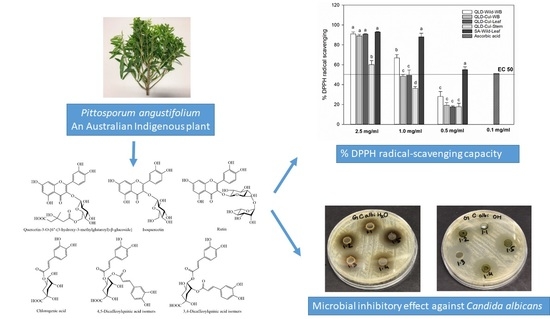
Nutritional Value and Antimicrobial Activity of Pittosporum angustifolium (Gumby Gumby), an Australian Indigenous Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents
2.3. Methods
2.3.1. Proximate Analysis
2.3.2. Mineral and Trace Element Analysis
2.3.3. Analysis of Polyphenols
Extraction of Free Phenolic Compounds
Extraction of Bound Phenolic Compounds
U(H)PLC-PDA-MS/MS Analysis
2.3.4. Analysis of Carotenoids
Extraction
U(H)PLC-PDA-APCI-MS/MS Analysis
2.3.5. Vitamins
Folates
Vitamin C
Non-Folate B Vitamins
2.3.6. Total Phenolic Content
2.3.7. DPPH Radical-Scavenging Capacity
2.3.8. Total (Condensed) Tannins
2.3.9. Total Saponins
2.3.10. Antimicrobial Activity
2.3.11. Statistical Analysis
3. Results and Discussion
3.1. Proximate, Minerals and Trace Elements
3.1.1. Proximate
3.1.2. Minerals and Trace Elements
3.2. Vitamins
3.2.1. Non-Folate B Vitamins
3.2.2. Vitamin C
3.2.3. Folates
3.3. Total Phenolic Content (TPC)
3.4. Total (Condensed) Tannins
3.5. Saponins
3.6. Individual Phenolic Compounds
3.7. Carotenoids
3.8. DPPH Radical-Scavenging Capacity
3.9. Antimicrobial Susceptibility Test
3.10. Pearson’s Correlation Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, T.; Gillespie, M.; Eckl, V.; Knepper, J.; Reynolds, C.M. Herbal Supplement Sales in US Increase by 9.4% in 2018. HerbalGram 2019, 123, 62–73. [Google Scholar]
- Cayzer, L.W.; Crisp, M.D.; Telford, I. Revision of Pittosporum (Pittosporaceae) in Australia. Aust. Syst. Bot. 2000, 13, 845–902. [Google Scholar] [CrossRef]
- Lassack, E.V.; Mccarthy, T. Australian Medicinal Plants; Methuen Australia: Sydney, Australia, 1983; p. 240. [Google Scholar]
- Latz, P. Bushfires and Bushtucker: Aboriginal Plant Use in Central Australia; Iad Press: Oxford, UK, 1995. [Google Scholar]
- Cock, I.; Winnett, V.; Sirdaarta, J.; Matthews, B. The potential of selected Australian medicinal plants with anti-Proteus activity for the treatment and prevention of rheumatoid arthritis. Pharmacogn. Mag. 2015, 11, S190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madikizela, B.; McGaw, L.J. Pittosporum viridiflorum Sims (Pittosporaceae): A review on a useful medicinal plant native to South Africa and tropical Africa. J. Ethnopharmacol. 2017, 205, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Errington, S.G.; Jefferies, P.R. Triterpenoid sapogenins of Pittosporum phillyraeoides. Phytochemistry 1988, 27, 543–545. [Google Scholar] [CrossRef]
- Backer, C.; Jenett-Siems, K.; Siems, K.; Wurster, M.; Bodtke, A.; Chamseddin, C.; Crusemann, M.; Lindequist, U. Triterpene glycosides from the leaves of Pittosporum angustifolium. Planta Med. 2013, 79, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Backer, C.; Jenett-Siems, K.; Siems, K.; Wurster, M.; Bodtke, A.; Niedermeyer, T.H.J.; Lindequist, U. New Mono and Bisdesmosidic Triterpene Glycosides from Pittosporum angustifolium Lodd. Z. Fur Nat. Sect. B A J. Chem. Sci. 2014, 69, 1026–1044. [Google Scholar] [CrossRef]
- Backer, C.; Jenett-Siems, K.; Siems, K.; Niedermeyer, T.H.J.; Wurster, M.; Bodtke, A.; Lindequist, U. Taraxastane-type triterpene saponins isolated from Pittosporum angustifolium Lodd. Z. Fur Nat. Sect. B A J. Chem. Sci. 2015, 70, 403–408. [Google Scholar] [CrossRef]
