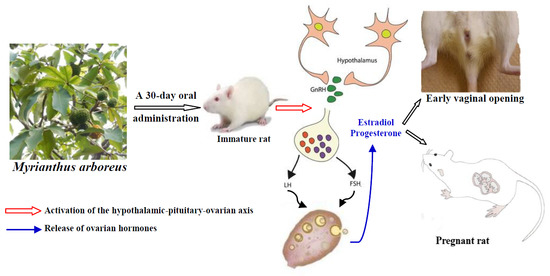
Myrianthus arboreus P. Beauv (Cecropiaceae) Extracts Accelerates Sexual Maturation, and Increases Fertility Index and Gestational Rate in Female Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Collection, Identification and Extracts
2.2. Animals
2.3. Experimental Protocol
2.4. Measurement of Biochemical Parameters
2.5. Histopathological Evaluation
2.6. Statistical Analysis
3. Results
3.1. Vaginal Opening
3.2. Relative Weight of Ovaries and Uterus
3.3. Epithelial Height of Uterus and Vaginas
3.4. Ovarian Follicles
3.5. Serum and Ovarian Total Cholesterol
3.6. Hormone Levels
3.7. Effects of M. arboreus Extracts on Fertility and Gestation
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| FSH | Follicle-stimulating hormone; |
| GnRH | Gonadotropin-releasing hormone; |
| LH | Luteinizing hormone. |
References
- Gnoth, C.; Godehardt, E.; Frank-Herrmann, P.; Friol, K.; Tigges, J.; Freundl, G. Definition and prevalence of subfertility and infertility. Hum. Reprod. 2005, 20, 1144–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chachamovich, J.R.; Chachamovich, E.; Ezer, H.; Fleck, M.P.; Knauth, D.; Passos, E.P. Investigating quality of life and health-related quality of life in infertility: A systematic review. J. Psychosom. Obstet. Gynaecol. 2010, 31, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Cui, W. Mother or nothing: The agony of infertility. Bull. World Health Organ. 2010, 88, 881–882. [Google Scholar] [CrossRef] [PubMed]
- Greil, A.L.; Slauson-Blevins, K.; McQuillan, J. The experience of infertility: A review of recent literature. Sociol. Health Illn. 2010, 32, 140–162. [Google Scholar] [CrossRef] [PubMed]
- Nguimfack, L.; Newsom, K.; Nguekeu, M.R. Brief report: A Cameroonian woman’s cultural-bound experience of infertility. J. Fem. Fam. Ther. 2016, 28, 100–110. [Google Scholar] [CrossRef]
- Papreen, N.; Sharma, A.; Sabin, K.; Begum, L.; Ahsan, S.K.; Baqui, A.H. Living with infertility: Experiences among urban slum populations in Bangladesh. Reprod. Health Matters 2000, 8, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Rouchou, B. Consequences of infertility in developing countries. Perspect. Public Health 2013, 133, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Wiersema, N.J.; Drukker, A.J.; Dung, M.B.T.; Nhu, G.H.; Nhu, N.T.; Lambalk, C.B. Consequences of infertility in developing countries: Results of a questionnaire and interview survey in the South of Vietnam. J. Transl. Med. 2006, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Deka, P.K.; Sarma, S. Psychological aspect of infertility. Br. J. Med. Pract. 2010, 3, 336. [Google Scholar]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A unique view on male infertility around the globe. Reprod. Biol. Endocrinol. 2015, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Boivin, J.; Bunting, L.; Collins, J.A.; Nygren, K.G. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum. Reprod. 2007, 22, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Ombelet, W. Reproductive healthcare systems should include accessible infertility diagnosis and treatment: An important challenge for resource-poor countries. Int. J. Gynecol. Obstet. 2009, 106, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Mascarenhas, M.N.; Flaxman, S.R.; Boerma, T.; Vanderpoel, S.; Stevens, G.A. National, regional, and global trends in infertility prevalence since 1990: A systematic analysis of 277 health surveys. PLoS Med. 2012, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Forti, G.; Krausz, C. Evaluation and treatment of the infertile couple. J. Clin. Endocrinol. Metab. 1998, 83, 4177–4188. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.E. Infertility and contraception. In Harrison’s Principles of Internal Medicine, 19th ed.; Kasper, D., Fauci, A., Hauser, S., Longo, D., Jameson, J.L., Loscalzo, J., Eds.; McGraw-Hill Medical: New York, NY, USA, 2015; pp. 2387–2391. [Google Scholar]
