Study of Edible Plants: Effects of Boiling on Nutritional, Antioxidant, and Physicochemical Properties
Abstract
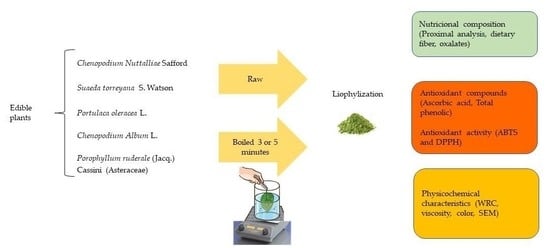
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
Sample Preparation
2.2. Nutritional Characteristics
2.2.1. Proximal Analysis
2.2.2. Total Dietary Fiber (TDF)
2.2.3. Total Oxalate
2.3. Antioxidant Compounds
2.3.1. Extraction of Antioxidants
2.3.2. Ascorbic Acid (AA)
2.3.3. Total Phenolic Compounds (TPC)
2.4. Antioxidant Capacity
2.4.1. ABTS
2.4.2. DPPH
2.5. Physicochemical Characteristics
2.5.1. Water Retention Capacity (WRC) and Viscosity
2.5.2. Color
2.5.3. SEM (Scanning Electron Microscopy)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Composition
3.2. Oxalic Acid
3.3. Antioxidant Properties
3.3.1. Ascorbic Acid
3.3.2. Total Phenolic Compounds (TPC)
3.3.3. Antioxidant Capacity
3.4. Physicochemical Properties
3.4.1. Water Retention Capacity (WRC) and Viscosity
3.4.2. Color
3.4.3. SEM (Scan Electronic Micrography)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toledo, V.M. La diversidad biológica de México. Ciencias 1994, 34, 43–59. [Google Scholar]
- Santiago-Saenz, Y.O.; Hernández-Fuentes, A.D.; López-Palestina, C.U.; Garrido-Cauich, J.H.; Alatorre-Cruz, J.M.; Monroy-Torres, R. Importancia nutricional y actividad biológica de los compuestos bioactivos de quelites consumidos en México. Rev. Chil. Nutr. 2019, 46, 593–605. [Google Scholar] [CrossRef] [Green Version]
- Santini, A.; Novellino, E.; Armini, V.; Ritieni, A. State of the art of ready-to-use therapeutic food: A tool for nutraceuticals addition to foodstuff. Food Chem. 2013, 40, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Fellows, P.J. Tecnología del Procesado de Los Alimentos: Principios y Práctica, 3rd ed.; Acribia SA: Zaragoza, Spain, 2000; ISBN 9788420011851. [Google Scholar]
- Southon, S.; Faulks, R. Health benefits of increased fruit and vegetable consumption. In Fruit and Vegetable Processing: Improving Quality, 9th ed.; Jongen, W., Ed.; Woodhead Publishing Ltd.: Cambridge, UK, 2002; Volume 1, pp. 2–20. ISBN 1855736640. [Google Scholar]
- Takeyama, E.; Yokokawa, N.; Tanimura, A. Changes in polysaccharide components and metal adsorption ability of soybean dietary fiber on heating. J. Jpn. Soc. Food. Sci. 1996, 43, 231–237. [Google Scholar] [CrossRef] [Green Version]
- Van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Scholz, G.; Lalljie, S. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 2010, 54, 1215–1247. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Hervert-Hernández, D.; García, O.P.; Rosado, J.L.; Goñi, I. The contribution of fruits and vegetables to dietary intake of polyphenols and antioxidant capacity in a Mexican rural diet: Importance of fruit and vegetable variety. Food Res. Int. 2011, 44, 1182–1189. [Google Scholar] [CrossRef]
- Villavicencio Nieto, M.A.; Pérez Escandón, B.E. Plantas Medicinales del Estado de Hidalgo; Universidad Autónoma del Estado de Hidalgo: Pachuca de Soto, México, 2013. [Google Scholar]
- Bergström, L. Nutrient losses and gains in the preparation of food. Food Chem. 1994, 57, 77–78. [Google Scholar] [CrossRef]