- Sadgrove, N.; Jones, G. Chemical and biological characterisation of solvent extracts and essential oils from leaves and fruit of two Australian species of Pittosporum (Pittosporaceae) used in aboriginal medicinal practice. J. Ethnopharmacol. 2013, 145, 813–821. [Google Scholar] [CrossRef]
- Bäcker, C.; Jenett-Siems, K.; Bodtke, A.; Lindequist, U. Polyphenolic compounds from the leaves of Pittosporum angustifolium. Biochem. Syst. Ecol. 2014, 55, 101–103. [Google Scholar] [CrossRef]
- Manase, M.J.; Mitaine-Offer, A.-C.; Miyamoto, T.; Tanaka, C.; Delemasure, S.; Dutartre, P.; Lacaille-Dubois, M.-A. New triterpenoid estersaponins from the root barks of Pittosporum verticillatum subsp. verticillatum and evaluation of cytotoxicities. Fitoterapia 2013, 91, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Vesoul, J.; Cock, I. An examination of the medicinal potential of Pittosporum phylliraeoides: Toxicity, antibacterial and antifungal activities. Pharmacogn. Commun. 2011, 1, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Association of Official Analytical Chemists. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1997. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1999. [Google Scholar]
- Association of Official Analytical Chemists. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Akter, S.; Netzel, M.E.; Fletcher, M.T.; Tinggi, U.; Sultanbawa, Y. Chemical and Nutritional Composition of Terminalia ferdinandiana (Kakadu Plum) Kernels: A Novel Nutrition Source. Foods 2018, 7, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rjeibi, I.; Ncib, S.; Ben Saad, A.; Souid, S. Evaluation of nutritional values, phenolic profile, aroma compounds and biological properties of Pittosporum tobira seeds. Lipids Health Dis. 2017, 16, 206. [Google Scholar] [CrossRef] [Green Version]
- Adom, K.K.; Liu, R.H. Antioxidant Activity of Grains. J. Agric. Food Chem. 2002, 50, 6182–6187. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.D.T.; Chaliha, M.; Sultanbawa, Y.; Netzel, M.E. Nutritional Characteristics and Antimicrobial Activity of Australian Grown Feijoa (Acca sellowiana). Foods 2019, 8, 376. [Google Scholar] [CrossRef] [Green Version]
- Djuikwo, V.; Ejoh, A.; Inocent, G.; Mbofung, C.; Tanumihardjo, S. Determination of Major Carotenoids in Processed Tropical Leafy Vegetables Indigenous to Africa. Food Nutr. Sci. 2011, 2, 9. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Sreelakshmi, Y.; Sharma, R. A rapid and sensitive method for determination of carotenoids in plant tissues by high performance liquid chromatography. Plant Methods. 2015, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Striegel, L.; Chebib, S.; Netzel, M.E.; Rychlik, M. Improved Stable Isotope Dilution Assay for Dietary Folates Using LC-MS/MS and Its Application to Strawberries. Front. Chem. 2018, 6, 11. [Google Scholar] [CrossRef]
- Campos, F.M.; Ribeiro, S.M.R.; Della Lucia, C.M.; Pinheiro-Sant’Ana, H.M.; Stringheta, P.C. Optimization of methodology to analyze ascorbic and dehydroascorbic acid in vegetables. Química. Nova 2009, 32, 87–91. [Google Scholar] [CrossRef]
