Bioactive Compounds and Quality of Extra Virgin Olive Oil
Abstract
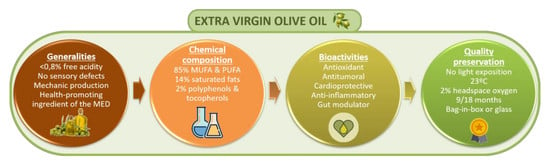
:1. Introduction
2. Main Components of EVOO
2.1. Primary Metabolites
2.1.1. Lipids
2.1.2. Tocopherols
2.1.3. Carbohydrates
2.2. Secondary Metabolites
2.2.1. Phenolic Compounds
2.2.2. Pigments
3. Biological Properties of EVOO
3.1. Cardioprotective Properties
3.2. Antioxidant Activity
3.3. Anti-Inflammatory Activity
3.4. Antitumoral Activity
3.5. Positive Modulation of Gut Microbiota
3.6. Other Bioactivities
4. EVOO Quality Regarding the Chemical Composition
4.1. Analytical Techniques
4.2. Effect of Olive Variety
4.3. Effect of Light
4.4. Effect of Temperature
4.5. Effect of Time
4.6. Effect of Oxygen Presence
4.7. Effect of Packaging Material
5. Possible Applications of EVOO beyond Nutritional Purposes
5.1. Extractive Solvent
5.2. Therapeutic Application
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations List
| Generic | |
| CHD | Coronary Heart Disease |
| CVD | Cardiovascular Diseases |
| DSC | Differential Scanning Calorimetry |
| DPPH | 2,2-Diphenyl-1-picryl-hydrazyl-hydrate free radical assay |
| EFSA | European Food Safety Authority |
| EU | European Union |
| EVOO | Extra Virgin Olive Oil |
| GC | Gas Chromatography |
| HPLC | High Performance Liquid Chromatography |
| HIV | Human Immunodeficiency Viruses |
| IC50 | Half Maximal Inhibitory Concentration |
| IOC | International Olive Council |
| MED | Mediterranean Diet |
| NMR | Nuclear Magnetic Resonance |
| PREDIMED | Prevention Through Mediterranean Diet |
| USA | United States of America |
| VOO | Virgin Olive Oil |
| Biological and Chemical Compounds | |
| CRP | C-Reactive Protein |
| DNA | Deoxyribonucleic Acid |
| HDL | High-Density Lipoprotein |
| Hg | Mercury |
| IL-6 | Interleukin-6 |
| LDL | Low-Density Lipoprotein |
| MAPK | Mitogen-Activated Protein Kinase |
| miRNA | Micro Ribonucleic Acid |
| mTOR | Mammalian Target of Rapamycin |
| MUFA | Monounsaturated Fatty Acids |
| OOL | Oleic–Oleic–Linoleic Triacylglycerol |
| OOO | Oleic–Oleic–Oleic Triacylglycerol |
| PET | Polyethylene Terephthalate |
| POO | Palmitic-Oleic-Oleic Triacylglycerol |
| POL | Palmitic–Oleic–Linoleic Triacylglycerol |
| PUFA | Polyunsaturated Fatty Acids |
| SOO | Stearic–Oleic–Oleic Triacylglycerol |
| TPC | Total Phenolic Content |
| TNF-α | Tumor Necrosis Factor-A |
References
- Foscolou, A.; Critselis, E.; Panagiotakos, D. Olive oil consumption and human health: A narrative review. Maturitas 2018, 118, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-López, M.J.; Berná, G.; Jurado-Ruiz, E.; López-García de la Serrana, H.; Martín, F. Consumption of extra-virgin olive oil rich in phenolic compounds has beneficial antioxidant effects in healthy human adults. J. Funct. Foods 2014, 10, 475–484. [Google Scholar] [CrossRef]
- Román, G.C.; Jackson, R.E.; Reis, J.; Román, A.N.; Toledo, J.B.; Toledo, E. Extra-virgin olive oil for potential prevention of Alzheimer disease. Rev. Neurol. 2019, 175, 705–723. [Google Scholar] [CrossRef] [PubMed]
- European Comission. EU Olive Oil Farms Report Based on FADN Data. L.3. Microeconomic Analysis of EU Agricultural Holdings; European Comission: Brussels, Belgium, 2012; p. 80. [Google Scholar]
- Rossi, R. The EU olive and olive oil sector: Main features, challenges and prospects. In European Parliamentary Research Service; European Parliament: Brussels, Belgium, 2017; p. 12. [Google Scholar]
- Letendre, D. Les mots de l’insoumis. Liberte 2014, 47, 278. [Google Scholar]
- Urpi-Sarda, M.; Casas, R.; Chiva-Blanch, G.; Romero-Mamani, E.S.; Valderas-Martínez, P.; Arranz, S.; Andres-Lacueva, C.; Llorach, R.; Medina-Remón, A.; Lamuela-Raventos, R.M.; et al. Virgin olive oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomakers related to atherosclerosis. Pharm. Res. 2012, 65, 577–583. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Martínez-González, M.A. Olive oil in the primary prevention of cardiovascular disease. Maturitas 2011, 68, 245–250. [Google Scholar] [CrossRef]
- Musumeci, G.; Trovato, F.M.; Pichler, K.; Weinberg, A.M.; Loreto, C.; Castrogiovanni, P. Extra-virgin olive oil diet and mild physical activity prevent cartilage degeneration in an osteoarthritis model: An in vivo and in vitro study on lubricin expression. J. Nutr. Biochem. 2013, 24, 2064–2075. [Google Scholar] [CrossRef]
- Seçmeler, Ö.; Galanakis, C.M. Chapter 8-olive fruit and olive oil. In Innovations in Traditional Foods; Woodhead Publishing: Sawston, UK, 2019; pp. 193–220. ISBN 9780128148884. [Google Scholar]
- Pérez-Rodrigo, C.; Aranceta, J. Olive oil: Its role in the diet. In The Encyclopedia of Healing Foods; Atria Books: New York, NY, USA, 2015; pp. 158–166. [Google Scholar]
- Yubero-Serrano, E.M.; Lopez-Moreno, J.; Gomez-Delgado, F.; Lopez-Miranda, J. Extra virgin olive oil: More than a healthy fat. Eur. J. Clin. Nutr. 2019, 72, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Beltrán, G.; Del Rio, C.; Sánchez, S.; Martínez, L. Influence of harvest date and crop yield on the fatty acid composition of virgin olive oils from cv. Picual. J. Agric. Food Chem. 2004, 52, 3434–3440. [Google Scholar] [CrossRef]
- Inglese, P.; Famiani, F.; Galvano, F.; Servili, M.; Esposto, S.; Urbani, S. Factors Affecting Extra-Virgin Olive Oil Composition. In Horticultural Reviews; John Wiley & Sons: Hoboken, NJ, USA, 2011; Volume 38, pp. 83–147. ISBN 9780470872376. [Google Scholar]
- Council of the European Union. Council Regulation (EC) No 1234/2007 of 22 October 2007 establishing a common organisation of agricultural markets and on specific provisions for certain agricultural products (Single CMO Regulation). Off. J. Eur. Union 2007, 299, 1–149. [Google Scholar]
- Baldo, M.A.; Oliveri, P.; Fabris, S.; Malegori, C.; Daniele, S. Fast determination of extra-virgin olive oil acidity by voltammetry and partial least squares regression. Anal. Chim. Acta 2019, 1056, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Peri, C. The Extra-Virgin Olive Oil Handbook, 1st ed.; Peri, C., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; ISBN 9781118460412. [Google Scholar]
- Mariotti, M. Virgin olive oil: Definition. In The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 11–19. [Google Scholar]
- Bertuccioli, M.; Monteleone, E. The sensory quality of extra-virgin olive oil. In The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 35–58. [Google Scholar]
- Zanoni, B. The role of oxygen and water in the extra-virgin olive oil process Bruno. In The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 69–74. ISBN 9781118460412. [Google Scholar]