- Templeton, A. Infertility and the establishment of pregnancy–overview. Br. Med. Bull. 2000, 56, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Unuane, D.; Tournaye, H.; Velkeniers, B.; Poppe, K. Endocrine disorders & female infertility. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Gorthi, S.; Balen, A.H.; Tang, T. Current issues in ovulation induction. Obstet. Gynaecol. 2012, 14, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Tamao, F.; Russo, G.L.; Spinelli, G.P.; Stati, V.; Prete, A.A.; Prinzi, N.; Sinjari, M.; Vici, P.; Papa, A.; Chiotti, M.S.; et al. Fertility drugs, reproductive strategies and ovarian cancer risk. J. Ovarian Res. 2014, 7, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duran, J.R.; Raja, M.L. Myocardial infarction in pregnancy associated with clomiphene citrate for ovulation induction: A case report. J. Reprod. Med. 2007, 52, 1059–1062. [Google Scholar] [PubMed]
- O’Donovan, O.; Al Chami, A.; Davies, M. Ovarian hyperstimulation syndrome. Obstet. Gynaecol. Reprod. Med. 2015, 25, 2. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Prescribing Information for Clomiphene Citrate. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/016131s026lbl.pdf (accessed on 15 September 2014).
- Amata, I.A. Nutritive value of the leaves of Myrianthus arboreus: A browse plant. Int. J. Agric. Res. 2010, 5, 576–581. [Google Scholar] [CrossRef]
- Burkill, H.M. The Useful Plants of West Tropical Africa; Families, J.L., Ed.; Royal Botanic Garden: Kew, UK, 1985; Volume 3. [Google Scholar]
- Konan, Y.; Witabouna, K.M.; Bassirou, B.; Kagoyire, K. Antioxidant activity and total phenolic content of nine plants from Cote d′Ivoire (West Africa). J. Appl. Pharm. Sci. 2014, 4, 36–41. [Google Scholar] [CrossRef]
- Oyeyemi, S.D.; Arowosegbe, S.; Adebiyi, A.O. Phytochemical and proximate evaluation of Myrianthus arboreus P. Beau. and Spargonophorus sporgonophora L. leaves. IOSR J. Agric. Vet. Sci. 2014, 7, 1–5. [Google Scholar]
- Agyare, C.; Ansah, A.O.; Ossei, P.P.S.; Apenteng, J.A.; Boakye, Y.D. Wound healing and anti-infective properties of Myrianthus arboreus and Alchornea cordifolia. Med. Chem. 2014, 4, 533–539. [Google Scholar] [CrossRef]
- Okafor, J.C. Myrianthus arboreus. P. Beauv. In PROTA 2; Vegetables legumes; Grubben, G.J.H., Denton, O.A., Eds.; PROTA. Fountain: Wageningen, The Netherlands, 2004; pp. 31–38. [Google Scholar]
- Olonode, E.T.; Aderibigbe, A.O.; Bakre, A.G. Antinociceptive activity of the crude extract of Myrianthus arboreus P. Beauv (Cecropiaceae) in mice. J. Ethnopharmacol. 2015, 171, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Adjanohoun, J.E.; Aboubakar, N.; Dramane, K.; Ebot, M.E.; Ekpere, J.A.; Enow-Orock, E.G.; Focho, D.; Gbile, Z.O.; Kamanyi, A.; KamsuKom, J.; et al. Traditional Medicine and Pharmacopoeia: Contribution to Ethnobotanical and Floristic Studies in Cameroon; OAU/STRC Publish House: Porto-Novo, Benin, 1996; pp. 12–607. [Google Scholar]
- Kasangana, P.B.; Selim Haddad, P.; Stevanovic, T. Study of polyphenol content and antioxidant capacity of Myrianthus arboreus (Cecropiaceae) root bark extracts. Antioxidants 2015, 4, 410–426. [Google Scholar] [CrossRef] [PubMed]