- Spinola, V.; Mendes, B.; Camara, J.S.; Castilho, P.C. An improved and fast UHPLC-PDA methodology for determination of L-ascorbic and dehydroascorbic acids in fruits and vegetables. Evaluation of degradation rate during storage. Anal. Bioanal. Chem. 2012, 403, 1049–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Moore, J.; Yu, L. Methods for Antioxidant Capacity Estimation of Wheat and Wheat-Based Food Products. In Wheat Antioxidants; Yu, L., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Karamac, M.; Kosinska, A.; Rybarczyk, A.; Amarowicz, R. Extraction and chromatographic separation of tannin fractions from tannin-rich plant material. Pol. J. Food Nutr. Sci. 2007, 57, 471–474. [Google Scholar]
- Price, M.L.; Vanscoyoc, S.; Butler, L.G. Critical Evaluation of Vanillin Reaction as an Assay for Tannin in Sorghum Grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Helaly, F.M.; Soliman, H.S.M.; Soheir, A.D.; Ahmed, A.A. Controlled release of migration of molluscicidal saponin from different types of polymers containing Calendula officinalis. Adv. Polym. Technol. 2001, 20, 305–311. [Google Scholar] [CrossRef]
- Dini, I.; Tenore, G.C.; Dini, A. Saponins in Ipomoea batatas tubers: Isolation, characterization, quantification and antioxidant properties. Food Chem. 2009, 113, 411–419. [Google Scholar] [CrossRef]
- Mosbah, H.; Louati, H.; Boujbiha, M.A.; Chahdoura, H.; Snoussi, M.; Flamini, G.; Ascrizzi, R.; Bouslema, A.; Achour, L.; Selmi, B. Phytochemical characterization, antioxidant, antimicrobial and pharmacological activities of Feijoa sellowiana leaves growing in Tunisia. Ind. Crop. Prod. 2018, 112, 521–531. [Google Scholar] [CrossRef]
- Food Standards Australia and New Zealand. Available online: https://www.foodstandards.gov.au/Pages/default.aspx (accessed on 15 April 2020).
- Otten, J.; Hellwig, J.; Meyers, L. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- European Food Safety Authority. Safety of aluminium from dietary intake—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Food Contact Materials (AFC). EFSA J. 2008, 754, 1–34. [Google Scholar] [CrossRef]
- Leblanc, J.-C.; Malmauret, L.; GuÉrin, T.; Bordet, F.; Boursier, B.; Verger, P. Estimation of the dietary intake of pesticide residues, lead, cadmium, arsenic and radionuclides in France. Food Addit. Contam. 2000, 17, 925–932. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain(CONTAM). Statement on tolerable weekly intake for cadmium. EFSA J. 2011, 9, 1975. [Google Scholar] [CrossRef]
- Cheung Chung, S.W.; Kwong, K.P.; Yau, J.C.; Wong, W.W. Dietary exposure to antimony, lead and mercury of secondary school students in Hong Kong. Food Addit. Contam. 2008, 25, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, W.-E.; Yan, J.-Q.; Liu, M.; Zhou, Y.; Shen, X.; Ma, Y.-L.; Feng, X.-S.; Yang, J.; Li, G.-H. A Review of the Extraction and Determination Methods of Thirteen Essential Vitamins to the Human Body: An Update from 2010. Molecules 2018, 23, 1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United States Department of Agriculture. USDA Food Composition Databases. 2019. Available online: https://ndb.nal.usda.gov/ndb/ (accessed on 15 April 2020).