- Alamprese, C. Extra-virgin olive oil contaminants. In The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 75–85. ISBN 9781118460412. [Google Scholar]
- Marjani, A. Product and process certification. In The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 251–261. ISBN 9781118460412. [Google Scholar]
- Zanoni, B. Extra-virgin olive oil traceability. In The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 245–250. ISBN 9781118460412. [Google Scholar]
- Mariotti, M.; Peri, C. The composition and nutritional properties of extra-virgin olive oil. In The Extra-Virgin Olive Oil Handbook; Peri, C., Ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2014; pp. 21–34. [Google Scholar]
- Sánchez-Villegas, A.; Sánchez-Tainta, A. Virgin olive oil. In The Prevention of Cardiovascular Disease through the Mediterranean Diet; Academic Press: Cambridge, MA, USA, 2018; pp. 59–87. ISBN 9780128112595. [Google Scholar]
- Lombardo, L.; Grasso, F.; Lanciano, F.; Loria, S.; Monetti, E. Broad-Spectrum Health Protection of Extra Virgin Olive Oil Compounds, 1st ed.; Elsevier BV: Amsterdam, The Netherlands, 2018; Volume 57, ISBN 9780444640574. [Google Scholar]
- Gavahian, M.; Mousavi Khaneghah, A.; Lorenzo, J.M.; Munekata, P.E.S.; Garcia-Mantrana, I.; Collado, M.C.; Meléndez-Martínez, A.J.; Barba, F.J. Health benefits of olive oil and its components: Impacts on gut microbiota antioxidant activities, and prevention of noncommunicable diseases. Trends Food Sci. Technol. 2019, 88, 220–227. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M.; et al. Nutrigenomics of extra-virgin olive oil: A review. BioFactors 2017, 43, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Storniolo, C.E.; Casillas, R.; Bulló, M.; Castañer, O.; Ros, E.; Sáez, G.T.; Toledo, E.; Estruch, R.; Ruiz-Gutiérrez, V.; Martínez-González, M.A.; et al. A Mediterranean diet supplemented with extra virgin olive oil or nuts improves endothelial markers involved in blood pressure control in hypertensive women. Eur. J. Nutr. 2017, 56, 89–97. [Google Scholar] [CrossRef]
- Pintó, X.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Mitjavila, M.T.; Moreno, J.J.; Casas, R.; Estruch, R.; Corella, D.; Bulló, M.; et al. A Mediterranean diet rich in extra-virgin olive oil is associated with a reduced prevalence of nonalcoholic fatty liver disease in older individuals at high cardiovascular risk. J. Nutr. 2019, 149, 1920–1929. [Google Scholar] [CrossRef]
- Mourouti, N.; Panagiotakos, D.B. The beneficial effect of a Mediterranean diet supplemented with extra virgin olive oil in the primary prevention of breast cancer among women at high cardiovascular risk in the PREDIMED Trial. Evid. Based Nurs. 2016, 19, 71. [Google Scholar] [CrossRef]
- Frankel, E.N. Nutritional and biological properties of extra virgin olive oil. J. Agric. Food Chem. 2011, 59, 785–792. [Google Scholar] [CrossRef]
- International Olive Council. World Olive Encyclopaedia; International Olive Oil Council: Madrid, Spain, 1996; ISBN 9788401618819. [Google Scholar]
- Rotondi, A.; Bendini, A.; Cerretani, L.; Mari, M.; Lercker, G.; Toschi, T.G. Effect of olive ripening degree on the oxidative stability and organoleptic properties of cv. Nostrana di Brisighella extra virgin olive oil. J. Agric. Food Chem. 2004, 52, 3649–3654. [Google Scholar] [CrossRef]
- Sánchez, J.; Harwood, J.L. Biosynthesis of triacylglycerols and volatiles in olives. Eur. J. Lipid Sci. Technol. 2002, 104, 564–573. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Echarte, M.M.; Puntel, L.A.; Aguirrezabal, L.A.N. Assessment of the critical period for the effect of intercepted solar radiation on sunflower oil fatty acid composition. F. Crop. Res. 2013, 149, 213–222. [Google Scholar] [CrossRef]
- Molina-Garcia, L.; Santos, C.S.P.; Cunha, S.C.; Casal, S.; Fernandes, J.O. Comparative fingerprint changes of toxic volatiles in low PUFA vegetable oils under deep-frying. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 271–284. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Neelakantan, N.; Wu, Y.; Lote-oke, R.; Pan, A.; Dam, R.M. Van palm oil consumption increases LDL cholesterol compared with vegetable oils low in saturated fat in a meta-analysis of clinical. J. Nutr. Nutr. Epidemiol. 2015, 145, 1549–1558. [Google Scholar]
- Criado, M.N.; Morelló, J.R.; Motilva, M.J.; Romero, M.P. Effect of growing area on pigment and phenolic fractions of virgin olive oils of the Arbequina variety in Spain. JAOCS J. Am. Oil Chem. Soc. 2004, 81, 633. [Google Scholar] [CrossRef]
- Boskou, D.; Blekas, G.; Tsimidou, M. Olive Oil Composition, 2nd ed.; AOCS Press: Urbana, IL, USA, 2006; ISBN 9780128043547. [Google Scholar]
- Conde, C.; Delrot, S.; Gerós, H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J. Plant Physiol. 2008, 165, 1545–1562. [Google Scholar] [CrossRef]
- Salvador, M.D.; Aranda, F.; Fregapane, G. Influence of fruit ripening on “Cornicabra” virgin olive oil quality: A study of four successive crop seasons. Food Chem. 2001, 73, 45–53. [Google Scholar] [CrossRef]
- Roca, M.; Mínguez-Mosquera, M.I. Change in the natural ratio between chlorophylls and carotenoids in olive fruit during processing for virgin olive oil. JAOCS J. Am. Oil Chem. Soc. 2001, 78, 133–138. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Kaliora, A.C. Effect of Fruit Maturity on Olive Oil Phenolic Composition and Antioxidant Capacity; AOCS Press: Urbana, IL, USA, 2015; ISBN 9781630670429. [Google Scholar]
- Caramia, G.; Gori, A.; Valli, E.; Cerretani, L. Virgin olive oil in preventive medicine: From legend to epigenetics. Eur. J. Lipid Sci. Technol. 2012, 114, 375–388. [Google Scholar] [CrossRef]
- López-Miranda, J.; Pérez-Jiménez, F.; Ros, E.; De Caterina, R.; Badimón, L.; Covas, M.I.; Escrich, E.; Ordovás, J.M.; Soriguer, F.; Abiá, R.; et al. Olive oil and health: Summary of the II international conference on olive oil and health consensus report, Jaén and Córdoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids and risk of cardiovascular disease: Synopsis of the evidence available from systematic reviews and meta-analyses. Nutrients 2012, 4, 1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, T.H.; Pereira, J.A.; Cabrera-Vique, C.; Lara, L.; Oliveira, A.F.; Seiquer, I. Characterization of Arbequina virgin olive oils produced in different regions of Brazil and Spain: Physicochemical properties, oxidative stability and fatty acid profile. Food Chem. 2017, 215, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Aranda, F.; Gómez-Alonso, S.; Rivera Del Álamo, R.M.; Salvador, M.D.; Fregapane, G. Triglyceride, total and 2-position fatty acid composition of Cornicabra virgin olive oil: Comparison with other Spanish cultivars. Food Chem. 2004, 86, 485–492. [Google Scholar] [CrossRef]