- Biapa, N.P.-C.; Agbor, G.A.; Oben, J.E.; Ngogang, J.Y. Phytochemical studies and antioxidant properties of four medicinal plants used in Cameroon. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Seukep, J.A.; Ngadjui, B.; Kuete, V. Antibacterial activities of Fagara macrophylla, Canarium schweinfurthii, Myrianthus arboreus, Dischistocalyx grandifolius and Tragia benthamii against multi-drug resistant Gram-negative bacteria. Springerplus 2015, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dickson, R.A.; Harley, B.K.; Berkoh, D.; Ngala, R.A.; Titiloye, N.A.; Fleischer, T.C. Antidiabetic and haematological effect of Myrianthus arboreus P. Beauv. Stem bark extract in streptozotocin-induced diabetic rats. Int. J. Pharm. Sci. Rev. Res. 2016, 7, 4812–4826. [Google Scholar] [CrossRef]
- Kasangana, P.B.; Nachar, A.; Eid, H.M.; Stevanovic, T.; Haddad, P.S. Root bark extracts of Myrianthus arboreus P. Beauv. (Cecropiaceae) exhibit anti-diabetic potential by modulating hepatocyte glucose homeostasis. J. Ethnopharmacol. 2018, 211, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Awounfack, C.F.; Ateba, S.B.; Zingue, S.; Mouchili, O.R.; Njamen, D. Safety evaluation (acute and sub-acute studies) of the aqueous extract of the leaves of Myrianthus arboreus P. Beauv. (Cecropiaceae) in Wistar rats. J. Ethnopharmacol. 2016, 194, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Lienou, L.L.; Telefo, B.P.; Bale, B.; Yemele, D.; Tagne, R.S.; Goka, S.C.; Lemfack, C.M.; Mouokeu, C.; Moundipa, P.F. Effect of the aqueous extract of Senecio biafrae (Oliv. & Hiern) J. Moore on sexual maturation of immature female rat. BMC Complement. Altern. Med. 2012, 12, 36. [Google Scholar] [CrossRef]
- Lienou, L.L.; Telefo, P.B.; Njimou, J.R.; Nangue, C.; Bayala, B.R.; Goka, S.C.; Biapa, P.; Yemele, M.D.; Donfack, N.J.; Mbemya, J.T.; et al. Effect of the aqueous extract of Senecio biafrae (Oliv. &Hiern) J. Moore on some fertility parameters in immature female rat. J. Ethnopharmacol. 2015, 161, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Medigović, I.; Manojlović-Stojanoski, M.; Trifunović, S.; Ristić, N.; Milošević, V.; Žikić, D.; Nestorović, N. Effects of genistein on gonadotropic cells in immature female rats. Acta Histochem. 2012, 114, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.S.; Silva, J.R.; Gomes, C.L.; Nery, M.B.L.; Navarro, D.M.A.F.; Santos, G.K.N.; Silva-Neto, J.C.; Costa-Silva, J.H.; Araújo, A.V.; Wanderleya, A.G. Effects of the oral treatment with Copaifera multijuga oil on reproductive performance of male Wistar rats. Braz. J. Pharmacog. 2014, 24, 355–362. [Google Scholar] [CrossRef]
- Cruz, G.; Barra, R.; Gonzalez, D.; Sotomayor-Zarate, R.; Lara, H.E. Temporal window in which exposure to estradiol permanently modifies ovarian function causing polycystic ovary morphology in rats. Fertil. Steril. 2012, 98, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhao, F.; Diao, H.; Xiao, S.; Ye, X. Postweaning dietary genistein exposure advances puberty without significantly affecting early pregnancy in C57BL/6J female mice. Reprod. Toxicol. 2014, 44, 85–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Do, K.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatic. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Tassinari, M.S.; Benson, K.; Elayan, I.; Espandiari, P.; Davis-Bruno, K. Juvenile animal studies and pediatric drug development retrospective review: Use in regulatory decisions and labeling. Birth Defects Res. B Dev. Reprod. Toxicol. 2011, 92, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Njamen, D.; Mvondo, M.A.; Nanbo, G.T.; Zingue, S.; Tanee, F.S.; Wandj, J. Erythrina lysistemon-derived flavonoids account only in part for the plant’s specific effects on rat uterus and vagina. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, S.C.; Deroo, B.J.; Hansen, K.; Collins, J.; Grissom, S.; Afshari, C.A. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol. Endocrinol. 2003, 17, 2070–2083. [Google Scholar] [CrossRef] [PubMed]
