The Pilot Study of Evaluating Fluctuation in the Blood Flow Volume of the Radial Artery, a Site for Traditional Pulse Diagnosis
Abstract
:1. Introduction
2. Experimental Section
2.1. Subjects
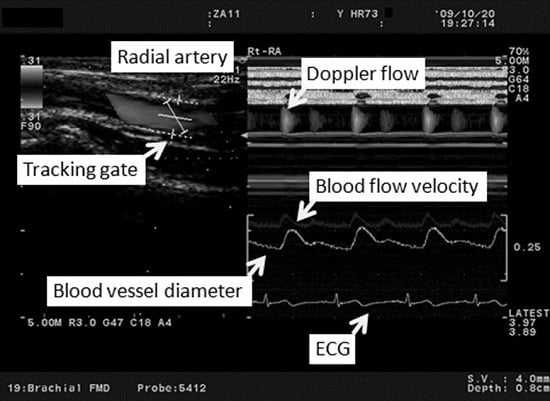
2.2. Study Protocol
2.3. Analysis
2.4. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- King, E.; Cobbin, D.; Ryan, D. The reliable measurement of radial pulse: Gender differences in pulse profiles. Acupunct. Med. 2002, 20, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Lad, V. Secrets of the Pulse: The Ancient Art of Ayurvedic Pulse Diagnosis, 2nd ed.; The Ayurvedic Press: Albuquerque, NM, USA, 2006. [Google Scholar]
- Subbarayappa, B.V. The roots of ancient medicine: An historical outline. J. Biosci. 2001, 26, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Hsu, E. A hybrid body technique: Does the pulse diagnostic cun guan chi method have Chinese-Tibetan origins? Gesnerus 2008, 65, 5–29. [Google Scholar] [PubMed]
- Steiner, R.P. Tibetan medicine: Part II: Pulse diagnosis in Tibetan medicine: Translated from the first chapter for the fourth tantra (rGyud-bzi). Am. J. Chin. Med. 1987, 15, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Steiner, R.P. Tibetan medicine. Part III: Pulse diagnosis in Tibetan medicine. Translated from the first chapter of the Fourth Tantra (rGyud-bzi). Am. J. Chin. Med. 1988, 16, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Steiner, R.P. Tibetan medicine Part IV: Pulse diagnosis in Tibetan medicine. Am. J. Chin. Med. 1989, 17, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W. Zhongyi Zhenduanxue (Study of the Chinese Medicine Diagnosis); Renmin Weisheng Chuban: Beijing, China, 1999. [Google Scholar]
- Yambe, T.; Yoshizawa, M.; Taira, R.; Tanaka, A.; Iquchi, A.; Tabayashi, K.; Tobita, S.; Nitta, S. Fluctuations of Emax of the left ventricle: Effect of atrial natriuretic polypeptide. Biomed. Pharmacother. 2001, 55 (Suppl. S1), 147s–152s. [Google Scholar] [CrossRef]
- Sato, T.; Nishinaga, M.; Kawamoto, A.; Ozawa, T.; Takatsuji, H. Accuracy of a continuous blood pressure monitor based on arterial tonometry. Hypertension 1993, 21, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, J.; Frattola, A.; Parati, G.; Rieenzo, M. 1/f-modelling of blood pressure and heart rate spectra: Relations to aging. In Proceedings of the 1992 14th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Paris, France, 29 October–1 November 1992; pp. 465–466.