- Alu, M.H.; Rababah, T.; Alhamad, M.N. Application of Olive Oil as Nutraceutical and Pharmaceutical Food: Composition and Biofunctional Constituents and Their Roles in Functionality, Therapeutic, and Nutraceutical Properties; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128114124. [Google Scholar]
- Ambra, R.; Natella, F.; Lucchetti, S.; Forte, V.; Pastore, G. α-Tocopherol, β-carotene, lutein, squalene and secoiridoids in seven monocultivar Italian extra-virgin olive oils. Int. J. Food Sci. Nutr. 2017, 68, 538–545. [Google Scholar] [CrossRef]
- Srigley, C.T.; Oles, C.J.; Kia, A.R.F.; Mossoba, M.M. Authenticity assessment of extra virgin olive oil: Evaluation of desmethylsterols and triterpene dialcohols. JAOCS J. Am. Oil Chem. Soc. 2016, 93, 171–181. [Google Scholar] [CrossRef]
- Mapelli-Brahm, P.; Hernanz-Vila, D.; Stinco, C.M.; Heredia, F.J.; Meléndez-Martínez, A.J. Isoprenoids composition and colour to differentiate virgin olive oils from a specific mill. LWT Food Sci. Technol. 2018, 89, 18–23. [Google Scholar] [CrossRef]
- Perrin, J.L. Minor components and natural antioxidants in olives and olive oil. Rev. Fr. Des Corps Gras 1992, 39, 25–32. [Google Scholar]
- Moyano, M.J.; Meléndez-Martínez, A.J.; Alba, J.; Heredia, F.J. A comprehensive study on the colour of virgin olive oils and its relationship with their chlorophylls and carotenoids indexes (I): CIEXYZ non-uniform colour space. Food Res. Int. 2008, 41, 505–512. [Google Scholar] [CrossRef]
- Grob, K.; Lanfranchi, M.; Mariani, C. Evaluation of olive oils through the fatty alcohols, the sterols and their esters by coupled LC-GC. J. Am. Oil Chem. Soc. 1990, 67, 626–634. [Google Scholar] [CrossRef]
- Itoh, T.; Tamura, T.; Matsumoto, T. Methylsterol compositions of 19 vegetable oils. J. Am. Oil Chem. Soc. 1973, 50, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Boskou, D.; Morton, I.D. Changes in the sterol composition of olive oil on heating. J. Sci. Food Agric. 1975, 26, 1149–1153. [Google Scholar] [CrossRef]
- Itoh, T.; Yoshida, K.; Yatsu, T.; Tamura, T.; Matsumoto, T.; Spencer, G.F. Triterpene alcohols and sterols of Spanish olive oil. J. Am. Oil Chem. Soc. 1981, 58, 545–550. [Google Scholar] [CrossRef]
- Boarelli, M.C.; Biedermann, M.; Peier, M.; Fiorini, D.; Grob, K. Ergosterol as a marker for the use of degraded olives in the production of olive oil. Food Control 2020, 112, 107136. [Google Scholar] [CrossRef]
- Gutiérrez, F.; Varona, I.; Albi, M.A. Relation of acidity and sensory quality with sterol content of olive oil from stored fruit. J. Agric. Food Chem. 2000, 48, 1106–1110. [Google Scholar] [CrossRef]
- Ranalli, A.; Modesti, G.; Patumi, M.; Fontanazza, G. The compositional quality and sensory properties of virgin olive oil from a new olive cultivar-I-77. Food Chem. 2000, 69, 37–46. [Google Scholar] [CrossRef]
- Rivera Del Álamo, R.M.; Fregapane, G.; Aranda, F.; Gómez-Alonso, S.; Salvador, M.D. Sterol and alcohol composition of Cornicabra virgin olive oil: The campesterol content exceeds the upper limit of 4% established by EU regulations. Food Chem. 2004, 84, 533–537. [Google Scholar] [CrossRef]
- Chryssafidis, D.; Maggos, P.; Kiosseoglou, V.; Boskou, D. Composition of total and esterified 4α-monomethylsterols and triterpene alcohols in virgin olive oil. J. Sci. Food Agric. 1992, 58, 581–583. [Google Scholar] [CrossRef]
- Aparicio, R.; Luna, G. Characterisation of monovarietal virgin olive oils. Eur. J. Lipid Sci. Technol. 2002, 104, 614–627. [Google Scholar] [CrossRef]
- Ranalli, A.; Ferrante, M.L.; De Mattia, G.; Costantini, N. Analytical evaluation of virgin olive oil of first and second extraction. J. Agric. Food Chem. 1999, 47, 417–424. [Google Scholar] [CrossRef]
- Mariani, C. On the complexity of sterol fraction in olive oil|Sulla complessità della frazione sterolica nell’olio di oliva. Riv. Ital. Delle Sostanze Grasse 2016, 93, 147. [Google Scholar]
- Rodrigues, N.; Casal, S.; Peres, A.M.; Baptista, P.; Bento, A.; Martín, H.; Asensio-S.-Manzanera, M.C.; Pereira, J.A. Effect of olive trees density on the quality and composition of olive oil from cv. Arbequina. Sci. Hortic. 2018, 238, 222–233. [Google Scholar] [CrossRef] [Green Version]
- Grams, G.W.; Eskins, K. Dye-sensitized photooxidation of tocopherols. Correlation between singlet oxygen reactivity and vitamin E activity. Biochemistry 1972, 11, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulos, N.K.; Hassapidou, M.N.; Manoukas, A.G. The tocopherol content of greek olive oils. J. Sci. Food Agric. 1989, 46, 503–509. [Google Scholar] [CrossRef]
- Rao, C.V.; Newmark, H.L.; Reddy, B.S. Chemopreventive effect of squalene on colon cancer. Carcinogenesis 1998, 19, 287–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, T.J.; Yang, G.Y.; Seril, D.N.; Liao, J.; Kim, S. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis by dietary olive oil and squalene. Carcinogenesis 1998, 19, 703–706. [Google Scholar] [CrossRef] [Green Version]
- Lanzón, A.; Albi, T.; Cert, A.; Gracián, J. The hydrocarbon fraction of virgin olive oil and changes resulting from refining. J. Am. Oil Chem. Soc. 1994, 71, 285–291. [Google Scholar] [CrossRef]
- Sánchez de Medina, V.; Riachy, M.E.; Priego-Capote, F.; Luque de Castro, M.D. Mass spectrometry to evaluate the effect of the ripening process on phenols of virgin olive oils. Eur. J. Lipid Sci. Technol. 2013, 115, 1053–1061. [Google Scholar] [CrossRef]
- Gimeno, E.; Castellote, A.I.; Lamuela-Raventós, R.M.; La Torre, M.C.D.; López-Sabater, M.C. The effects of harvest and extraction methods on the antioxidant content (phenolics, α-tocopherol, and β-carotene) in virgin olive oil. Food Chem. 2002, 78, 207–211. [Google Scholar] [CrossRef]
- Fito Colomer, M. Efectos Antioxidantes del Aceite de Oliva y de Sus Compuestos Fenólicos. Ph.D. Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, 2003. [Google Scholar]
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S.J. Chemistry and health of olive oil phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236. [Google Scholar] [CrossRef] [Green Version]
- Boskou, D. Olive Oil: Minor Constituents and Health; CRC Press: Boca Raton, FL, USA, 2009; ISBN 9780367387143. [Google Scholar]
- Covas, M.I.; Ruiz-Gutiérrez, V.; De La Torre, R.; Kafatos, A.; Lamuela-Raventós, R.M.; Osada, J.; Owen, R.W.; Visioli, F. Minor components of olive oil: Evidence to date of health benefits in humans. Nutr. Rev. 2006, 64, S20–S30. [Google Scholar] [CrossRef]
- Konstantinidou, V.; Covas, M.; Muñoz-Aguayo, D.; Khymenets, O.; Torre, R.; Saez, G.; Carmen Tormos, M.; Toledo, E.; Marti, A.; Ruiz-Gutiérrez, V.; et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the Mediterranean diet: A randomized controlled trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsimidou, M. Polyphenols and quality of virgin olive oil in retrospect. Ital. J. Food Sci. 1998, 10, 99–116. [Google Scholar]