- Shieh, P.; Govindasamy, A.; Kuo, D. Evaluation of antioxidant polyphenols in Taiwan’s medicinal plants. Asian J. Chem. 2009, 21, 5556–5562. [Google Scholar]
- Wandji, B.A.; Bomba, F.D.T.; Awouafack, M.D.; Nkeng-Efouet, P.A.; Kamanyi, A.; Nguelefack, T.B. Antinociceptive effects of the aqueous and methanol extracts of the leaves of Pittosporum mannii Hook. F. (Pittosporaceae) in mice. J. Ethnopharmacol. 2016, 187, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Amoo, S.O.; Aremu, A.O.; Moyo, M.; Van Staden, J. Antioxidant and acetylcholinesterase-inhibitory properties of long-term stored medicinal plants. BMC Complement. Altern. Med. 2012, 12, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olaokun, O.; Mkolo, N.; Mogale, M.; King, P. Phytochemical Screening, Antioxidant, Anti-inflammatory and Glucose Utilization Activities of Three South African Plants Used Traditionally to Treat Diseases. Biol. Med. 2017, 9, 2. [Google Scholar] [CrossRef]
- Khokhar, S.; Magnusdottir, S.G.M. Total Phenol, Catechin, and Caffeine Contents of Teas Commonly Consumed in the United Kingdom. J. Agric. Food Chem. 2002, 50, 565–570. [Google Scholar] [CrossRef]
- Momeni, J.; Djialeu Ntchatchoua, W.P.; Akam, F.M.T.; Ngassoum, M.B. Antioxidant activities of some cameroonian plants extracts used in the treatment of intestinal and infectious diseases. Indian J. Pharm. Sci. 2010, 72, 140–144. [Google Scholar] [CrossRef] [Green Version]
- Savage, G.P. Saponins. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 5095–5098. [Google Scholar] [CrossRef]
- O’Hare, T.J.; Fanning, K.J.; Martin, I.F. Zeaxanthin biofortification of sweet-corn and factors affecting zeaxanthin accumulation and colour change. Arch. Biochem. Biophys. 2015, 572, 184–187. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for human disease: An update on plant-derived compounds antibacterial activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef]
- Rivera, S.M.; Christou, P.; Canela-Garayoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2014, 33, 353–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Proximate Composition | QLD_Wild_WB | QLD_Cul_WB | |
|---|---|---|---|
| Quantity (g/100 g DW) * | |||
| Protein | 7.2 | 10.6 | |
| Fat | Total fat content | 4.6 | 3.4 |
| Saturated fatty acids | 1.8 | 0.9 | |
| Monounsaturated fatty acids | 0.4 | 0.2 | |
| Polyunsaturated fatty acids | 2.4 | 2.3 | |
| Trans fatty acids | <0.01 | <0.01 | |
| Carbohydrate | Total carbohydrate | 26.6 | 31.3 |
| Soluble sugars | 6.3 | 9.5 | |
| Total dietary fiber | 45.2 | 43.3 | |
| Moisture | 5.2 | 1.7 | |
| Dry matter | 94.8 | 98.3 | |
| Ash | 11.2 | 9.6 | |
| Chemical Elements | QLD–Wild–WB | QLD–Cul–WB | QLD–Cul–Leaf | QLD–Cul–Stem | Nutritional Information | |
|---|---|---|---|---|---|---|
| mg/kg DW * | ||||||