- Kuusela, T.A.; Kaila, T.J.; Kahonen, M. Fine structure of the low-frequency spectra of heart rate and blood pressure. BMC Physiol. 2003, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsh, D.J.; Osborn, J.L.; Cowley, A.W., Jr. 1/f fluctuations in arterial pressure and regulation of renal blood flow in dogs. Am. J. Physiol. 1990, 258, F1394–F1400. [Google Scholar] [PubMed]
- Pomeranz, B.; Macaulay, R.J.; Caudill, M.A.; Kutz, L.; Adam, D.; Gordon, D.; Kilborn, K.M.; Barger, A.C.; Shannon, D.C.; Cohen, R.J.; et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol. 1985, 248, H151–H153. [Google Scholar] [PubMed]
- Parati, G.; Saul, J.P.; di Rienzo, M.; Mancia, G. Spectral analysis of blood pressure and heart rate variability in evaluating cardiovascular regulation. A critical appraisal. Hypertension 1995, 25, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Akselrod, S.; Gordon, D.; Ubel, F.A.; Shannon, D.C.; Berger, A.C.; Cohen, R.J. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science 1981, 213, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Pagani, M.; Lombardi, F.; Guzzetti, S.; Rimoldi, O.; Furlan, R.; Pizzinelli, P.; Sandrone, G.; Malfatto, G.; Dell’Orto, S.; Piccaluga, E.; et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 1986, 59, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Guyton and Hall, Textbook of Medical Physiology; SAUNDERS ELSEVIE: Philaclclphia, PA, USA, 2011. [Google Scholar]
- Griffith, T.M. Vasomotioin: The case for chaos. J. Biorheol. 2009, 23, 11–23. [Google Scholar] [CrossRef]
- Soga, J.; Nishioka, K.; Nakamura, S.; Umemura, T.; Jitsuiki, D.; Hidaka, T.; Teragawa, H.; Takemoto, H.; Goto, C.; Yoshizumi, M.; et al. Measurement of flow-mediated vasodilation of the brachial artery: A comparison of measurements in the seated and supine positions. Circ. J. 2007, 71, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Burns, P.N.; Jaffe, C.C. Quantitative flow measurements with Doppler ultrasound: Techniques, accuracy, and limitations. Radiol. Clin. N. Am. 1986, 23, 641–657. [Google Scholar]
- Taylor, K.J.; Holland, S. Doppler US. Part I. Basic principles, instrumentation, and pitfalls. Radiology 1990, 174, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.W. Measurement of blood flow by ultrasound: Accuracy and sources of error. Ultrasound. Med. Biol. 1985, 11, 625–641. [Google Scholar] [CrossRef]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J. Am. Coll. Cardiol. 2002, 39, 257–265. [Google Scholar] [CrossRef]
- Deanfield, J.; Donald, A.; Ferri, C.; Giannattasio, C.; Halcox, J.; Halligan, S.; Lerman, A.; Mancia, G.; Oliver, J.J.; Pessina, A.C.; et al. Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds: A statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J. Hypertens. 2005, 23, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.D.; Tarassenko, L. Quantifying errors in spectral estimates of HRV due to beat replacement and resampling. IEEE Trans. Biomed. Eng. 2005, 52, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Daskalov, I.; Christov, I. Improvement of resolution in measurement of electrocardiogram RR intervals by interpolation. Med. Eng. Phys. 1997, 19, 375–379. [Google Scholar] [CrossRef]
- Hayano, J. Therapeutic Research; Life Science Publishing: Tokyo, Japan, 1996. [Google Scholar]
- Berger, R.D.; Saul, J.P.; Cohen, R.J. Transfer function analysis of autonomic regulation. I. Canine atrial rate response. Am. J. Physiol. 1989, 256, H142–H152. [Google Scholar] [PubMed]