- Segura-Carretero, A.; Menéndez-Menéndez, J.; Fernández-Gutiérrez, A. Polyphenols in olive oil: The importance of phenolic compounds in the chemical composition of olive oil. In Olives and Olive Oil in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2010; ISBN 9780123744203. [Google Scholar]
- Morelló, J.R.; Vuorela, S.; Romero, M.P.; Motilva, M.J.; Heinonen, M. Antioxidant activity of olive pulp and olive oil phenolic compounds of the arbequina cultivar. J. Agric. Food Chem. 2005, 53, 2002–2008. [Google Scholar] [CrossRef]
- Murkovic, M.; Lechner, S.; Pietzka, A.; Bratacos, M.; Katzogiannos, E. Analysis of minor components in olive oil. J. Biochem. Biophys. Methods 2004, 61, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; García, A.; García, P.; Garrido, A. Rapid and complete extraction of phenols from olive oil and determination by means of a coulometric electrode array system. J. Agric. Food Chem. 2000, 48, 5178–5183. [Google Scholar] [CrossRef]
- De La Torre-Carbot, K.; Jauregui, O.; Gimeno, E.; Castellote, A.I.; Lamuela-Raventós, R.M.; López-Sabater, M.C. Characterization and quantification of phenolic compounds in olive oils by solid-phase extraction, HPLC-DAD, and HPLC-MS/MS. J. Agric. Food Chem. 2005, 53, 4331–4340. [Google Scholar] [CrossRef]
- Rovellini, P.; Cortesi, N. Liquid chromatography-mass spectrometry in the study of oleuropein and ligstroside aglycons in virgin olive oil: Aldehydic, dialdehydic forms and their oxidized products. Riv. Ital. Sostanze Grasse 2002, 79, 1–14. [Google Scholar]
- Mateos, R.; Cert, A.; Carmen Pérez-Camino, M.; García, J.M. Evaluation of virgin olive oil bitterness by quantification of secoiridoid derivatives. JAOCS J. Am. Oil Chem. Soc. 2004, 81, 71–75. [Google Scholar] [CrossRef]
- Bianco, A.; Coccioli, F.; Guiso, M.; Marra, C. The occurrence in olive oil of a new class of phenolic compounds: Hydroxy-isochromans. Food Chem. 2002, 77, 405–411. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; Salvador, M.D.; Fregapane, G. Phenolic compounds profile of Cornicabra virgin olive oil. J. Agric. Food Chem. 2002, 50, 6812–6817. [Google Scholar] [CrossRef]
- Montealegre, C.; Alegre, M.L.M.; García-Ruiz, C. Traceability markers to the botanical origin in olive oils. J. Agric. Food Chem. 2010, 58, 28–38. [Google Scholar] [CrossRef]
- Lazzerini, C.; Cifelli, M.; Domenici, V. Pigments in extra-virgin olive oil: Authenticity and quality. In Products from Olive Tree; Books on Demand: McFarland, WI, USA, 2016. [Google Scholar]
- Lazzerini, C.; Domenici, V. Pigments in extra-virgin olive oils produced in Tuscany (Italy) in different years. Foods 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uncu, O.; Ozen, B. Importance of some minor compounds in olive oil authenticity and quality. Trends Food Sci. Technol. 2020, 100, 164–176. [Google Scholar] [CrossRef]
- Lazzerini, C.; Cifelli, M.; Domenici, V. Pigments in extra virgin olive oils produced in different mediterranean countries in 2014: Near UV-vis spectroscopy versus HPLC-DAD. LWT Food Sci. Technol. 2017, 84, 586–594. [Google Scholar] [CrossRef]
- Gandul-Rojas, B.; Roca, M.; Gallardo-Guerrero, L. Chlorophylls and carotenoids in food products from olive tree. In Products from Olive Tree; Books on Demand: McFarland, WI, USA, 2016. [Google Scholar]
- Tena, N.; Wang, S.C.; Aparicio-Ruiz, R.; García-González, D.L.; Aparicio, R. In-depth assessment of analytical methods for olive oil purity, safety, and quality characterization. J. Agric. Food Chem. 2015, 63, 4509–4526. [Google Scholar] [CrossRef] [PubMed]
- Gandul-Rojas, B.; Cepero, M.R.L.; Mínguez-Mosquera, M.I. Use of chlorophyll and carotenoid pigment composition to determine authenticity of virgin olive oil. JAOCS J. Am. Oil Chem. Soc. 2000, 77, 853–858. [Google Scholar] [CrossRef]
- Roca, M.; Gandul-Rojas, B.; Gallardo-Guerrero, L.; Mínguez-Mosquera, M.I. Pigment parameters determining spanish virgin olive oil authenticity: Stability during storage. JAOCS J. Am. Oil Chem. Soc. 2003, 80, 1237–1240. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Gandul-Rojas, B. Decoloration kinetics of chlorophylls and carotenoids in virgin olive oil by autoxidation. Food Res. Int. 2014, 65, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.; Cerretani, L.; Cichelli, A. Chlorophylls in olive and in olive oil: Chemistry and occurrences. Crit. Rev. Food Sci. Nutr. 2011, 51, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P.E. How the seven countries study contributed to the definition and development of the Mediterranean diet concept: A 50-year journey. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ros, E.; Covas, M.I.; Fiol, M.; Warnberg, J.; Aros, F.; Ruiz-Gutierrez, V.; Lamuela-Raventos, R.M.; et al. Cohort profile: Design and methods of the PREDIMED study. Int. J. Epidemiol. 2012, 41, 377–385. [Google Scholar] [CrossRef] [Green Version]
- Hernáez, Á.; Castañer, O.; Elosua, R.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; Fiol, M.; et al. Mediterranean diet improves high-density lipoprotein function in high-cardiovascular-risk individuals. Circulation 2017, 135, 633–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernáez, Á.; Castañer, O.; Goday, A.; Ros, E.; Pintó, X.; Estruch, R.; Salas-Salvadó, J.; Corella, D.; Arós, F.; Serra-Majem, L.; et al. The Mediterranean diet decreases LDL atherogenicity in high cardiovascular risk individuals: A randomized controlled trial. Mol. Nutr. Food Res. 2017, 61, 1601015. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean diet and invasive breast cancer risk among women at high cardiovascular risk in the PREDIMED trial. JAMA Intern. Med. 2015, 175, 1752. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.-I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of diabetes with mediterranean diets. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Guasch-Ferré, M.; Hu, F.B.; Martínez-González, M.A.; Fitó, M.; Bulló, M.; Estruch, R.; Ros, E.; Corella, D.; Recondo, J.; Gómez-Gracia, E.; et al. Olive oil intake and risk of cardiovascular disease and mortality in the PREDIMED Study. BMC Med. 2014, 12, 78. [Google Scholar] [CrossRef] [Green Version]
- Zamora-Zamora, F.; Martínez-Galiano, J.M.; Gaforio, J.J.; Delgado-Rodríguez, M.; Delgado-Rodríguez, M. Effects of olive oil on blood pressure: A systematic review and meta-analysis. Grasas Aceites 2018, 69, 272. [Google Scholar] [CrossRef] [Green Version]
