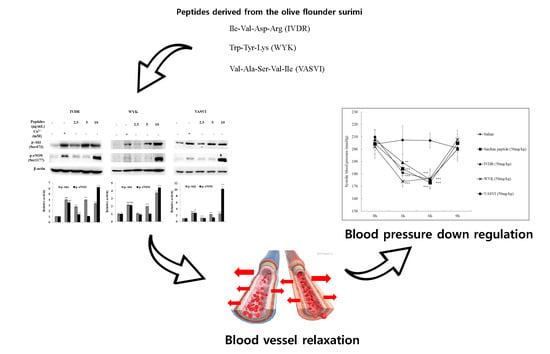
Anti-Hypertensive Activity of Novel Peptides Identified from Olive Flounder (Paralichthys olivaceus) Surimi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Antihypertensive Peptides
2.2. ACE Inhibitory Activity Assay
2.3. Cell Culture and NO Assay
2.4. Western Blot Analysis
2.5. Animals and Measurement of Blood Pressure
2.6. Statistical Analysis
3. Results and Discussion
3.1. ACE Inhibition Activity of Peptides
3.2. NO Production in HUVECs Treated with Peptides
3.3. Antihypertensive Activity of Peptides via the Akt/eNOS Pathway
3.4. Antihypertensive Effect of Peptides on SHRs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Lee, E.T.; Devereux, R.B.; Yeh, J.; Best, L.G.; Fabsitz, R.R.; Howard, B.V. Prehypertension, diabetes, and cardiovascular disease risk in a population-based sample: The Strong Heart Study. Hypertension 2006, 47, 410–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Causes of Death 2008: Data Sources and Methods; World Health Organization: Geneva, Switzerland, 2011.
- Wu, S.; Feng, X.; Lan, X.; Xu, Y.; Liao, D. Purification and identification of Angiotensin-I Converting Enzyme (ACE) inhibitory peptide from lizard fish (Saurida elongata) hydrolysate. J. Funct. Foods 2015, 13, 295–299. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Kwon, J.Y.; Kim, S.-K. A novel angiotensin I converting enzyme inhibitory peptide from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. J. Agric. Food Chem. 2004, 52, 7842–7845. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.J.; Hooper, N.M. The angiotensin–converting enzyme gene family: Genomics and pharmacology. Trends Pharmacol. Sci. 2002, 23, 177–183. [Google Scholar] [CrossRef]
- Kohama, Y.; Matsumoto, S.; Oka, H.; Teramoto, T.; Okabe, M.; Mimura, T. Isolation of angiotensin-converting enzyme inhibitor from tuna muscle. Biochem. Biophys. Res. Commun. 1988, 155, 332–337. [Google Scholar] [CrossRef]
- Byun, H.-G.; Kim, S.-K. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process Biochem. 2001, 36, 1155–1162. [Google Scholar] [CrossRef]
- Ukeda, H.; Matsufuji, H.; Matsui, T.; Osajima, Y.; Matsuda, H.; Osajima, K. Peptides from peptic hydrolyzate of heated sardine meat that inhibit angiotensin I converting enzyme. Nippon Nogeikagaku Kaishi 1992, 66, 25–29. [Google Scholar] [CrossRef]
- Kim, H.-S.; Je, J.-G.; Ryu, B.; Kang, N.; Fernando, I.S.; Jayawardena, T.U.; Sanjeewa, K.A.; Oh, J.-Y.; Lee, T.-G.; Jeon, Y.-J. Antioxidant and angiotensin-I converting enzyme inhibitory peptides from Hippocampus abdominalis. Eur. Food Res. Technol. 2019, 245, 479–487. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Lee, J.-H.; Samarakoon, K.; Kim, J.-S.; Jeon, Y.-J. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food Chem. Toxicol. 2013, 52, 113–120. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Je, J.-Y.; Kim, S.-K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef]
- Ryan, J.T.; Ross, R.P.; Bolton, D.; Fitzgerald, G.F.; Stanton, C. Bioactive peptides from muscle sources: Meat and fish. Nutrients 2011, 3, 765–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, S.-C.; Jeon, Y.-J. Marine peptides for preventing metabolic syndrome. Curr. Protein Pept. Sci. 2013, 14, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W. Surimi and Surimi Seafood; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Vidal-Giraud, B.; Chateau, D. World surimi market. Globefish Res. Programme 2007, 89, 1–128. [Google Scholar]
