 ± 0.0 | nd | |
| T3 | 22.27 ± 0.0 | 16.27 ± 0.1 | 11.73 ± 0.3 | 4.44 ± 1.1 | 5.45 ± 0.7 | nd | 8.12 ± 0.9 | 12.51 ± 0.0 | 2.60 ± 0.0 | 2.85 ± 0.0 | |
| 18 | T1 | nd | nd | nd | nd | nd | nd | nd | nd | 15.45 ± 0.0 | 0.87 ± 0.0 |
| T2 | 25.66 ± 0.1 | nd | 17.24 ± 2.1 | nd | nd | nd | nd | 15.14 ± 3.2 | nd | nd | |
| T3 | 11.77 ± 0.1 | nd | 20.60 ± 1.6 | nd | nd | nd | nd | 16.94 ± 1.9 | nd | nd | |
| Sample | Anthocyanins (mg g−1DFW) | Sign. | TPC (mg GAE g−1DFW) | Sign. | Catechin (mg g−1DFW) | Sign. | Epicatechin (mg g−1DFW) | Sign. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |||||
| 1 | 2.28a | 1.36c | 2.08b | * | 43.00c | 68.79b | 83.85a | * | nd | 8.41b | 11.49a | ** | 0.5b | 0.87a | 0.51b | * |
| 2 | 3.36a | 1.77b | 2.00b | * | 22.97b | 100.73a | 110.17a | *** | 0.18b | 4.36a | nd | *** | 0.76 | 0.9 | 0.54 | ** |
| 3 | 0.24c | 0.31b | 0.37a | * | 47.38b | 33.54a | 45.56b | * | 1.47b | 1.64b | 2.17a | * | 1.72a | nd | 0.7b | ** |
| 4 | 0.76a | 0.59b | 0.51b | * | 85.75a | 48.56c | 56.42b | * | 1.82b | 2.23b | 3.72a | * | 1.41 | nd | nd | * |
| 5 | 2.33a | 1.79b | 1.80b | * | 40.66b | 79.91a | 83.76a | * | n.d | 3.36b | 4.66a | * | 1.16a | nd | 1.1a | * |
| 6 | 0.36b | 0.50a | 0.56c | * | 65.12a | 68.36a | 60.39b | * | 2.16b | 5.93a | 6.12a | ** | 1.07a | 0.47b | nd | * |
| 7 | 0.35b | 0.33b | 0.45a | * | 70.61a | 33.29b | 44.41b | * | 0.16b | 1.69a | 0.16b | ** | 1.29 | nd | nd | * |
| 8 | 0.29c | 0.33b | 0.45a | * | 34.04b | 29.33b | 43.24a | * | 0.49a | 0.17b | 0.18b | * | 1.22 | nd | nd | * |
| 9 | 1.42a | 0.97c | 0.86b | * | 47.34c | 83.17a | 57.77b | * | nd | 0.65 | nd | * | 1.26 | nd | nd | * |
| 10 | 1.94c | 1.86b | 2.29a | * | 54.73c | 75.15b | 89.98a | * | 0.03b | 13.02a | 0.19b | *** | 0.45b | nd | 0.83a | * |
| 11 | 0.57a | 0.49b | 0.52ab | * | 53.02a | 40.38c | 46.23b | * | 4.35a | 3.06b | 0.13c | * | 5.7a | nd | 3.58b | ** |
| 12 | 0.46 | 0.48 | 0.44 | n.s. | 49.59b | 55.46a | 58.36a | ** | 3.42b | 4.39a | 0.42c | * | 1.61b | nd | 5.26a | *** |
| 13 | 0.17c | 0.23b | 0.31a | * | 22.62b | 18.99b | 38.49a | * | 4.43a | 0.74b | 0.05c | * | 0.59b | nd | 2.07a | ** |
| 14 | 0.92b | 0.48c | 1.15a | * | 42.02b | 28.78c | 48.02a | * | 0.66 | 0.13 | 0.33 | n.s. | 0.79b | nd | 2.62a | ** |
| 15 | 0.52b | 0.64a | 0.66a | * | 32.90c | 46.56b | 57.72a | ** | 4.43b | 6.61a | nd | ** | 2.34a | 1.62b | nd | ** |
| 16 | 1.39a | 0.63c | 0.84b | * | 23.89b | 60.64a | 62.91a | ** | 4.00b | 0.50b | nd | ** | 0.97b | 5.87a | 0.78b | *** |
| 17 | 0.72a | 0.71a | 0.35b | * | 33.83b | 87.43a | 88.57a | *** | 0.65b | 0.27b | nd | * | 0.7c | 2.27a | 1.14b | * |
| 18 | 0.35b | 0.52a | 0.60a | * | 49.53a | 35.56c | 40.32b | * | 0.09b | 0.11b | 1.65a | * | 1.58a | 0.95b | 0.53c | * |