- Martínez-González, M.A.; Dominguez, L.J.; Delgado-Rodríguez, M. Olive oil consumption and risk of CHD and/or stroke: A meta-analysis of case-control, cohort and intervention studies. Br. J. Nutr. 2014, 112, 248–259. [Google Scholar] [CrossRef] [Green Version]
- George, E.S.; Marshall, S.; Mayr, H.L.; Trakman, G.L.; Tatucu-Babet, O.A.; Lassemillante, A.C.M.; Bramley, A.; Reddy, A.J.; Forsyth, A.; Tierney, A.C.; et al. The effect of high-polyphenol extra virgin olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2019, 59, 2772–2795. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Panel on dietetic products nutrition and allergies (NDA) scientific opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL cholesterol concentrations (ID 1639), mainte. EFSA J. 2011, 9, 1–25. [Google Scholar]
- Covas, M.-I.; de la Torre, K.; Farré-Albaladejo, M.; Kaikkonen, J.; Fitó, M.; López-Sabater, C.; Pujadas-Bastardes, M.A.; Joglar, J.; Weinbrenner, T.; Lamuela-Raventós, R.M.; et al. Postprandial LDL phenolic content and LDL oxidation are modulated by olive oil phenolic compounds in humans. Free Radic. Biol. Med. 2006, 40, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M.; Cladellas, M.; de la Torre, R.; Martí, J.; Alcántara, M.; Pujadas-Bastardes, M.; Marrugat, J.; Bruguera, J.; López-Sabater, M.C.; Vila, J.; et al. Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: A randomized, crossover, controlled, clinical trial. Atherosclerosis 2005, 181, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Hernáez, Á.; Remaley, A.T.; Farràs, M.; Fernández-Castillejo, S.; Subirana, I.; Schröder, H.; Fernández-Mampel, M.; Muñoz-Aguayo, D.; Sampson, M.; Solà, R.; et al. Olive oil polyphenols decrease LDL concentrations and LDL atherogenicity in men in a randomized controlled trial. J. Nutr. 2015, 145, 1692. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, C.D.; Cramer, H.; Michalsen, A.; Kessler, C.; Steckhan, N.; Choi, K.; Dobos, G. Effects of high phenolic olive oil on cardiovascular risk factors: A systematic review and meta-analysis. Phytomedicine 2015, 22, 631–640. [Google Scholar] [CrossRef] [PubMed]
- López-Biedma, A.; Sánchez-Quesada, C.; Delgado-Rodríguez, M.; Gaforio, J.J. The biological activities of natural lignans from olives and virgin olive oils: A review. J. Funct. Foods 2016, 26, 36–47. [Google Scholar] [CrossRef]
- Fernandes, J.; Fialho, M.; Santos, R.; Peixoto-Plácido, C.; Madeira, T.; Sousa-Santos, N.; Virgolino, A.; Santos, O.; Vaz Carneiro, A. Is olive oil good for you? A systematic review and meta-analysis on anti-inflammatory benefits from regular dietary intake. Nutrition 2020, 69, 110559. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of olive oil on markers of inflammation and endothelial function—A systematic review and meta-analysis. Nutrients 2015, 7, 5356. [Google Scholar] [CrossRef] [Green Version]
- Camargo, A.; Rangel-Zuñiga, O.A.; Haro, C.; Meza-Miranda, E.R.; Peña-Orihuela, P.; Meneses, M.E.; Marin, C.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Delgado-Lista, J.; et al. Olive oil phenolic compounds decrease the postprandial inflammatory response by reducing postprandial plasma lipopolysaccharide levels. Food Chem. 2014, 162, 161–171. [Google Scholar] [CrossRef]
- Psaltopoulou, T.; Kosti, R.I.; Haidopoulos, D.; Dimopoulos, M.; Panagiotakos, D.B. Olive oil intake is inversely related to cancer prevalence: A systematic review and a meta-analysis of 13800 patients and 23340 controls in 19 observational studies. Lipids Health Dis. 2011, 10, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Brandt, P.A.; Schulpen, M. Mediterranean diet adherence and risk of postmenopausal breast cancer: Results of a cohort study and meta-analysis. Int. J. Cancer 2017, 140, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean diet and risk of cancer: An updated systematic review and meta-analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Vazquez-Martin, A.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Oliveras-Ferraros, C.; Fernandez-Gutierrez, A.; Segura-Carretero, A. tabAnti-HER2 (erbB-2) oncogene effects of phenolic compounds directly isolated from commercial extra-virgin olive oil (EVOO). BMC Cancer 2008, 8, 377. [Google Scholar] [CrossRef] [Green Version]
- Menendez, J.A.; Vazquez-Martin, A.; Oliveras-Ferraros, C.; Garcia-Villalba, R.; Carrasco-Pancorbo, A.; Fernandez-Gutierrez, A.; Segura-Carretero, A. Analyzing effects of extra-virgin olive polyphenols on breast cancer-associated fatty acid synthase protein expression using reverse-phase protein microarrays. Int. J. Mol. Med. 2008, 22, 433–439. [Google Scholar] [CrossRef] [Green Version]
- Fini, L.; Hotchkiss, E.; Fogliano, V.; Graziani, G.; Romano, M.; De Vol, E.B.; Qin, H.; Selgrad, M.; Boland, C.R.; Ricciardiello, L. Chemopreventive properties of pinoresinol-rich olive oil involve a selective activation of the ATM–p53 cascade in colon cancer cell lines. Carcinogenesis 2008, 29, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Martín-Peláez, S.; Mosele, J.I.; Pizarro, N.; Farràs, M.; de la Torre, R.; Subirana, I.; Pérez-Cano, F.J.; Castañer, O.; Solà, R.; Fernandez-Castillejo, S.; et al. Effect of virgin olive oil and thyme phenolic compounds on blood lipid profile: Implications of human gut microbiota. Eur. J. Nutr. 2017, 56, 119–131. [Google Scholar] [CrossRef]
- Moorthy, M.; Chaiyakunapruk, N.; Jacob, S.A.; Palanisamy, U.D. Prebiotic potential of polyphenols, its effect on gut microbiota and anthropometric/clinical markers: A systematic review of randomised controlled trials. Trends Food Sci. Technol. 2020, 99, 634–649. [Google Scholar] [CrossRef]
- Rosignoli, P.; Fuccelli, R.; Fabiani, R.; Servili, M.; Morozzi, G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J. Nutr. Biochem. 2013, 24, 1513–1519. [Google Scholar] [CrossRef]
- Luque-Sierra, A.; Alvarez-Amor, L.; Kleemann, R.; Martín, F.; Varela, L.M. Extra-virgin olive oil with natural phenolic content exerts an anti-inflammatory effect in adipose tissue and attenuates the severity of atherosclerotic lesions in Ldlr−/−.Leiden mice. Mol. Nutr. Food Res. 2018, 62, 1800295. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Wolf, A.; Parian, A.M. Nutritional Interventions in the patient with inflammatory bowel disease. Gastroenterol. Clin. North Am. 2018, 47, 155–177. [Google Scholar] [CrossRef] [PubMed]
- Cabré, E.; Domènech, E. Impact of environmental and dietary factors on the course of inflammatory bowel disease. World J. Gastroenterol. 2012, 18, 3814. [Google Scholar] [CrossRef] [PubMed]