- Oh, J.-Y.; Kim, E.-A.; Lee, H.; Kim, H.-S.; Lee, J.-S.; Jeon, Y.-J. Antihypertensive effect of surimi prepared from olive flounder (Paralichthys olivaceus) by angiotensin-I converting enzyme (ACE) inhibitory activity and characterization of ACE inhibitory peptides. Process Biochem. 2019, 80, 164–170. [Google Scholar] [CrossRef]
- Hai Bang, T.; Suhara, H.; Ishikawa, H.; Fukami, K.; Parajuli, G.P.; Katakura, Y.; Yamashita, S.; Watanabe, K.; Adhikari, M.K.; Manandhar, H.K. Wild mushrooms in Nepal: Some potential candidates as antioxidant and ACE-inhibition sources. Evid. Based Complement. Altern. Med. 2014, 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, S.-C.; Jung, W.-K.; Kang, S.-M.; Lee, S.-H.; Kang, M.C.; Heo, S.-J.; Kang, K.-H.; Kim, Y.-T.; Park, S.-J.; Jeong, Y.; et al. Angiotensin I-converting enzyme (ACE) inhibition and nitric oxide (NO)-mediated antihypertensive effect of octaphlorethol A isolated from Ishige sinicola: In vitro molecular mechanism and in vivo SHR model. J. Funct. Foods 2015, 18, 289–299. [Google Scholar] [CrossRef]
- Vasdev, S.; Stuckless, J. Antihypertensive effects of dietary protein and its mechanism. Int. J. Angiol. 2010, 19, e7–e20. [Google Scholar] [CrossRef] [Green Version]
- Escudero, E.; Sentandreu, M.A.; Arihara, K.; Toldra, F. Angiotensin I-converting enzyme inhibitory peptides generated from in vitro gastrointestinal digestion of pork meat. J. Agric. Food Chem. 2010, 58, 2895–2901. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Zhao, Y.; Zhu, X.; Yu, R.; Dong, S.; Wu, H. Novel natural angiotensin converting enzyme (ACE)-inhibitory peptides derived from sea cucumber-modified hydrolysates by adding exogenous proline and a study of their structure–activity relationship. Mar. Drugs 2018, 16, 271. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Hur, S.J. Antihypertensive peptides from animal products, marine organisms, and plants. Food Chem. 2017, 228, 506–517. [Google Scholar] [CrossRef]
- Fu, W.; Chen, C.; Zeng, H.; Lin, J.; Zhang, Y.; Hu, J.; Zheng, B.J.L. Novel angiotensin-converting enzyme inhibitory peptides derived from Trichiurus lepturus myosin: Molecular docking and surface plasmon resonance study. LWT 2019, 110, 54–63. [Google Scholar] [CrossRef]
- Matsufuji, H.; Matsui, T.; Ohshige, S.; Kawasaki, T.; Osajima, K.; Osajima, Y. Antihypertensive effects of angiotensin fragments in SHR. Biosci. Biotechnol. Biochem. 1995, 59, 1398–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, E.S.; Lee, Y.J.; Seo, C.S.; Yoon, J.J.; Han, B.H.; Park, M.C.; Kang, D.G.; Lee, H.S. Vascular protective role of Samul-Tang in HUVECs: Involvement of Nrf2/HO-1 and NO. Evid. Based Complement. Altern. Med. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Udenigwe, C.C.; Mohan, A. Mechanisms of food protein-derived antihypertensive peptides other than ACE inhibition. J. Funct. Foods 2014, 8, 45–52. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Wu, J. Bioactive peptides on endothelial function. Food Sci. Hum. Wellness 2016, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.-L.; Ge, X.-L.; Gao, X.-Y.; Zhan, H.-Y.; Shi, T.; Su, N.; Zhang, Z.-Q. Two angiotensin-converting enzyme-inhibitory peptides from almond protein and the protective action on vascular endothelial function. Food Funct. 2016, 7, 3733–3739. [Google Scholar] [CrossRef]
- Ugusman, A.; Zakaria, Z.; Chua, K.H.; Nordin, M.M.; Anita, N.; Abdullah Mahdy, Z. Role of rutin on nitric oxide synthesis in human umbilical vein endothelial cells. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef]