- Latimer, G.W. Official Methods of Analysis of the Association of Official Analytical Chemists (AOAC), 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Oderiz, M.L.V.; Blanco, M.E.V.; Hernandez, J.L.; Lozano, J.S.; Romero-Rodríguez, A. Simultaneous determination of organic acids and vitamin C in green beans by liquid chromatography. J. AOAC Int. 1998, 77, 1056–1059. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M. A current shot and re-thinking of antioxidant research strategy. Br. J. Anal. Chem. 2018, 5, 9–11. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Rubio, M.E.D.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Dürüst, N.; Sümengen, D.; Dürüst, Y. Ascorbic Acid and Element Contents of Foods of Trabzon (Turkey). J. Agric. Food. Chem. 1997, 45, 2085–2087. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Kuskoski, E.M.; Asuero, A.G.; Troncoso, A.M.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de frutos. Food Sci. Technol. 2005, 25, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Morales, F.J.; Jiménez-Pérez, S. Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence. Food Chem. 2001, 72, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J.A.; de Monredon, F.D.; Dysseler, P.; Guillon, F.; Amado, R.; Thibault, J.F. Hydration Properties of Dietary Fibre and Resistant Starch: A European Collaborative Study. LWT Food Sci. Technol. 2000, 33, 72–79. [Google Scholar] [CrossRef]
- Francis, F.J. Color quality evaluation of horticultural crops. Hortic. Sci. 1980, 15, 58–59. [Google Scholar]
- Uusiku, N.P.; Oelofse, A.; Duodu, K.G.; Bester, M.J.; Faber, M. Nutritional value of leafy vegetables of sub-Saharan Africa and their potential contribution to human health: A review. J. Food Compos. Anal. 2010, 23, 499–509. [Google Scholar] [CrossRef]
- Rodríguez-Sevilla, M.D.; Suárez, M.J.V.; Cuenca, A.R. Effects of processing conditions on soluble sugars content of carrot, beetroot and turnip. Food Chem. 1999, 66, 81–85. [Google Scholar] [CrossRef]
- Nyman, M. Importance of processing for physicochemical and physiological properties of dietary fibre. Proc. Nutr. Soc. 2003, 62, 187–192. [Google Scholar] [CrossRef]
- Bressani, R. Grain quality of common beans. Food Rev. 1993, 9, 237–297. [Google Scholar] [CrossRef]
- Siljeström, M.; Westerlund, E.; Björck, I.; Holm, J.; Asp, N.G.; Theander, O. The effects of various thermal processes on dietary fibre and starch content of whole grain wheat and white flour. J. Cereal Sci. 1986, 4, 315–323. [Google Scholar] [CrossRef]
- Slavin, J.L.; Marlett, J.A.; McBurney, M.I. Position of the American Dietetic Association: Health Implications of Dietary Fiber. J. Am. Diet. Assoc. 2008, 102, 1716–1731. [Google Scholar] [CrossRef]
- Holmes, R.P.; Kennedy, M. Estimation of the oxalate content of foods and daily oxalate intake. Kidney Int. 2000, 57, 1662–1667. [Google Scholar] [CrossRef] [Green Version]
- Horner, H.T.; Wagner, B.L. Calcium Oxalate Formation in Higher Plants. In Calcium Oxalate in Biological Systems, 1st ed.; Khan, S., Ed.; CRC Press: Boca Raton, FL, USA, 1995; pp. 53–72. [Google Scholar]
- Lane, B.G. Oxalate, germin, and the extracellular matrix of higher plants. FASEB J. 1994, 8, 294–301. [Google Scholar] [CrossRef]
- Savage, G.P.; Vanhanen, L.; Mason, S.M.; Ross, A.B. Effect of Cooking on the Soluble and Insoluble Oxalate Content of Some New Zealand Foods. J. Food Compost. Anal. 2000, 13, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Chai, W.; Liebman, M. Effect of Different Cooking Methods on Vegetable Oxalate Content. J. Agric. Food Chem. 2005, 53, 3027–3030. [Google Scholar] [CrossRef]