- Rosillo, M.A.; Sánchez-Hidalgo, M.; Sánchez-Fidalgo, S.; Aparicio-Soto, M.; Villegas, I.; Alarcón-de-la-Lastra, C. Dietary extra-virgin olive oil prevents inflammatory response and cartilage matrix degradation in murine collagen-induced arthritis. Eur. J. Nutr. 2016, 55, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Soto, M.; Sánchez-Hidalgo, M.; Cárdeno, A.; González-Benjumea, A.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Dietary hydroxytyrosol and hydroxytyrosyl acetate supplementation prevent pristane-induced systemic lupus erythematous in mice. J. Funct. Foods 2017, 29, 84–92. [Google Scholar] [CrossRef]
- Martín, R.; Carvalho-Tavares, J.; Hernández, M.; Arnés, M.; Ruiz-Gutiérrez, V.; Nieto, M.L. Beneficial actions of oleanolic acid in an experimental model of multiple sclerosis: A potential therapeutic role. Biochem. Pharm. 2010, 79, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Martín, R.; Hernández, M.; Córdova, C.; Nieto, M. Natural triterpenes modulate immune-inflammatory markers of experimental autoimmune encephalomyelitis: Therapeutic implications for multiple sclerosis. Br. J. Pharm. 2012, 166, 1708–1723. [Google Scholar] [CrossRef] [Green Version]
- Klimova, B.; Novotný, M.; Kuca, K.; Valis, M. Effect of an extra-virgin olive oil intake on the delay of cognitive decline: Role of secoiridoid oleuropein? Neuropsychiatr. Dis. Treat. 2019, 15, 3033–3040. [Google Scholar] [CrossRef] [Green Version]
- Trichopoulou, A.; Lagiou, P.; Kuper, H.; Trichopoulos, D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol. Prev. Biomark. 2000, 9, 869–873. [Google Scholar]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Carrasco-Pancorbo, A.; Simal-Gándara, J.; Giampieri, F.; et al. Characterization of phenolic extracts from Brava extra virgin olive oils and their cytotoxic effects on MCF-7 breast cancer cells. Food Chem. Toxicol. 2018, 119, 73–85. [Google Scholar] [CrossRef]
- Nanda, N.; Mahmood, S.; Bhatia, A.; Mahmood, A.; Dhawan, D.K. Chemopreventive role of olive oil in colon carcinogenesis by targeting noncoding RNAs and methylation machinery. Int. J. Cancer 2019, 144, 1180–1194. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Wang, Z.; Lam, K.L.; Zeng, S.; Tan, B.K.; Hu, J. Role of intestinal microecology in the regulation of energy metabolism by dietary polyphenols and their metabolites. Food Nutr. Res. 2019, 63, 1518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The reciprocal interactions between polyphenols and gut microbiota and effects on bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Borzì, A.M.; Biondi, A.; Basile, F.; Luca, S.; Vicari, E.S.D.; Vacante, M. Olive oil effects on colorectal cancer. Nutrients 2019, 11, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokkala, K.; Houttu, N.; Cansev, T.; Laitinen, K. Interactions of dietary fat with the gut microbiota: Evaluation of mechanisms and metabolic consequences. Clin. Nutr. 2020, 39, 994–1018. [Google Scholar] [CrossRef]
- De Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharm. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential health benefits of olive oil and plant polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [Green Version]
- Farr, S.A.; Price, T.O.; Dominguez, L.J.; Motisi, A.; Saiano, F.; Niehoff, M.L.; Morley, J.E.; Banks, W.A.; Ercal, N.; Barbagallo, M. Extra virgin olive oil improves learning and memory in SAMP8 Mice. J. Alzheimer’s Dis. 2012, 28, 81–92. [Google Scholar] [CrossRef]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef]
- Aroca-Santos, R.; Lastra-Mejías, M.; Cancilla, J.C.; Torrecilla, J.S. Intelligent modelling to monitor the evolution of quality of extra virgin olive oil in simulated distribution conditions. Biosyst. Eng. 2018, 172, 49–56. [Google Scholar] [CrossRef]
- Calabria, D.; Mirasoli, M.; Guardigli, M.; Simoni, P.; Zangheri, M.; Severi, P.; Caliceti, C.; Roda, A. Paper-based smartphone chemosensor for reflectometric on-site total polyphenols quantification in olive oil. Sens. Actuators B Chem. 2020, 305, 127522. [Google Scholar] [CrossRef]
- Domenici, V.; Ancora, D.; Cifelli, M.; Serani, A.; Veracini, C.A.; Zandomeneghi, M. Extraction of pigment information from near-UV vis absorption spectra of extra virgin olive oils. J. Agric. Food Chem. 2014, 62, 9317–9325. [Google Scholar] [CrossRef] [PubMed]
- Lerma-García, M.J.; Simó-Alfonso, E.F.; Chiavaro, E.; Bendini, A.; Lercker, G.; Cerretani, L. Study of chemical changes produced in virgin olive oils with different phenolic contents during an accelerated storage treatment. J. Agric. Food Chem. 2009, 57, 7834–7840. [Google Scholar] [CrossRef] [PubMed]
- Nedic, V.; Despotovic, D.; Cvetanovic, S.; Despotovic, M.; Babic, S. Comparison of classical statistical methods and artificial neural network in traffic noise prediction. Environ. Impact Assess. Rev. 2014, 49, 24–30. [Google Scholar] [CrossRef]
- Meenu, M.; Cai, Q.; Xu, B. A critical review on analytical techniques to detect adulteration of extra virgin olive oil. Trends Food Sci. Technol. 2019, 91, 391–408. [Google Scholar] [CrossRef]
- Mallamace, D.; Longo, S.; Corsaro, C. Proton NMR study of extra virgin olive oil with temperature: Freezing and melting kinetics. Phys. A Stat. Mech. Its Appl. 2018, 499, 20–27. [Google Scholar] [CrossRef]
- Song, W.; Song, Z.; Vincent, J.; Wang, H.; Wang, Z. Quantification of extra virgin olive oil adulteration using smartphone videos. Talanta 2020, 216, 120920. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J.; Trujillo, I. Genotypic and phenotypic identification of olive cultivars from north-western Spain and characterization of their extra virgin olive oils in terms of fatty acid composition and minor compounds. Sci. Hortic. 2018, 232, 269–279. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J.; Trujillo, I. Corrigendum to “Genotypic and phenotypic identification of olive cultivars from north-western Spain and characterization of their extra virgin olive oils in terms of fatty acid composition and minor compounds” (Scientia Horticulturae (2018) 232 (269–279). Sci. Hortic. 2019, 108624. [Google Scholar] [CrossRef]
- Bakhouche, A.; Lozano-Sánchez, J.; Ballus, C.A.; Martínez-García, M.; Velasco, M.G.; Govantes, Á.O.; Gallina-Toschi, T.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Monitoring the moisture reduction and status of bioactive compounds in extra-virgin olive oil over the industrial filtration process. Food Control 2014, 40, 292–299. [Google Scholar] [CrossRef]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Improvements in the malaxation process to enhance the aroma quality of extra virgin olive oils. Food Chem. 2014, 158, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Veneziani, G.; Di Maio, I.; Sordini, B.; Servili, M. Effect of light exposure on the quality of extra virgin olive oils according to their chemical composition. Food Chem. 2017, 229, 726–733. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Morales-Pérez, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship of quality parameters, antioxidant capacity and total phenolic content of EVOO with ripening state and olive variety. Food Chem. 2020, 325, 126926. [Google Scholar] [CrossRef] [PubMed]