- Ateba, S.B.; Njamen, D.; Medjakovic, S.; Hobiger, S.; Mbanya, J.C.; Jungbauer, A.; Krenn, L. Eriosema laurentii De Wild (Leguminosae) methanol extract has estrogenic properties and prevents menopausal symptoms in ovariectomized Wistar rats. J. Ethnopharmacol. 2013, 150, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.C.; Giuliana, G.K.; BotelhoAedra, C.B.; Boareto, C.A.; Rattmann, D.Y.; Martins, E.S.; Cabrini, D.A.; Otuki, M.F.; Paulo, R.D. Morinda citrifolia Linn (Noni): In vivo and in vitro reproductive toxicology. J. Ethnopharmacol. 2009, 121, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Onyeka, C.A.; Aligwekwe, A.U.; Olawuyi, T.S.; Nwakama, E.A.; Kalu, E.C.; Oyeyemi, A.W. Antifertility effects of ethanolic root bark extract of Chrysophyllum albidum in male albino rats. Int. J. Appl. Res. Nat. Prod. 2012, 5, 12–17. [Google Scholar]
- Picut, C.A.; Remick, A.K.; Asakawa, M.G.; Simons, M.L.; Parker, G.A. Histologic features of prepubertal and pubertal reproductive development in female Sprague-Dawley rats. Toxicol. Pathol. 2014, 42, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Henderson, K.M.; Gorban, A.M.; Boyd, G.S. Effect of LH factors regulating ovarian cholesterol metabolism and progesterone synthesis in PMSG-primed immature rats. J. Reprod. Fertil. 1981, 61, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Marieb, E.N.; Hoehn, K. Anatomie et Physiologie Humaines. Adaptation de la 8e édition Américaine; Nouveau Horizons-ARS: Paris, France, 2010; pp. 1–1293. [Google Scholar]
- Terranova, P.F. Female Reproductive System. In Medical Phisiology, 2nd ed.; Rhoades, R.A., Tanner, G.A., Eds.; Williams & Wilkins: Lippincott, NY, USA, 2004; pp. 640–679. [Google Scholar]
- Calabrese, E.J.; Baldwin, L.A. The frequency of U-shaped dose responses in the toxicological literature. Toxicol. Sci. 2001, 62, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Baldwin, L.A. Hormesis: The dose-response revolution. Ann. Rev. Pharmacol. Toxicol. 2003, 43, 175–197. [Google Scholar] [CrossRef] [PubMed]
- Mvondo, M.A.; Njamen, D.; Kretzschmar, G.; Bader, M.I.; Fomum, S.T.; Wandji, J.; Vollmer, G. Alpinumisoflavone and abyssinone V 4′-methylether derived from Erythrina lysistemon (Fabaceae) promote HDL-cholesterol synthesis and prevent cholesterol gallstone formation in ovariectomized rats. J. Pharm. Pharmacol. 2015, 67, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Oludare, G.O.; Iranloye, B.O. Implantation and pregnancy outcome of Sprague-Dawley rats fed with low and high salt diet. Middle East Fertil. Soc. J. 2016, 21, 228–235. [Google Scholar] [CrossRef]
- Hiremath, S.P.; Rudresh, K.; Shrishailappa, B.; Patil, S.B.; Patil, S.R. Post-coital antifertility activity of Acalypha indica L. J. Ethnopharmacol. 1999, 67, 253–258. [Google Scholar] [CrossRef]
- Deb, K.; Reese, J.; Paria, B.C. Methodologies to study implantation in mice. Methods Mol. Med. 2006, 121, 9–34. [Google Scholar] [PubMed]
- Watcho, P.; Ngadjui, E.; Alango, N.-E.P.; Benoît, N.T.; Kamanyi, A. Reproductive effects of Ficus asperifolia (Moraceae) in female rats. Afr. Health Sci. 2009, 9, 49–53. [Google Scholar] [PubMed]
- U.S. Department of Health & Human Services. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers; Office of New Drugs in the Center for Drug Evaluation and Research (CDER) at the FDA: Rockville, MD, USA, July 2005. Available online: http://www.fda.gov/cder/guidance/index.htm (accessed on 5 March 2015).