- Kamchan, A.; Puwastien, P.; Sirichakwal, P.P.; Kongkachuichai, R. In vitro calcium bioavailability of vegetables, legumes and seeds. J. Food Compos. Anal. 2004, 17, 311–320. [Google Scholar] [CrossRef]
- Deruelle, F.; Baron, B. Vitamin C: Is Supplementation Necessary for Optimal Health. J. Altern. Complement. Med. 2008, 14, 1291–1298. [Google Scholar] [CrossRef]
- Davey, M.W.; Montagu, M.V.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Vincieri, F.F.; Romani, A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem. 2006, 99, 464–469. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, G.J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Sulaiman, S.; Sajak, A.A.B.; Ooi, K.L.; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Conde-Hernandez, L.A.; Guerrero-Beltrán, J.Á. Total phenolics and antioxidant activity of Piper auritum and Porophyllum ruderale. Food Chem. 2014, 142, 455–460. [Google Scholar] [CrossRef]
- Laghari, A.H.; Memon, S.; Nelofar, A.; Khan, K.M.; Yasmin, A. Determination of free phenolic acids and antioxidant activity of methanolic extracts obtained from fruits and leaves of Chenopodium album. Food Chem. 2011, 126, 1850–1855. [Google Scholar] [CrossRef]
- Perez-Jimenez, J.; Saura-Calixto, F. Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Res. Int. 2006, 39, 791–800. [Google Scholar] [CrossRef]
- Faller, A.L.K.; Fialho, E. The antioxidant capacity and polyphenol content of organic and conventional retail vegetables after domestic cooking. Food Res. Int. 2009, 42, 210–215. [Google Scholar] [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of Different Cooking Methods on Nutritional and Physicochemical Characteristics of Selected Vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Guillon, F.; Cham, M. Structural and physical properties of dietary fibres and consequences of processing on human physiology. Food Res. Int. 2000, 33, 233–245. [Google Scholar] [CrossRef]
- Champ, M.; Langkilde, A.M.; Brouns, F.; Kettlitz, B.; Collet, Y.L.B. Advances in dietary fibre characterization. Definition of dietary fiber, physiological relevance, health benefits and analytical aspects. Nutr. Res. Rev. 2003, 16, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Lecumberri, E.; Mateos, R.; Izquierdo-Pulido, M.; Ruérez, P.; Goya, L.; Bravo, L. Dietary fibre composition, antioxidant capacity and physico-chemical properties of a fibre-rich product from cocoa (Theobroma cacao L). Food Chem. 2007, 104, 948–954. [Google Scholar] [CrossRef]
- Marcotte, M.; Taherian, H.; Ramaswamy, H. Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Res. Int. 2001, 34, 695–703. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M. Total antioxidant capacity of plants, Beverages and oils consumed in Italy assessed by three different In vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, M.H.; Tang, J.; Swanson, B.G. Kinetics of textural and color changes in green asparagus during thermal treatments. J. Food Eng. 2000, 45, 231–236. [Google Scholar] [CrossRef]
- Grote, M.; Fromme, H.G. Electron microscopic investigations of the cell structure in fresh and processed vegetables (carrots and green bean pods). Food Struct. 1984, 3, 8. [Google Scholar]
