Characterization of Key Aroma-Active Compounds Isolated from Omija Fruit Treated Differently Based on Odor Activity Values and Descriptive Sensory Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Omija Extract Samples and Chemical Reagents
2.2. Gas Chromatography-Mass Spectrometry (GC-MS)
2.3. Orthonasal Threshold Test
2.4. Odor Activity Value (OAV) Calculation
2.5. Descriptive Sensory Analysis
2.6. Statistical Analysis
3. Results
3.1. Instrumental Flavor Analysis Result
3.2. Orthonasal Threshold and Odor Activity Values of Key Flavor Compounds
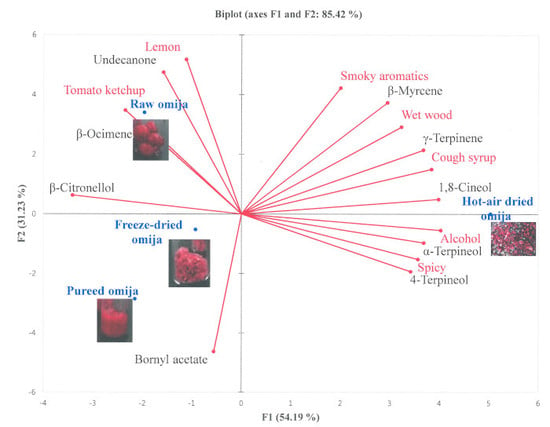
3.3. Descriptive Sensory Analysis Result
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, H.J.; Cho, I.H.; Lee, K.E.; Kim, Y.S. The compositions of volatiles and aroma-active compounds in dried Omija fruits (Schisandra chinensis Baillon) according to the cultivation areas. J. Agric. Food Chem. 2011, 59, 8338–8346. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.D. A study on quality characteristics of medicinal demi-glace sauce with added omija. Cul. Sci. Hosp. Res. 2006, 12, 119–133. [Google Scholar]
- Moon, S.W.; Kim, B.K.; Jang, M.S. Effects of Omija (Schizandra chinensis Baillon) extract on the physic-chemical properties of Nabakkimchi during fermentation. Food Sci. Biotechnol. 2006, 15, 564–571. [Google Scholar]
- Jeong, T.S.; Jeong, E.J.; Lee, S.H. Effects on the quality characteristics of Mul-kimchi with omija (Schizandra chinensis Baillon) water extract. J. Korean Soc. Food Sci. Nutr. 2008, 37, 1301–1306. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, J.M.; Do, J.S.; Bang, W.S. Antioxidant activities and quality characteristics of Omija (Schizandra chinesis Baillon) Cookies. Food Sci. Biotechnol. 2015, 24, 931–937. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, M.H. Comparison of physicochemical and organoleptic characteristics of omija wines made by different methods. J. Korean Soc. Food Sci. Nutr. 2009, 38, 182–187. [Google Scholar] [CrossRef]
- Song, Y.R.; Lim, B.U.; Song, G.S.; Baik, S.H. Quality characteristics and antioxidant activity of Makgeolli supplemented with Omija berries (Schizandra chinensis Baillon). Korean J. Food Sci. Technol. 2015, 47, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Lee, Y.G.; Choi, Y.W.; Kim, Y.C. Effects of drying conditions on the profile of volatile terpenoid and colour of Schizandra fruit (Schizandra Chinensis fructus). J. Life Sci. 2008, 18, 1066–1071. [Google Scholar] [CrossRef]
- Kim, M.K.; Drake, S.L.; Drake, M.A. Evaluation of key flavor compounds in reduced- and full-fat Cheddar cheeses using sensory studies on model systems. J. Sens. Stud. 2011, 26, 278–290. [Google Scholar] [CrossRef]
- Qian, M.; Reineccius, G.A. Quantification of aroma compounds in Parmigiano Reggiano cheese by a dynamic headspace gas chromatography-mass spectrometry technique and calculation of odor activity value. J. Dairy Sci. 2003, 86, 770–776. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Plotto, A.; Baldwin, E.; Rouseff, R. Evaluation of volatiles from two subtropical strawberry cultivars using GC-Olfactometry, GC-MS Odor activity values and sensory analysis. J. Agric. Food Chem. 2011, 59, 12569–12577. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Guo, X.; Qin, Z.; Yao, Y.; Hu, X.; Wu, J. Identification of aroma-active compounds in Jiashi Muskmelon juice by GC-O-MS and OAV calculation. J. Agric. Food Chem. 2012, 60, 4179–4185. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, F.; Wang, L.Y.; Niu, Y.W.; Shu, C.; Chen, H.X.; Xiao, Z.B. Comparison of aroma-active compounds and sensory characteristics of Durian (Durio zibethinus L.) wines using strains of Saccharomyces cerevisiae with odor activity values and partial least-squares regression. J. Agric. Food Chem. 2015, 63, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Umano, K.; Shibamoto, T.; Lee, K.G. Identification of volatile components in basil (Ocimum basilicum L.) and thyme leaves (Thymus vulgaris L.) and their antioxidant properties. Food Chem. 2015, 91, 131–137. [Google Scholar] [CrossRef]
- Leksrisompong, P.P.; Barbano, D.M.; Foegeding, A.E.; Gerard, P.; Drake, M.A. The roles of fat and pH on the detection thresholds and partition coefficients of three compounds: Diacetyl, δ-decalactone and furaneol. J. Sens. Stud. 2010, 25, 347–370. [Google Scholar] [CrossRef]
- Lawless, H.; Harono, C.; Hernandez, S. Thresholds and supra-threshold intensity functions for capsaicin in oil and aqueous based carriers. J. Sens. Stud. 2010, 15, 437–447. [Google Scholar] [CrossRef]
- Milo, C.; Reineccius, G.A. Identification and quantification of potent odorants in regular-fat and low-fat mild Cheddar cheese. J. Agric. Food Chem. 1997, 45, 3590–3594. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, Y.Y.; Lee, K.G.; Jang, H.W. Instrumental volatile flavor analyses of omija (Schisandra chinensis Baillon) using headspace stir-bar sorptive extraction-gas chromatography-mass spectrometry and its relationship to human sensory perceptions. Food Res. Int. 2019, 120, 650–655. [Google Scholar] [CrossRef]
- Van Germert, L.J. Odour thresholds Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Utrecht, The Netherlands, 2003. [Google Scholar]
- Takeoka, G.R.; Flath, R.A.; Mon, T.R.; Teranishi, R.; Guentert, M. Volatile constituents of apricot (Prunus armeniaca). J. Agric. Food Chem. 1990, 39, 471–477. [Google Scholar] [CrossRef]
- Buttery, R.G.; Seifert, R.M.; Guadagni, D.G.; Black, D.R.; Ling, L.C. Characterization of some volatile constituents of carrots. J. Agric. Food Chem. 1968, 16, 1009–1015. [Google Scholar] [CrossRef]
- Amoore, J.E.; Venstrom, D. Sensory analysis of odor qualities in terms of the streochemical theory. J. Food Sci. 1966, 31, 118–128. [Google Scholar] [CrossRef]
- Yang, X. Aroma constituents and Alkylamides of red and green Huajiao (Zanthoxylum bungeanum and Zanthoxylum schinifolium). J. Agric. Food Chem. 2008, 56, 1689–1696. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, M.; Wang, L.; Muhumdar, A.S.; Sun, D. Influence of combination drying methods on composition, texture, aroma and microstructure of apple slices. LWT-Food Sci. Technol. 2012, 47, 183–188. [Google Scholar] [CrossRef]
- Lee, C.W.; Oh, H.J.; Han, S.H.; Lim, S.B. Effects of hot air and freeze drying methods on physicochemical properties of citrus “Hallabong” powders. Food Sci. Biotechnol. 2012, 21, 1633–1639. [Google Scholar] [CrossRef]
- Her, J.Y.; Kim, M.S.; Kim, M.K.; Lee, K.G. Development of a spray freeze-drying method for preparation of volatile shiitake mushroom (Lentinus edodes) powder. Int. J. Food Sci. Technol. 2015, 50, 2222–2228. [Google Scholar] [CrossRef]