- Malik, M. Heart rate bvariability: Standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Montano, N.; Ruscone, T.G.; Porta, A.; Lombardi, F.; Pagani, M.; Malliani, A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994, 90, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Perini, R.; Veicsteinas, A. Heart rate variability and autonomic activity at rest and during exercise in various physiological conditions. Eur. J. Appl. Physiol. 2003, 90, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Persson, P.B. Spectrum analysis of cardiovascular time series. Am. J. Physiol. 1997, 273, R1201–R1210. [Google Scholar] [PubMed]
- Kudaiberdieva, G.; Gorenek, B.; Timuralp, B. Heart rate variability as a predictor of sudden cardiac death. Anadolu Kardiyol. Derg. 2007, 7 (Suppl. S1), 68–70. [Google Scholar] [PubMed]
- Hirsch, J.A.; Bishop, B. Respiratory sinus arrhythmia in humans: How breathing pattern modulates heart rate. Am. J. Physiol. 1981, 241, H620–H629. [Google Scholar] [PubMed]
- De Angelis, C.; Perelli, P.; Trezza, R.; Casagrande, M.; Biselli, R.; Pannitteri, G.; Marino, B.; Farrace, S. Modified autonomic balance in offsprings of diabeties detected by spectral analysis of heart rate variability. Metab. Clin. Exp. 2001, 50, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, G.V.; Fernhall, B.; Heffernan, K.S.; Pereira, F.D. Spectral methods of heart rate variability analysis during dynamic exercise. Clin. Auton. Res. 2009, 19, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Pichon, A.; Roulaud, M.; Antoine-Jonville, S.; Bisschop, C.D.; Denjean, A. Spectral analysis of heart rate variability: Interchangeability between autoregressive analysis and fast Fourier transform. J. Electrocardiol. 2006, 39, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Takada, H.T.; Katayama, M.A. The significance of “LF-component and HF-component which resulted from frequency analysis of heart rate” and “the coefficient of the heart rate variability”: Evaluation of autonomic nerve function by acceleration plethysmography. Health Eval. Promot. 2005, 32, 504–512. [Google Scholar] [CrossRef]




| Characteristic | Variable | |
|---|---|---|
| Age (y) | 34.2 ± 7.6 | |
| Sex (n) | Male | 26 |
| Female | 7 | |
| Body height (cm) | 168.4 ± 6.7 | |
| Body weight (kg) | 67.0 ± 13.0 | |
| Participant | Volume | Velocity | Sys-VD | Dia-VD | HR | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | LF (Hz) | HF (Hz) | LF (Hz) | HF (Hz) | LF (Hz) | HF (Hz) | LF (Hz) | HF (Hz) | LF (Hz) | HF (Hz) |
| 1 | 0.137 | 0.156 | 0.137 | 0.156 | 0.049 | 0.156 | 0.049 | 0.166 | 0.049 | 0.146 |
| 2 | 0.049 | 0.166 | 0.049 | 0.166 | 0.137 | 0.176 | 0.049 | 0.156 | 0.068 | 0.166 |
| 3 | 0.137 | 0.166 | 0.137 | 0.156 | 0.137 | 0.166 | 0.049 | 0.166 | 0.137 | 0.166 |
| 4 | 0.049 | 0.156 | 0.098 | 0.166 | 0.059 | 0.166 | 0.068 | 0.146 | 0.059 | 0.166 |
| 5 | 0.059 | 0.166 | 0.059 | 0.166 | 0.049 | 0.156 | 0.049 | 0.156 | 0.059 | 0.156 |
| 6 | 0.078 | 0.156 | 0.088 | 0.186 | 0.107 | 0.156 | 0.049 | 0.156 | 0.137 | 0.146 |
| 7 | 0.059 | 0.234 | 0.059 | 0.313 | 0.078 | 0.273 | 0.078 | 0.225 | 0.068 | 0.156 |
| 8 | 0.078 | 0.146 | 0.088 | 0.410 | 0.137 | 0.146 | 0.137 | 0.146 | 0.137 | 0.146 |
| 9 | 0.049 | 0.146 | 0.088 | 0.146 | 0.137 | 0.156 | 0.049 | 0.488 | 0.137 | 0.146 |
| 10 | 0.049 | 0.303 | 0.049 | 0.293 | 0.078 | 0.264 | 0.088 | 0.332 | 0.088 | 0.273 |
| 11 | 0.049 | 0.146 | 0.049 | 0.303 | 0.049 | 0.176 | 0.088 | 0.488 | 0.049 | 0.498 |
| 12 | 0.049 | 0.156 | 0.059 | 0.332 | 0.049 | 0.156 | 0.049 | 0.146 | 0.127 | 0.146 |
| 13 | 0.049 | 0.146 | 0.117 | 0.342 | 0.049 | 0.146 | 0.049 | 0.146 | 0.137 | 0.146 |