- Fuchigami, M.; Hyakumoto, N.; Miyazaki, K. Programmed Freezing Affects Texture, Pectic Composition and Electron Microscopic Structures of Carrots. J. Food Sci. 1995, 60, 137–141. [Google Scholar] [CrossRef]
- Escarpa, A.; González, M.C. Tecnología del almidón resistente / Technology of resistant starch. Food Sci. Technol. Int. 1997, 3, 149–161. [Google Scholar] [CrossRef]
- Marconi, E.; Ruggeri, S.; Cappelloni, M.; Leonardi, D.; Carnovale, E. Physicochemical, Nutritional, and Microstructural Characteristics of Chickpeas (Cicer arietinum L.) and Common Beans (Phaseolus vulgaris L.) Following Microwave Cooking. J. Agric. Food Chem. 2000, 48, 5986–5994. [Google Scholar] [CrossRef]


| Plants | Scientific Name (Common Name) |
|---|---|
 | Chenopodium Nuttalliae Safford (Huauzontle, quelite cenizo) Herbaceous plant of erect bearing with a prominent, angular stem and with grooves. The stem presents branching and its branches emerge obliquely from the main stem. The number of primary branches ranges from 32 to 41. The leaves have a serrated edge generally of 3 to 12 teeth. The inflorescences are amarantiform. This plant is considered to be a condiment or the main ingredient in Mexican regional food, which is available throughout the year. |
 | Suaeda torreyana S. Watson (Romerito) The perennial shrub can reach a height of two meters. Its stems are woody and erect with many branches and firm needles. Its color is dark green in the beam and white at the bottom. The flowers vary in their strain and their small seeds have a camphoric aroma. It is an aromatic plant used in food preparation and is developed in the base of the Mexican mountains, where humidity is the main factor for development of arboreal species throughout the year but mainly from May to September. |
 | Portulaca oleracea L. (Verdolaga) It has a smooth stem and red branches. Alternate leaves, obovate-cuneate to spatulated, 0.5–3 cm long, and 0.2–1.5 cm wide. Sessile flowers, solitary or grouped by few, are surrounded by scarce (sometimes none) inconspicuous hairs. Oval to orbicular sepals are 2.5–4.5 mm long and wide and somewhat caked. The yellow petals are 3–5 mm long. There are 6 to 10 stamens in which 4 to 6 are lobed. The seeds are formed in a small pod, which opens when the seeds are ripe. The consumption of this plant is in an unmatured state and could be found in irrigation crops throughout the year, while, in seasonal crops, the production is from March to August. |
 | Chenopodium Album L. (Quelite) This plant is similar to grass up to 70 cm tall, erect, and red-colored. It has elongated and pointed leaves. Its flowers are greenish, small, and are grouped in long spikes. This plant contributes with different aromas, colors, and flavors to the human diet and it is used in many cases as an ingredient of dishes or a condiment available throughout the year. |
 | Porophyllum ruderale (Jacq.) Cassini (Asteraceae) (Papaloquelite) It is a straight plant that generally grows between 20 and 100 cm in height. The leaves are a little divided and pale green and the flowers are yellow. This specie is an herb with a strong and characteristic flavor. The leaves and stems are commonly consumed raw. It is used as salad dressings as a condiment in some rural and semi-rural regions of Mexico. It is available all year but is mainly found from June to October. |