- Noble, A.C.; Arnold, R.A.; Masuda, B.M.; Pecore, S.D.; Schimidt, J.O.; Stern, P.M. Progress towards a standardized system of wine aroma terminology. Am. J. Enol. Vitic. 1984, 35, 107–109. [Google Scholar]
- Drake, M.A.; McIngvale, S.C.; Gerard, P.D.; Cadwallder, K.R.; Civille, G.C. Development of a descriptive language for Cheddar cheese. J. Food Sci. 2001, 66, 1422–1427. [Google Scholar] [CrossRef]
- Koch, I.S.; Muller, M.; Joubert, E.; van der Rijst, M.; Naes, T. Sensory characterization of rooibos tea and the development of a rooibos sensory wheel and lexicon. Food Res. Int. 2012, 46, 217–228. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, Y.J.; Kwak, H.S.; Kang, M.W. Identification of sensory attributes that drive consumer liking of commercial orange juice products in Korea. J. Food Sci. 2013, 78, S1451–S1458. [Google Scholar] [CrossRef]
- Koppel, K.; Chambers, E. Development and application of a lexicon to describe the flavor of pomegranate juice. J. Sens. Stud. 2010, 25, 819–837. [Google Scholar] [CrossRef]
- Lawless, L.J.R.; Hottenstein, A.; Ellingsworth, J. The McCormick spice wheel: A systematic and visual approach to sensory lexicon development. J. Sens. Stud. 2012, 27, 37–47. [Google Scholar] [CrossRef]
- Yajima, I.; Yanai, T.; Nakamura, M.; Sakakibara, H.; Hayashi, K. Volatile flavor components of cooked Kaorimai (Scented rice, O. Sativa japonica). J. Agric. Food Chem. 1979, 43, 2425–2429. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Chen, L.; Xu, Y.; Wang, L.; Wang, L. Identification of floarla fragrances in tree peony cultivars by gas chromatography-mass spectrometry. Sci. Horticult. 2012, 142, 158–165. [Google Scholar] [CrossRef]
- Kadow, D.; Bohlmann, J.; Phillips, W.; Lieberei, R. Identification of main fine or flavor components in two genotypes of the cocoa tree (Theobroma cacao L.). J. Appl. Bot. Food Qual. 2013, 86, 90–98. [Google Scholar] [CrossRef]

| Peak No. | Compound | Kovats Index 1 | mg/kg | |||||
|---|---|---|---|---|---|---|---|---|
| ID Method | KI | KI (Ref) | Raw Omija Fruit | Pureed Omija Fruit | Freeze Dried Omija Fruits | Hot Air-Dried Omija Fruits | ||
| 1 | β-Myrcene | MS, Co, KI | 1177 | 1172 | 1.88 | 2.5 | 0.74 | |
| 2 | 1,8-Cineol | MS, Co, KI | 1232 | 1224 | 0.22 | 0.13 | 0.71 | 0.37 |
| 3 | γ-Terpinene | MS, Co, KI | 1264 | 1261 | 0.63 | 1.49 | 0.14 | |
| 4 | β-Ocimene | MS, Co | 1245 | 0.84 | 1.03 | |||
| 6 | 4-Cymene | MS, Co, KI | 1289 | 1287 | 0.76 | |||
| 7 | Cyclohexenone | MS | 0.37 | 0.67 | ||||
| 8 | Cyclohexenol | MS | 0.27 | 0.74 | 1.15 | 0.22 | ||
| 9 | Bornyl acetate | MS, Co, KI | 1611 | 1613 | 0.54 | 2.51 | 1.19 | 0.81 |
| 10 | Undecanone | MS, Co, KI | 1619 | 1615 | 0.34 | |||
| 11 | 4-Terpineol | MS, Co, KI | 1631 | 1633 | 3.74 | 4.82 | 6.08 | 3.73 |
| 12 | Dimethyloctadiene | MS | 1.00 | 3.42 | ||||
| 13 | Citronellylpropianoate | MS | 2.42 | |||||
| 14 | γ-Selinene | MS, Co, KI | 1724 | 1756 | 0.77 | 4.07 | 5.87 | |
| 15 | α-Terpineol | MS, Co, KI | 1586 | 1576 | 1.51 | 1.63 | 2.58 | 2.16 |
| 16 | β-Selinene | MS | 0.91 | 5.82 | 0.23 | 2.26 | ||
| 17 | α-Selinene | MS | 0.55 | 3.4 | 3.16 | |||
| 18 | β-Citronellol | MS, Co, KI | 1783 | 1774 | 1.41 | 1.07 | 1.9 | |
| 19 | β-Farnesene | MS, Co, KI | 1683 | 1674 | 1.76 | 0.82 | 1.23 | 0.94 |
| 20 | β-Verbesinol | MS | 0.23 | |||||
| 21 | γ-Gurjunene | MS | 0.27 | |||||
| 22 | α-Copaene | MS, KI | 1515 | 1509 | 1.19 | |||
| 23 | Bicyclosesqui-phellandrene | MS, KI | 1795 | 1782 | 0.86 | 1.12 | ||