- Reboredo-Rodríguez, P.; González-Barreiro, C.; Cancho-Grande, B.; Fregapane, G.; Salvador, M.D.; Simal-Gándara, J. Characterisation of extra virgin olive oils from Galician autochthonous varieties and their co-crushings with Arbequina and Picual cv. Food Chem. 2015, 176, 493–503. [Google Scholar] [CrossRef]
- Pristouri, G.; Badeka, A.; Kontominas, M.G. Effect of packaging material headspace, oxygen and light transmission, temperature and storage time on quality characteristics of extra virgin olive oil. Food Control 2010, 21, 412–418. [Google Scholar] [CrossRef]
- Trypidis, D.; García-González, D.L.; Lobo-Prieto, A.; Nenadis, N.; Tsimidou, M.Z.; Tena, N. Real time monitoring of the combined effect of chlorophyll content and light filtering packaging on virgin olive oil photo-stability using mesh cell-FTIR spectroscopy. Food Chem. 2019, 395, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.F.; Anjos, C.A.R.; Cavalcanti, R.N.; Celeghini, R.M.D.S. Evaluation of extra virgin olive oil stability by artificial neural network. Food Chem. 2015, 179, 35–43. [Google Scholar] [CrossRef]
- Rotich, V.; Al Riza, D.F.; Giametta, F.; Suzuki, T.; Ogawa, Y.; Kondo, N. Thermal oxidation assessment of Italian extra virgin olive oil using an UltraViolet (UV) induced fluorescence imaging system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 237, 118373. [Google Scholar] [CrossRef]
- Laddomada, B.; Colella, G.; Tufariello, M.; Durante, M.; Angiuli, M.; Salvetti, G.; Mita, G. Application of a simplified calorimetric assay for the evaluation of extra virgin olive oil quality. Food Res. Int. 2013, 54, 2062–2068. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; Di Maio, I.; Sordini, B.; Servili, M. Cooling treatment of olive paste during the oil processing: Impact on the yield and extra virgin olive oil quality. Food Chem. 2017, 221, 107–113. [Google Scholar] [CrossRef]
- Esposto, S.; Selvaggini, R.; Taticchi, A.; Veneziani, G.; Sordini, B.; Servili, M. Quality evolution of extra-virgin olive oils according to their chemical composition during 22 months of storage under dark conditions. Food Chem. 2020, 311, 126044. [Google Scholar] [CrossRef] [PubMed]
- Iqdiam, B.M.; Welt, B.A.; Goodrich-Schneider, R.; Sims, C.A.; Baker, G.L.; Marshall, M.R. Influence of headspace oxygen on quality and shelf life of extra virgin olive oil during storage. Food Packag. Shelf Life 2020, 23, 100433. [Google Scholar] [CrossRef]
- Kontominas, M.G. Olive oil packaging: Recent developments. In Olives and Olive Oil as Functional Foods; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Abbadi, J.; Afaneh, I.; Ayyad, Z.; Al-Rimawi, F.; Sultan, W.; Kanaan, K. Evaluation of the effect of packaging materials and storage temperatures on quality degradation of extra virgin olive oil from olives grown in Palestine. Am. J. Food Sci. Technol. 2014, 2, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Lolis, A.; Badeka, A.V.; Kontominas, M.G. Effect of bag-in-box packaging material on quality characteristics of extra virgin olive oil stored under household and abuse temperature conditions. Food Packag. Shelf Life 2019, 21, 100368. [Google Scholar] [CrossRef]
- Yu, J.; Gleize, B.; Zhang, L.; Caris-Veyrat, C.; Renard, C.M.G.C. Microwave heating of tomato puree in the presence of onion and EVOO: The effect on lycopene isomerization and transfer into oil. LWT 2019, 113, 108284. [Google Scholar] [CrossRef]
- Collado-González, J.; Grosso, C.; Valentão, P.; Andrade, P.B.; Ferreres, F.; Durand, T.; Guy, A.; Galano, J.M.; Torrecillas, A.; Gil-Izquierdo, Á. Inhibition of α-glucosidase and α-amylase by Spanish extra virgin olive oils: The involvement of bioactive compounds other than oleuropein and hydroxytyrosol. Food Chem. 2017, 235, 298–307. [Google Scholar] [CrossRef]
- Nikou, T.; Liaki, V.; Stathopoulos, P.; Sklirou, A.D.; Tsakiri, E.N.; Jakschitz, T.; Bonn, G.; Trougakos, I.P.; Halabalaki, M.; Skaltsounis, L.A. Comparison survey of EVOO polyphenols and exploration of healthy aging-promoting properties of oleocanthal and oleacein. Food Chem. Toxicol. 2019, 125, 403–412. [Google Scholar] [CrossRef]
- Khalil, S.; Awad, A.; Elewa, Y. Antidotal impact of extra virgin olive oil against genotoxicity, cytotoxicity and immunotoxicity induced by hexavalent chromium in rat. Int. J. Vet. Sci. Med. 2013, 1, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Roselli, L.; Cicia, G.; Del Giudice, T.; Cavallo, C.; Vecchio, R.; Carfora, V.; Caso, D.; Sardaro, R.; Carlucci, D.; De Gennaro, B. Testing consumers’ acceptance for an extra-virgin olive oil with a naturally increased content in polyphenols: The case of ultrasounds extraction. J. Funct. Foods 2020, 69, 103940. [Google Scholar] [CrossRef]
- Hernández-Hernández, C.; Morales-Sillero, A.; Fernández-Prior, M.Á.; Fernández-Bolaños, J.; de la Aguilera-Herrera, M.P.; Rodríguez-Gutiérrez, G. Extra virgin olive oil jam enriched with cocoa bean husk extract rich in theobromine and phenols. LWT 2019, 111, 278–283. [Google Scholar] [CrossRef]
- Demisli, S.; Theochari, I.; Christodoulou, P.; Zervou, M.; Xenakis, A.; Papadimitriou, V. Structure, activity and dynamics of extra virgin olive oil-in-water nanoemulsions loaded with vitamin D3 and calcium citrate. J. Mol. Liq. 2020, 306, 112908. [Google Scholar] [CrossRef]
- Vargas-Ramella, M.; Pateiro, M.; Barba, F.J.; Franco, D.; Campagnol, P.C.B.; Munekata, P.E.S.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Microencapsulation of healthier oils to enhance the physicochemical and nutritional properties of deer pâté. LWT 2020, 125, 109223. [Google Scholar] [CrossRef]




| Type of Oil | Characteristics | Free Acidity | |
|---|---|---|---|
| Virgin olive oils | EVOO | They are characterized for being obtained by mechanical or other physical processes under specific thermal conditions that do not cause alterations in the oil and have not suffered any treatment other than washing, decantation, centrifugation or filtration. Excluded are oils obtained using solvents or adjuvants with chemical actions, by re-esterification process or any mixture with oils of other types. | <0.8 g per 100 g |
| Virgin olive oil | ≤2 g per 100 g | ||
| Lampante olive oil | >2 g per 100 g | ||
| Refined olive oil | In this case, virgin olive oil is submitted to a refining process. | ≤0.3 g per 100 g | |