| Scientific Name Plant | Common Name | Edible Parts | Time (min) | Dilution |
|---|---|---|---|---|
| Chenopodium nuttalliae Safford | Huazontle | Leaves and stems | 5 | 1:15 |
| Suaeda torreyana S. Watson | Romerito | Leaves and stems | 3 | 1:10 |
| Portulaca oleracea L. | Verdolaga | Leaves and stems | 5 | 1:10 |
| Chenopodium album L. | Quelite | Leaves and stems | 5 | 1:15 |
| Porophyllum ruderale (Jacq.) Cassini (Asteraceae) | Papaloquelite | Leaves and stems | Raw | Raw |
| Scientific Name of Plant | Treatment | Moisture | Proteins | Lipids | Ash | Total Dietary Fiber | Carbohydrates B |
|---|---|---|---|---|---|---|---|
| Chenopodium nuttalliae Safford | Raw | 86.3 ± 0.4 a | 30.1 ± 0.0 f | 10.1 ± 1.3 b | 0.6 ± 0.1 abcd | 22.4 ± 0.3 c* | 36.9 ± 1.3 d |
| Boiled A | 85.6 ± 0.2 a | 29.4 ± 0.0 e | 9.4 ± 0.7 b | 0.5 ± 0.0 abcd | 18.8 ± 1.2 a | 42.0 ± 0.5 e | |
| Suaeda torreyana S. Watson | Raw | 88.8 ± 0.6 b* | 31.3 ± 0.4 h* | 19.2 ± 0.8 ef | 0.6 ± 0.3 abcd* | 24.9 ± 0.3 d* | 24.0 ± 1.2 b* |
| Boiled A | 91.8 ± 0.0 c | 23.6 ± 0.1 a | 17.1 ± 0.3 cd | 0.4± 0.1 abc | 18.5 ± 0.6 a | 40.4 ± 0.2 e | |
| Portulaca oleracea L. | Raw | 94.0 ± 0.3 d | 30.7 ± 0.1 g* | 22.8 ± 0.7 g* | 0.3 ± 0.0 ab | 26.9 ± 1.6 e | 19.3 ± 0.7 a |
| Boiled A | 90.7 ± 0.1 c | 26.1 ± 0.2 b | 20.7 ± 0.5 f | 0.2 ± 0.8 a | 29.2 ± 0.0 f | 23.8 ± 0.3 b | |
| Chenopodium album L. | Raw | 88.4 ± 0.2 b* | 30.8 ± 0.1 gh | 18.6 ± 0.2 de* | 0.8 ± 0.0 d | 26.3 ± 1.7 e | 23.7 ± 1.0 b |
| Boiled A | 90.5 ± 0.1 c | 27.8 ± 0.0 d | 16.2 ± 0.7 c | 0.7 ± 0.1 cde | 25.3 ± 0.9 d | 30.3 ± 0.4 c | |
| Porophyllum ruderale (Jacq.) Cassini (Asteraceae) | Raw | 85.3 ± 0.8 a | 26.9 ± 0.0 c | 5.0 ± 0.3 a | 0.6 ± 0.2 cd | 19.7 ± 0.6 b | 47.4 ± 0.3 f |
| Scientific Name Plant | Treatment | Dietary Fiber Fractions | |
|---|---|---|---|
| Soluble | Insoluble | ||
| Chenopodium nuttalliae Safford | Raw | 10.6 ± 0.5 e* | 11.7 ± 0.4 a |
| Boiled A | 6.1 ± 1.0 d | 12.6 ± 1.2 a | |
| Suaeda torreyana S. Watson | Raw | 0.4 ± 0.0 a | 24.6 ± 0.3 d* |
| Boiled A | 0.2 ±0.0 a | 18.3 ± 0.6 c | |
| Portulaca oleracea L | Raw | 4.7 ±0.3 c | 22.3 ± 1.6 c |
| Boiled A | 4.3 ± 0.4 c | 24.9 ± 0.0 d | |
| Chenopodium album L. | Raw | 1.0 ± 0.1 b | 25.3 ± 1.7 d* |
| Boiled A | 0.7 ± 0.1 b | 24.6 ± 1.0 d | |
| Porophyllum ruderale (Jacq.) Cassini (Asteraceae) | Raw | 4.9 ± 0.6 c | 14.9 ± 0.6 b |
| Scientific Name Plant | Treatment | Soluble | Insoluble |
|---|---|---|---|
| Chenopodium nuttalliae Safford | Raw | 985.2 ± 27.6 f* | 197.6 ± 58.3 b* |
| BoiledA | 678.7 ± 78.1 e | Traces | |
| Suaeda torreyana S. Watson | Raw | 598.3 ± 35.0 d* | 242.5 ± 52.3 c* |
| BoiledA | 137.1 ±2.9 b | 155.7 ± 4.9 a | |
| Portulaca oleracea L. | Raw | 1045.8 ±3.7 g* | 478.3 ± 16.3 e* |
| BoiledA | 538.2 ± 11.1 d | 749.9 ± 10.9 f | |
| Chenopodium album L. | Raw | 623.9 ± 20.4 e* | 324.5 ± 16.1 d* |
| BoiledA | 326.2 ± 4.4 c | 303.7 ± 11.9 d | |
| Porophyllum ruderale (Jacq.) Cassini (Asteraceae) | Raw | 107.6 ± 1.8 a | Traces |
| Scientific Name Plant | Treatment | Ascorbic Acid (mg AA/100 g db) | Total Phenolics (mg GAE/100 g db) | ABTS (µmol TE/100 g db) | DPPH (µmol TE/100 g db) |