- Choi, S.; Kwon, H.J.; Song, H.J.; Choi, S.W.; Nagar, H.; Piao, S.; Jung, S.B.; Jeon, B.H.; Kim, D.W.; Kim, C.S. Nafamostat mesilate promotes endothelium-dependent vasorelaxation via the Akt-eNOS dependent pathway. Korean J. Physiol. Pharmacol. 2016, 20, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, S.-J.; Han, Z.-H.; Li, Y.-Q.; Xue, J.-H.; Gao, D.-F.; Wu, X.-S.; Wang, C.-X. PI3K/AKT signaling pathway plays a role in enhancement of eNOS activity by recombinant human angiotensin converting enzyme 2 in human umbilical vein endothelial cells. Int. J. Clin. Exp. Pathol. 2014, 7, 8112. [Google Scholar]
- Quillon, A.; Fromy, B.; Debret, R. Endothelium microenvironment sensing leading to nitric oxide mediated vasodilation: A review of nervous and biomechanical signals. Nitric Oxide 2015, 45, 20–26. [Google Scholar] [CrossRef]
- Wang, B.; Liu, D.; Wang, C.; Wang, Q.; Zhang, H.; Liu, G.; Tao, X.; Zhang, L. Mechanism of endothelial nitric oxide synthase phosphorylation and activation by tentacle extract from the jellyfish Cyanea capillata. PeerJ 2017, 5, e3172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, S.A.; Costa, S.L.; Gholamin, S.; Nitta, R.T.; Dubois, L.G.; Fève, M.; Zeniou, M.; Coelho, P.L.C.; El-Habr, E.; Cadusseau, J. The anti-hypertensive drug prazosin inhibits glioblastoma growth via the PKCδ-dependent inhibition of the AKT pathway. EMBO Mol. Med. 2016, 8, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Suzuki, J.; Yamawaki, H.; Sharma, V.K.; Sheu, S.-S.; Berk, B.C. Losartan Metabolite EXP3179 Activates Akt and Endothelial Nitric Oxide Synthase via Vascular Endothelial Growth Factor Receptor-2 in Endothelial Cells: Angiotensin II Type 1 Receptor-Independent. Circulation 2005, 112, 1798–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-H.; Qian, Z.-J.; Kim, S.-K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Ichimura, T.; Yamanaka, A.; Otsuka, T.; Yamashita, E.; Maruyama, S. Antihypertensive effect of enzymatic hydrolysate of collagen and Gly-Pro in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2009, 10, 2317–2319. [Google Scholar] [CrossRef]
- Jung, W.-K.; Mendis, E.; Je, J.-Y.; Park, P.-J.; Son, B.W.; Kim, H.C.; Choi, Y.K.; Kim, S.-K. Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2006, 94, 26–32. [Google Scholar] [CrossRef]
- Otani, L.; Ninomiya, T.; Murakami, M.; Osajima, K.; Kato, H.; Murakami, T. Sardine peptide with angiotensin I-converting enzyme inhibitory activity improves glucose tolerance in stroke-prone spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2009, 73, 2203–2209. [Google Scholar] [CrossRef]



| Peptide | IC50 Value (µM) |
|---|---|
| IVDR | 46.90 ± 0.32 |
| WYK | 32.97 ± 0.68 |
| VASVI | 32.66 ± 1.32 |
| * Sardine peptide (Val-Tyr) | 26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.-Y.; Je, J.-G.; Lee, H.-G.; Kim, E.-A.; Kang, S.I.; Lee, J.-S.; Jeon, Y.-J. Anti-Hypertensive Activity of Novel Peptides Identified from Olive Flounder (Paralichthys olivaceus) Surimi. Foods 2020, 9, 647. https://doi.org/10.3390/foods9050647
Oh J-Y, Je J-G, Lee H-G, Kim E-A, Kang SI, Lee J-S, Jeon Y-J. Anti-Hypertensive Activity of Novel Peptides Identified from Olive Flounder (Paralichthys olivaceus) Surimi. Foods. 2020; 9(5):647. https://doi.org/10.3390/foods9050647
Chicago/Turabian StyleOh, Jae-Young, Jun-Geon Je, Hyo-Geun Lee, Eun-A Kim, Sang In Kang, Jung-Suck Lee, and You-Jin Jeon. 2020. "Anti-Hypertensive Activity of Novel Peptides Identified from Olive Flounder (Paralichthys olivaceus) Surimi" Foods 9, no. 5: 647. https://doi.org/10.3390/foods9050647
APA StyleOh, J.-Y., Je, J.-G., Lee, H.-G., Kim, E.-A., Kang, S. I., Lee, J.-S., & Jeon, Y.-J. (2020). Anti-Hypertensive Activity of Novel Peptides Identified from Olive Flounder (Paralichthys olivaceus) Surimi. Foods, 9(5), 647. https://doi.org/10.3390/foods9050647