| 14 | 0.049 | 0.176 | 0.049 | 0.215 | 0.088 | 0.488 | 0.078 | 0.498 | 0.088 | 0.195 |
| 15 | 0.049 | 0.166 | 0.049 | 0.166 | 0.137 | 0.156 | 0.137 | 0.156 | 0.137 | 0.156 |
| 16 | 0.059 | 0.156 | 0.059 | 0.156 | 0.137 | 0.166 | 0.117 | 0.166 | 0.059 | 0.166 |
| 17 | 0.049 | 0.156 | 0.049 | 0.322 | 0.137 | 0.166 | 0.088 | 0.166 | 0.068 | 0.166 |
| 18 | 0.049 | 0.146 | 0.049 | 0.371 | 0.049 | 0.215 | 0.049 | 0.166 | 0.137 | 0.146 |
| 19 | 0.049 | 0.166 | 0.049 | 0.244 | 0.049 | 0.146 | 0.117 | 0.156 | 0.049 | 0.166 |
| 20 | 0.049 | 0.166 | 0.107 | 0.166 | 0.049 | 0.166 | 0.049 | 0.166 | 0.137 | 0.166 |
| 21 | 0.059 | 0.166 | 0.068 | 0.166 | 0.137 | 0.166 | 0.098 | 0.166 | 0.137 | 0.156 |
| 22 | 0.049 | 0.166 | 0.078 | 0.361 | 0.137 | 0.156 | 0.137 | 0.156 | 0.117 | 0.166 |
| 23 | 0.098 | 0.176 | 0.127 | 0.176 | 0.049 | 0.166 | 0.098 | 0.166 | 0.088 | 0.166 |
| 24 | 0.068 | 0.166 | 0.068 | 0.166 | 0.098 | 0.166 | 0.107 | 0.176 | 0.049 | 0.166 |
| 25 | 0.137 | 0.166 | 0.137 | 0.166 | 0.049 | 0.166 | 0.049 | 0.166 | 0.137 | 0.166 |
| 26 | 0.049 | 0.146 | 0.049 | 0.205 | 0.049 | 0.166 | 0.049 | 0.156 | 0.137 | 0.146 |
| 27 | 0.049 | 0.195 | 0.049 | 0.146 | 0.049 | 0.146 | 0.049 | 0.244 | 0.078 | 0.156 |
| 28 | 0.049 | 0.166 | 0.049 | 0.166 | 0.049 | 0.166 | 0.049 | 0.166 | 0.137 | 0.166 |
| 29 | 0.127 | 0.146 | 0.049 | 0.166 | 0.137 | 0.166 | 0.137 | 0.166 | 0.137 | 0.156 |
| 30 | 0.059 | 0.146 | 0.137 | 0.166 | 0.127 | 0.186 | 0.127 | 0.186 | 0.078 | 0.186 |
| 31 | 0.117 | 0.146 | 0.127 | 0.146 | 0.127 | 0.146 | 0.127 | 0.146 | 0.117 | 0.146 |
| 32 | 0.049 | 0.146 | 0.049 | 0.146 | 0.137 | 0.156 | 0.137 | 0.156 | 0.137 | 0.156 |
| 33 | 0.049 | 0.166 | 0.068 | 0.166 | 0.049 | 0.166 | 0.049 | 0.166 | 0.137 | 0.166 |
| 34 | 0.049 | 0.166 | 0.049 | 0.322 | 0.068 | 0.156 | 0.107 | 0.176 | 0.137 | 0.166 |
| Mean ± SD | 0.066 ± 0.030 | 0.166 ± 0.030 | 0.076 ± 0.033 | 0.219 ± 0.082 | 0.089 ± 0.040 | 0.179 ± 0.062 | 0.082 ± 0.035 | 0.200 ± 0.098 | 0.104 ± 0.036 | 0.173 ± 0.062 |
| LF/HF Mean ± SD | 2.1 ± 1.6 | 0.9 ± 0.5 | 0.8 ± 0.5 | 0.9 ± 0.5 | 0.8 ± 0.4 | |||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, M.; Kaneko, S.; Takayama, S.; Shiraishi, Y.; Numata, T.; Saito, N.; Seki, T.; Sugita, N.; Konno, S.; Yambe, T.; et al. The Pilot Study of Evaluating Fluctuation in the Blood Flow Volume of the Radial Artery, a Site for Traditional Pulse Diagnosis. Medicines 2016, 3, 11. https://doi.org/10.3390/medicines3020011
Watanabe M, Kaneko S, Takayama S, Shiraishi Y, Numata T, Saito N, Seki T, Sugita N, Konno S, Yambe T, et al. The Pilot Study of Evaluating Fluctuation in the Blood Flow Volume of the Radial Artery, a Site for Traditional Pulse Diagnosis. Medicines. 2016; 3(2):11. https://doi.org/10.3390/medicines3020011
Chicago/Turabian StyleWatanabe, Masashi, Soichiro Kaneko, Shin Takayama, Yasuyuki Shiraishi, Takehiro Numata, Natsumi Saito, Takashi Seki, Norihiro Sugita, Satoshi Konno, Tomoyuki Yambe, and et al. 2016. "The Pilot Study of Evaluating Fluctuation in the Blood Flow Volume of the Radial Artery, a Site for Traditional Pulse Diagnosis" Medicines 3, no. 2: 11. https://doi.org/10.3390/medicines3020011
APA StyleWatanabe, M., Kaneko, S., Takayama, S., Shiraishi, Y., Numata, T., Saito, N., Seki, T., Sugita, N., Konno, S., Yambe, T., Yoshizawa, M., Yaegashi, N., & Ishii, T. (2016). The Pilot Study of Evaluating Fluctuation in the Blood Flow Volume of the Radial Artery, a Site for Traditional Pulse Diagnosis. Medicines, 3(2), 11. https://doi.org/10.3390/medicines3020011