|---|---|---|---|---|---|
| Chenopodium nuttalliae Safford | Raw | 273.1 ± 5.9 ab* | 1463.3 ± 9.6 f* | 326.7 ± 3.4 d | 2440.7 ± 245.0 e* |
| Boiled A | 172.2 ± 4.9 a | 1268.4 ± 12.9 d | 304.6 ± 3.4 d | 1864.8 ± 172.0 d | |
| Suaeda torreyana S. Watson | Raw | 434.2 ± 14.4 b* | 1081.4 ± 25.4 b* | 325.9± 2.7 d* | 1957.4 ± 198.5 e |
| Boiled A | 293.0 ± 5.8 ab | 753.0 ± 15.5 a | 122.3± 2.4 a | 1591.2 ± 285.7 b | |
| Portulaca oleracea L. | Raw | 810.6 ± 11.8 c* | 1477.2 ± 83.3 g* | 250.5 ±3. 4c* | 2526.4 ± 337.0 f* |
| Boiled A | 305.9 ± 4.6 ab | 1310.7 ± 12.3 e | 170.2 ± 4.6 ab | 875.3 ± 95.6 a | |
| Chenopodium album L. | Raw | 420.3 ± 8.3 b* | 1123.1 ± 17.3 c* | 192.1 ± 2.7b | 2378.2 ± 152.3 e* |
| Boiled A | 276.0 ± 11.1 b | 950.5 ± 8.4 a | 148.8 ±15.7ab | 1694.9 ± 28.1 c | |
| Porophyllum ruderale (Jacq.) Cassini (Asteraceae) | Raw | 952.2 ± 16.7 c | 960.8 ± 12.7 a | 437.0 ± 3.4e | 6355.0 ± 191.9 g |
| Scientific Name Plant | Treatment | WRC g/g | Viscosity (MPa) | Color | ||
|---|---|---|---|---|---|---|
| L * | a * | b * | ||||
| Chenopodium nuttalliae Safford | Raw | 11.4 ± 0.8 ab | 6.4 ± 0.0 d* | 34.9 ± 0.8 g* | −9.1 ± 0.0 a* | 22.9 ± 0.1 f* |
| BoiledA | 9.4 ± 1.2 ab | 3.8 ± 0.0 b | 19.6 ± 0.3 b | −4.0 ± 0.1 d | 9.1 ± 0.2 c | |
| Suaeda torreyana S. Watson | Raw | 12.2 ± 1.1 b | 7.7 ± 0.0 e* | 28.3 ± 0.6 e | −5.7 ± 0.1 c | 9.8 ± 0.2 d |
| BoiledA | 11.3 ± 2.4 ab | 3.7 ± 0.2 b | 21.9 ± 0.4 c | −4.1 ± 0.1 d | 10.1 ± 0.3 d | |
| Portulaca oleracea L. | Raw | 10.5 ± 0.4 ab | 6.5 ± 0.0 d* | 33.7 ± 0.2 f* | −3.8 ± 0.1 d* | 12.3 ± 0.3d* |
| BoiledA | 8.6 ± 1.3 a | 5.1 ± 0.0 c | 23.8 ± 0.2 d | −1.0 ± 0.0 g | 7.8 ± 0.1 b | |
| Chenopodium album L. | Raw | 12.4 ± 1.3 b | 7.7 ± 0.0 e* | 24.1 ± 0.2 d | −3.4 ± 0.0 e | 10.0 ± 0.1 d |
| BoiledA | 10.1 ± 1.6 ab | 5.1 ± 0.0 c | 17.6 ± 0.4 a | −2.5 ± 0.1 f | 6.8 ± 0.3 a | |
| Porophyllum ruderale (Jacq.) Cassini (Asteraceae) | Raw | 8.4 ± 0.9 a | 2.6 ± 0.0 a | 33.5 ± 0.2 f | −8.1 ± 0.0 b | 12.8 ± 0.0 e |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Rico, J.; Macías-León, F.J.; Alanís-García, E.; Cruz-Cansino, N.d.S.; Jaramillo-Morales, O.A.; Barrera-Gálvez, R.; Ramírez-Moreno, E. Study of Edible Plants: Effects of Boiling on Nutritional, Antioxidant, and Physicochemical Properties. Foods 2020, 9, 599. https://doi.org/10.3390/foods9050599
Arias-Rico J, Macías-León FJ, Alanís-García E, Cruz-Cansino NdS, Jaramillo-Morales OA, Barrera-Gálvez R, Ramírez-Moreno E. Study of Edible Plants: Effects of Boiling on Nutritional, Antioxidant, and Physicochemical Properties. Foods. 2020; 9(5):599. https://doi.org/10.3390/foods9050599
Chicago/Turabian StyleArias-Rico, José, Francisco Jesús Macías-León, Ernesto Alanís-García, Nelly del Socorro Cruz-Cansino, Osmar Antonio Jaramillo-Morales, Rosario Barrera-Gálvez, and Esther Ramírez-Moreno. 2020. "Study of Edible Plants: Effects of Boiling on Nutritional, Antioxidant, and Physicochemical Properties" Foods 9, no. 5: 599. https://doi.org/10.3390/foods9050599
APA StyleArias-Rico, J., Macías-León, F. J., Alanís-García, E., Cruz-Cansino, N. d. S., Jaramillo-Morales, O. A., Barrera-Gálvez, R., & Ramírez-Moreno, E. (2020). Study of Edible Plants: Effects of Boiling on Nutritional, Antioxidant, and Physicochemical Properties. Foods, 9(5), 599. https://doi.org/10.3390/foods9050599