| Sample | TEAC (µmol TE g−1) | Sign. | FRAP (µmol Fe2+ g−1) | Sign. | ||||
|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | |||
| 1 | 293.6b | 270.6c | 374.0a | * | 374.9c | 594.7a | 439.4b | * |
| 2 | 125.0c | 529.8b | 578.6a | *** | 144.6c | 714.8b | 790.6a | ** |
| 3 | 380.1a | 100.9c | 193.5b | * | 382.4a | 217.5c | 261.9b | * |
| 4 | 304.7a | 181.7b | 158.2c | * | 486.6a | 301.5c | 405.6b | * |
| 5 | 200.4c | 313.5b | 384.6a | * | 249.2b | 521.5a | 488.0a | * |
| 6 | 268.4a | 220.7b | 260.9a | * | 510.1 | 481.2 | 488.3 | n.s. |
| 7 | 411.0a | 94.5c | 170.6b | *** | 562.3a | 242.3b | 243.0b | * |
| 8 | 101.5b | 91.4b | 187.9a | * | 259.2a | 187.0b | 269.9a | * |
| 9 | 161.4c | 396.9a | 188.9b | * | 282.6c | 321.0b | 431.2a | * |
| 10 | 217.1c | 389.0b | 459.3a | * | 432.2c | 562.7b | 703.5a | ** |
| 11 | 238.4a | 135.2c | 168.3b | * | 401.9a | 282.0b | 279.5b | * |
| 12 | 246.3b | 284.8a | 279.2a | * | 313.8c | 352.0b | 440.1a | * |
| 13 | 98.2b | 69.6c | 141.3a | ** | 174.5b | 135.7c | 222.1a | *** |
| 14 | 195.5a | 136.1c | 161.1b | * | 264.3b | 252.8b | 334.3a | * |
| 15 | 161.3b | 202.5a | 111.3c | * | 205.6c | 349.9b | 466.3a | ** |
| 16 | 184.1c | 224.8b | 351.0a | * | 151.1c | 388.4b | 498.8a | *** |
| 17 | 191.0c | 391.9b | 441.5a | *** | 214.6c | 636.9b | 789.1a | *** |
| 18 | 231.0a | 167.2b | 122.3c | * | 275.3 | 229.1 | 273.8 | n.s. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Ospina, J.; Di Mattia, C.D.; Paparella, A.; Mastrocola, D.; Martuscelli, M.; Chaves-Lopez, C. Effect of Fermentation, Drying and Roasting on Biogenic Amines and Other Biocompounds in Colombian Criollo Cocoa Beans and Shells. Foods 2020, 9, 520. https://doi.org/10.3390/foods9040520
Delgado-Ospina J, Di Mattia CD, Paparella A, Mastrocola D, Martuscelli M, Chaves-Lopez C. Effect of Fermentation, Drying and Roasting on Biogenic Amines and Other Biocompounds in Colombian Criollo Cocoa Beans and Shells. Foods. 2020; 9(4):520. https://doi.org/10.3390/foods9040520
Chicago/Turabian StyleDelgado-Ospina, Johannes, Carla Daniela Di Mattia, Antonello Paparella, Dino Mastrocola, Maria Martuscelli, and Clemencia Chaves-Lopez. 2020. "Effect of Fermentation, Drying and Roasting on Biogenic Amines and Other Biocompounds in Colombian Criollo Cocoa Beans and Shells" Foods 9, no. 4: 520. https://doi.org/10.3390/foods9040520
APA StyleDelgado-Ospina, J., Di Mattia, C. D., Paparella, A., Mastrocola, D., Martuscelli, M., & Chaves-Lopez, C. (2020). Effect of Fermentation, Drying and Roasting on Biogenic Amines and Other Biocompounds in Colombian Criollo Cocoa Beans and Shells. Foods, 9(4), 520. https://doi.org/10.3390/foods9040520