| Types of Follicles | Control | Aqueous extract of M. arboreus (mg/kg BW/d) | Methanol extract of M. arboreus (mg/kg BW/d) | ||||
|---|---|---|---|---|---|---|---|
| AE 20 | AE 110 | AE 200 | ME 20 | ME 110 | ME 200 | ||
| Primary follicles | 5.00 ± 0.83 | 5.40 ± 0.92 | 5.20 ± 0.48 | 6.80 ± 0.86 | 8.00 ± 1.14 | 5.80 ± 0.37 | 6.20 ± 0.73 |
| Preantral follicles | 9.00 ± 2.12 | 14.20 ± 3.08 | 17.60 ± 2.22 | 12.60 ± 1.28 | 16.00 ± 3.11 | 15.00 ± 2.81 | 15.40 ± 5.51 |
| Antral follicles | 1.60 ± 0.24 | 1.80 ± 1.11 | 0.80 ± 0.37 | 1.60 ± 0.67 | 3.40 ± 0.67 | 4.4 ± 0.74* | 2.40 ± 0.50 |
| Graafian follicles | 0.40 ± 0.24 | 1.00 ± 0.31 | 0.60 ± 0,24 | 2.00 ± 0.31** | 1.00 ± 0.31 | 1.40 ± 0.24 | 1.00 ± 0.31 |
| Corpora lutea | 0.60 ± 0.40 | 1.80 ± 0.86 | 0.50 ± 0.22 | 1.40 ± 0.67 | 1.40 ± 0.50 | 3.00 ± 0.89* | 1.80 ± 0.20 |
| Experimental Groups | Fertility Index (%) | Gestation Rate (%) |
|---|---|---|
| Control (distilled water 10 mL/kg) | 14.28 | 100 |
| AE 20 (aqueous extract 20 mg/kg) | 28.57 | 100 |
| AE 110 (aqueous extract 110 mg/kg) | 57.14 | 100 |
| AE 200 (aqueous extract 200 mg/kg) | 28.57 | 50 |
| ME 20 (methanol extract 20 mg/kg) | 71.42 | 100 |
| ME 110 (methanol extract 110 mg/kg) | 71.42 | 100 |
| ME 200 (methanol extract 200 mg/kg) | 42.85 | 33.33 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awounfack, C.F.; Mvondo, M.A.; Zingue, S.; Ateba, S.B.; Djiogue, S.; Megnekou, R.; Tantoh Ndinteh, D.; Njamen, D. Myrianthus arboreus P. Beauv (Cecropiaceae) Extracts Accelerates Sexual Maturation, and Increases Fertility Index and Gestational Rate in Female Wistar Rats. Medicines 2018, 5, 73. https://doi.org/10.3390/medicines5030073
Awounfack CF, Mvondo MA, Zingue S, Ateba SB, Djiogue S, Megnekou R, Tantoh Ndinteh D, Njamen D. Myrianthus arboreus P. Beauv (Cecropiaceae) Extracts Accelerates Sexual Maturation, and Increases Fertility Index and Gestational Rate in Female Wistar Rats. Medicines. 2018; 5(3):73. https://doi.org/10.3390/medicines5030073
Chicago/Turabian StyleAwounfack, Charline Florence, Marie Alfrede Mvondo, Stéphane Zingue, Sylvin Benjamin Ateba, Sefirin Djiogue, Rosette Megnekou, Derek Tantoh Ndinteh, and Dieudonné Njamen. 2018. "Myrianthus arboreus P. Beauv (Cecropiaceae) Extracts Accelerates Sexual Maturation, and Increases Fertility Index and Gestational Rate in Female Wistar Rats" Medicines 5, no. 3: 73. https://doi.org/10.3390/medicines5030073
APA StyleAwounfack, C. F., Mvondo, M. A., Zingue, S., Ateba, S. B., Djiogue, S., Megnekou, R., Tantoh Ndinteh, D., & Njamen, D. (2018). Myrianthus arboreus P. Beauv (Cecropiaceae) Extracts Accelerates Sexual Maturation, and Increases Fertility Index and Gestational Rate in Female Wistar Rats. Medicines, 5(3), 73. https://doi.org/10.3390/medicines5030073