| Minerals | Ca | 27,302 ± 190 a | 7879 ± 99 c | 10,095 ± 31 b | 3295 ± 194 d | 1200 mg/day AI [35] |
| K | 15,403 ± 8 d | 22,766 ± 179 b | 26,180 ± 120 a | 19,131 ± 528 c | 4.7 g/day AI [35] | |
| Mg | 2547 ± 9 b | 2590 ± 21 b | 3284 ± 10 a | 989 ± 43 c | 350 mg/day EAR [35] | |
| Na | 878 ± 3 c | 1161 ± 5 b | 2083 ± 15 a | 686 ± 19 d | 1300 mg/day AI [35] | |
| P | 1194 ± 14 d | 2669 ± 31 b | 2844 ± 17 a | 2286 ± 37 c | 700 mg/day AI [35] | |
| Trace elements | B | 68 ± 1.0 a | 48 ± 0.6 c | 57.7 ± 0.6 b | 16.7 ± 1.0 d | – |
| Al | 89 ± 4.4 a | 18 ± 0.6 c | 28.3 ± 0.2 b | 7.5 ± 0.1 d | 1.0 mg/kg BW/week TWI [36] | |
| V ** | 0.14 a | 0.04 c | 0.05 b | 0.02 d | – | |
| Cr ** | 0.33 a | 0.11 b | 0.16 b | 0.14 b | 35 µg/day AI [35] | |
| Mn | 41.7 ± 0.6 b | 39.7 ± 0.6 c | 60.3 ± 0.6 a | 12.3 ± 0.6 d | 2.3 mg/day AI [35] | |
| Fe | 116.7 ± 5.8 a | 48.3 ± 1.0 b | 56 ± 0.1 b | 17 ± 1.2 c | 8 mg/day RDA [35] | |
| Co ** | 0.1 a | 0.05 b | 0.05 b | 0.02 c | – | |
| Ni | 0.9 ± 0.1 b | 1.1 ± 0.1 b | 1.57 ± 0.1 a | 1 ± 0.1 b | – | |
| Cu | 8.1 ± 0.1 b | 8.9 ± 0.1 a | 6.8 ± 0.1 c | 7.6 ± 0.2 d | 900 µg/day AI [35] | |
| Zn | 57 ± 1.0 c | 62 ± 0.1 b | 76.7 ± 1.2 a | 19.7 ± 1.0 d | 11 mg/day RDA [35] | |
| As ** | 0.03 c | 0.04 b | 0.06 a | 0.02 d | 128 µg/week for a 60 kg BW TWI [37] | |
| Se ** | 0.06 b | 0.08 a | 0.08 a | 0.04 c | 55 µg/day AI [35] | |
| Sr | 140 ± 0.1 a | 47.3 ± 0.6 b | 57.7 ± 0.4 b | 32 ± 0.6 d | – | |
| Mo ** | 0.2 c | 0.7 b | 0.8 a | 0.2 c | 45 µg/day AI [35] | |
| Ag | <0.01 | <0.01 | <0.01 | <0.01 | – | |
| Cd ** | 0.05 c | 0.06 b | 0.07 a | 0.06 b | 2.5 µg/kg BW/week TWI [38] | |
| Sn | <0.05 | <0.05 | <0.05 | <0.05 | – | |
| Sb | <0.01 | <0.01 | <0.01 | <0.01 | – | |
| Ba | 36 ± 0.3 a | 3.3 ± 0.1 c | 4.0 ± 0.2 b | 3.4 ± 0.1 c | – | |
| Hg ** | 0.03 b | 0.05 a | 0.01 c | <0.01 | 5 µg/kg BW/week TWI [39] | |
| Pb ** | 0.12 a | 0.06 c | 0.08 bc | 0.09 b | 25 µg/kg BW/week TWI [39] | |
| Vitamin | QLD–Wild–WB | QLD–Cul–WB | Nutrition Information [34] |
|---|---|---|---|
| Quantity (per 100 g DW) * | |||
| B1 (Thiamin) | <5 µg | <5 µg | 1.1–1.2 mg/day RDI |
| B2 (Riboflavin) | <5 µg | 73 µg | 1.6 mg/day RDI |
| B3 (Niacin) | 840 µg | 1300 µg | 14–16 mg/day RDI |
| B5 (Pantothenic acid) | <5 µg | 300 µg | 4–6 mg/day RDI |
| B6 (Pyridoxine) | 180 µg | 59 µg | 1.7 mg/day RDI |
| B7 (Biotin) | <5 µg | <5 µg | 25–30 µg/day RDI |
| B12 (Cyanocobalamin) | <5 µg | <5 µg | 2.4 µg/day RDI |
| Samples | TPC (mg GAE/100 g DW) | Total (Condensed) Tannins (mg CaE/100 g DW) | Total Saponins (mg OE/100 g DW) | ||
|---|---|---|---|---|---|
| Free | Bound | Total Free and Bound | |||
| QLD_Wild_WB | 1306 ± 41 b | 44.2 ± 7.7 c | 1350 ± 47 b | 32 ± 1.2 b | 3645 ± 351 ab |
| QLD_Cul_WB | 1177 ± 110 b | 44.6 ± 2 c | 1222 ± 112 b | 20 ± 2.6 bc | 2869 ± 343 b |
| QLD_Cul_Leaf | 1254 ± 59 b | 40.9 ± 1.4 c | 1295 ± 60 b | 26.1 ± 6.6 bc | 3871 ± 157 a |