| Olive oil(composed of refined olive oils and virgin olive oils) | It is the result of the blending of the two previous oils: virgin olive oils (not lampante oil) with refined olive oil. | ≤1 g per 100 g | |
| Crude olive pomace oil | This type refers to oil obtained from olive pomace by using solvents, physical treatments or oil corresponding to lampante olive oil type, except for certain specified characteristics. As well as in the case of virgin olive oils, excluded are oils obtained by means of re-esterification and mixtures with oils of other types. | ||
| Refined olive pomace oil | This type is obtained from refining crude olive pomace oil. | ≤0.3 g per 10 g | |
| Olive pomace oil | It if the resultant oil from mixing refined olive pomace oil and virgin olive oil different than lampante oil. | ≤1 g per 100 g | |
| Component | Concentration | References | |
|---|---|---|---|
| Lipids | |||
| Fatty acids (%) | |||
| Myristic acid | C14:0 | 0.05 | [53] |
| Palmitic acid | C16:0 | 9.4–19.5 | [51,54] |
| Palmitoleic acid | C16:1 | 0.6–3.2 | [51,54] |
| Heptadecanoic acid | C17:0 | 0.07–0.13 | [51] |
| Heptadecenoic acid | C17:1 | 0.17–0.24 | [51] |
| Stearic acid | C18:0 | 1.4–3.0 | [51,54] |
| Oleic acid | C18:1 | 63.1–79.7 | [51,54] |
| Linoleic acid | C18:2 | 6.6–14.8 | [51,54] |
| α-Linolenic acid | C18:3 | 0.46–0.69 | [51,54] |
| Arachidic acid | C20:0 | 0.3–0.4 | [51,54] |
| Eicosenoic acid | C20:1 | 0.2–0.3 | [51,54] |
| Docosanoic acid | C22:0 | 0.09–0.12 | [51,54] |
| Lignoceric acid | C24:0 | 0.04–0.05 | [51] |
| MUFA | 65.2–80.8 | [51] | |
| PUFA | 7.0-15.5 | [51] | |
| Other lipids | |||
| Diacylglycerols (%) | 1–2.8 | [53] | |
| Monoacylglycerols (%) | 0.25 | [53] | |
| Total sterol content (mg/kg) | 1000–3040 | [43,55] | |
| Tocopherols (mg/kg) | |||
| α- Tocopherol | 10.2–208 | [51,54,56] | |
| β- Tocopherol | 0.75–1.05 | [51] | |
| γ- Tocopherol | 0.7–2.1 | [51] | |
| Carbohydrates (mg/kg) | |||
| Squalene | 200–8260 | [43,54,56,57] | |
| Pigments (mg/kg) | |||
| Total chlorophylls (mg/kg) | 0.15–61.96 | [51,58] | |
| Pheophytin-a (mg/kg) | 0.08–0.49 | [56] | |
| Total carotenoids (mg/kg) | 0.53–31.51 | [51,54,58] | |
| β-carotene (mg/kg) | 0.15–0.67 | [56] | |
| Lutein (mg/kg) | 0.65–3.60 | [56] | |
| Other Compounds | |||
| Total phenolic compounds (mg/kg) | 213–450 | [54] | |
| Triterpene dialcohols (% of total sterols) | 0.9–2.8 | [55] | |
| β-sitosterol (mg/kg) | 530.2–2638.6 | [56] | |
| Bioactivity | Studies Description | Main Results | Ref |
|---|---|---|---|
| Cardioprotection | RCT, PREDIMED (n = 7447 participants at high CVD risk) | Following a MED enriched with EVOO decreases CVD risk by 30% | [30,107] |
| PREDIMED observational study (n = 7216 participants) | For each 10g EVOO/day intake, CVD risk decreases by 10% | [112] | |
| Systematic review of 15 RCTs | 10–50 mL/day EVOO reduced diastolic blood pressure by 0.7 mm Hg | [113] | |
| Meta-analysis of 9 studies (38,673 stroke and 101,460 CHD cases from RCT, case-control and prospective studies) | For every increase of 25 g of olive oil consumption the risk of CVD, stroke and CHD was reduced by 18%, 26% and 4% respectively | [114] | |
| Meta-analysis of 26 RCTs | High polyphenol olive oil intake significantly reduced CVD and inflammatory markers | [115] | |
| Antioxidant properties | European Food Safety Authority health claim. | 5 mg/day of olive oils polyphenols protects blood lipids from oxidation | [116] |
| RCTs evaluating the effect of olive oils consumption on blood lipids oxidative state. | EVOO and high-phenolic olive oils consumption reduces LDL oxidation in a dose-dependent manner | [117,118,119,120] | |
| Controlled trials with sub-samples of PREDIMED cohort (n = 296) and (n = 210) | Adherence to a MED enriched with EVOO improves HDL function and protects against LDL oxidation | [108,109] | |
| In vitro studies review. | Lignans present in EVOO show antioxidant activity in vitro | [121] | |
| Anti-inflammatory capacity | Meta-analysis of 13 studies based on 9 RCTs | Regular consumption of EVOO reduces IL-6, CRP and TNF-α levels | [122] |
| Meta-analysis of RCTs (3106 participants) | Diet supplemented or enriched in olive oil reduces IL-6 and CRP plasmatic levels | [123] | |
| Randomized crossover study (49 patients) | High-phenolic virgin olive oil in breakfast reduces postprandial inflammatory response. | [124] | |
| Antitumoral activity | Meta-analysis of 19 case-control studies (comprising 13,800 cancer cases and 23,340 controls) | Olive oil consumption is associated with lower odds of developing digestive and breast cancers | [125] |
| Cohort-study follow up (2321 breast cancer cases and 1665 controls) and meta-analysis | Inverse association between adherence to MED and ERN breast cancer | [126] | |
| RCT with a sub-sample of the PREDIMED cohort (n = 4152 women) | Women following MED enriched in EVOO showed 62% relatively lower risk of breast cancer compared to control low-fat diet | [110] | |
| Systematic review and meta-analysis of 83 studies, comprising a total of 2,130,753 subjects | The adherence to MED is associated with lower risk of cancer mortality and lower risk of breast, colorectal, gastric and liver cancers, among others | [127] | |
| In vitro experiments of antitumoral activity of phenolic compounds on cancer cell lines | The phenolic fraction of EVOO, as well as isolated phenolic compounds, shows antitumoral and cytotoxic effect on different cancer cell lines | [128,129,130] | |
| Gut microbiota modulation | RCT with 12 hypercholesterolemic participants | Virgin olive oil enriched in phenolic compounds consumption favors gut bifidobacteria growth and decreases serum levels of oxidized LDL | [131] |
| Systematic review and meta-analysis of 17 RCTs | Polyphenols exert a prebiotic action on gut microbiota, improving also CVD and CRC | [132] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. https://doi.org/10.3390/foods9081014
Jimenez-Lopez C, Carpena M, Lourenço-Lopes C, Gallardo-Gomez M, Lorenzo JM, Barba FJ, Prieto MA, Simal-Gandara J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods. 2020; 9(8):1014. https://doi.org/10.3390/foods9081014
Chicago/Turabian StyleJimenez-Lopez, Cecilia, Maria Carpena, Catarina Lourenço-Lopes, Maria Gallardo-Gomez, Jose M. Lorenzo, Francisco J. Barba, Miguel A. Prieto, and Jesus Simal-Gandara. 2020. "Bioactive Compounds and Quality of Extra Virgin Olive Oil" Foods 9, no. 8: 1014. https://doi.org/10.3390/foods9081014
APA StyleJimenez-Lopez, C., Carpena, M., Lourenço-Lopes, C., Gallardo-Gomez, M., Lorenzo, J. M., Barba, F. J., Prieto, M. A., & Simal-Gandara, J. (2020). Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods, 9(8), 1014. https://doi.org/10.3390/foods9081014