| 24 | Ylangene | MS, Co, KI | 1515 | 1499 | 0.2 | |||
| 25 | α-Cadinol | MS, Co, KI | 2264 | 2264 | 3.85 | 4.22 | 2.17 | 0.77 |
| 26 | 11-Selinenol | MS, KI | 5.02 | 0.21 | 2.3 | |||
| Peak No. | Compound | BET (µg/kg) | OAV | |||
|---|---|---|---|---|---|---|
| Raw Omija Fruit Extract | Pureed Omija Fruit Extract | Freeze Dried Omija Fruit Extract | Hot Air-Dried Omija fruit Extract | |||
| 1 | β-Myrcene | 9.46 | 0.20 | 0.00 | 0.26 | 0.08 |
| 2 | 1,8-Cineol | 1.86 | 0.12 | 0.07 | 0.38 | 0.20 |
| 3 | γ-Terpinene | 389.40 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4 | β-Ocimene | 38.51 | 0.02 | 0.00 | 0.00 | 0.03 |
| 9 | Bornyl acetate | 13.13 | 0.04 | 0.19 | 0.09 | 0.06 |
| 10 | Undecanone | 98.50 | 0.00 | 0.00 | 0.00 | 0.00 |
| 11 | 4-Terpineol | 3.31 | 1.13 | 1.46 | 1.84 | 1.13 |
| 15 | α-Terpineol | 1.00 | 1.51 | 1.63 | 2.58 | 2.16 |
| 18 | β-Citronellol | 3.02 | 0.47 | 0.35 | 0.00 | 0.63 |
| Aroma Attribute | Definition | Raw Omija Fruit Extract | Pureed Omija Fruit Extract | Freeze Dried Omija Fruit Extract | Hot Air-Dried Omija Fruit Extract | p-Value |
|---|---|---|---|---|---|---|
| Overall Aroma Intensity | Overall aroma impact | 1.23 b | 1.77 b | 2.50 a | 2.83 a | 0.002 |
| Cough syrup | Characteristic aromatics associated with cherry cough syrup (Reference: Bruffen®, Samil-pharm) | 1.23 a | 1.00 a | 1.03 a | 1.77 a | 0.053 |
| Spicy | Characteristic aromatics associated with whole black pepper (Reference: whole black pepper, Ottugi®) | 0.53 b | 0.83 b | 1.43 a | 1.90 a | 0.002 |
| Smoky aromatics | Characteristic aromatics associated with burning wood at campfire | 0.70 a | 0.17 b | 0.17 b | 0.67 a | 0.033 |
| Wet wood | Characteristic aromatics associated with wet wood | 1.50 a | 0.50 b | 1.50 a | 2.17 a | 0.014 |
| Lemon | Characteristic aromatics associated with cooked lemon (Reference: freshly shaved lemon peel) | 0.90 a | 0.60 a | 0.73 a | 0.67 a | 0.606 |
| Tomato ketchup | Characteristic aromatics associated with tomato ketchup (Reference: tomato ketchup, Ottugi®) | 1.17 a | 0.43 b | 0.00 b | 0.00 b | 0.001 |
| Alcohol | Characteristic aromatics associated with ethyl alcohol (Reference: 10% (v/v) ethyl alcohol in distilled water) | 1.10 c | 1.23 c | 1.83 b | 3.27 a | <0.0001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.K.; Jang, H.w.; Lee, K.-G. Characterization of Key Aroma-Active Compounds Isolated from Omija Fruit Treated Differently Based on Odor Activity Values and Descriptive Sensory Analysis. Foods 2020, 9, 638. https://doi.org/10.3390/foods9050638
Kim MK, Jang Hw, Lee K-G. Characterization of Key Aroma-Active Compounds Isolated from Omija Fruit Treated Differently Based on Odor Activity Values and Descriptive Sensory Analysis. Foods. 2020; 9(5):638. https://doi.org/10.3390/foods9050638
Chicago/Turabian StyleKim, Mina K., Hae won Jang, and Kwang-Geun Lee. 2020. "Characterization of Key Aroma-Active Compounds Isolated from Omija Fruit Treated Differently Based on Odor Activity Values and Descriptive Sensory Analysis" Foods 9, no. 5: 638. https://doi.org/10.3390/foods9050638
APA StyleKim, M. K., Jang, H. w., & Lee, K. -G. (2020). Characterization of Key Aroma-Active Compounds Isolated from Omija Fruit Treated Differently Based on Odor Activity Values and Descriptive Sensory Analysis. Foods, 9(5), 638. https://doi.org/10.3390/foods9050638