| QLD_Cul_Stem | 653 ± 31 c | 76.3 ± 7.8 b | 730 ± 27 c | 15.9 ± 3.2 c | 1590 ± 223 c |
| SA_Wild_Leaf | 3985 ± 103 a | 90.9 ± 4.2 a | 4075 ± 99 a | 52 ± 6.4 a | 3250 ± 319 ab |
| Samples | E. coli | S. aureus | C. albicans | |||
|---|---|---|---|---|---|---|
| Water Extraction | MeOH Extraction | Water Extraction | MeOH Extraction | Water Extraction | MeOH Extraction | |
| Inhibition Zone (mm) * | ||||||
| QLD–Wild–WB | – | – | – | – | 13.6 ± 2.2 c | 19.5 ± 1.7 bc |
| QLD–Cul–WB | – | – | – | – | 24.7 ± 0.7 ab | 20.6 ± 0.6 ab |
| QLD–Cul–Leaf | – | – | – | – | 23.1 ± 1.6 ab | 21.2 ± 1.9 ab |
| QLD–Cul–Stem | – | – | – | – | 24.0 ± 0.8 ab | 21.0 ± 1.8 ab |
| SA–Wild–Leaf | – | - | 12.8 ± 0.9 | – | 26.9 ± 1.6 a | 25.3 ± 2.4 ab |
| Antibiotic/antifungal control ** | 29.2 | 55.8 | 27.1 | |||
| Bioactive Compounds | % DPPH Free Radical-Scavenging | C. albicans Inhibition | |||
|---|---|---|---|---|---|
| 2.5 mg/mL | 1 mg/mL | 0.5 mg/mL | Water Extract (0.5 mg/mL) | MeOH Extract (0.5 mg/mL) | |
| TPC | 0.78 ** | 0.87 *** | 0.92 *** | 0.24 | 0.76 * |
| Rutin | 0.76 ** | 0.69 * | 0.66 * | 0.57 | 0.64 |
| Isoquercetin | −0.03 | −0.40 | −0.18 | −0.48 | −0.05 |
| Quercetin 3-O-[6″-(3-hydroxy-3-methylglutaroyl)-β-glucoside] | 0.71 ** | 0.65 * | 0.64 * | 0.60 | 0.81 * |
| Dicaffeoylquinic acid | 0.48 | 0.58 | 0.60 * | 0.48 | 0.36 |
| Chlorogenic acid | 0.36 | 0.45 | 0.41 | 0.57 | 0.48 |
| Vitamin C | −0.58 * | −0.65 * | −0.73 ** | −0.32 | −0.42 |
| Folates | −0.59 * | −0.68 * | −0.64 * | −0.36 | −0.36 |
| Carotenoids | −0.07 | −0.10 | −0.21 | −0.55 | 0.048 |
| Saponins | 0.26 | 0.37 | 0.26 | 0.79 * | 0.31 |
| Tannins | 0.75 ** | 0.92 *** | 0.83 *** | 0.38 | 0.55 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, A.D.T.; Chaliha, M.; Hong, H.T.; Tinggi, U.; Netzel, M.E.; Sultanbawa, Y. Nutritional Value and Antimicrobial Activity of Pittosporum angustifolium (Gumby Gumby), an Australian Indigenous Plant. Foods 2020, 9, 887. https://doi.org/10.3390/foods9070887
Phan ADT, Chaliha M, Hong HT, Tinggi U, Netzel ME, Sultanbawa Y. Nutritional Value and Antimicrobial Activity of Pittosporum angustifolium (Gumby Gumby), an Australian Indigenous Plant. Foods. 2020; 9(7):887. https://doi.org/10.3390/foods9070887
Chicago/Turabian StylePhan, Anh Dao Thi, Mridusmita Chaliha, Hung Trieu Hong, Ujang Tinggi, Michael E. Netzel, and Yasmina Sultanbawa. 2020. "Nutritional Value and Antimicrobial Activity of Pittosporum angustifolium (Gumby Gumby), an Australian Indigenous Plant" Foods 9, no. 7: 887. https://doi.org/10.3390/foods9070887
APA StylePhan, A. D. T., Chaliha, M., Hong, H. T., Tinggi, U., Netzel, M. E., & Sultanbawa, Y. (2020). Nutritional Value and Antimicrobial Activity of Pittosporum angustifolium (Gumby Gumby), an Australian Indigenous Plant. Foods, 9(7), 887. https://doi.org/10.3390/foods9070887