Development of Continuous Gas Generation Method for Hydrogen Chloride Using Azeotropic Hydrochloric Acid System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Diffusion Materials
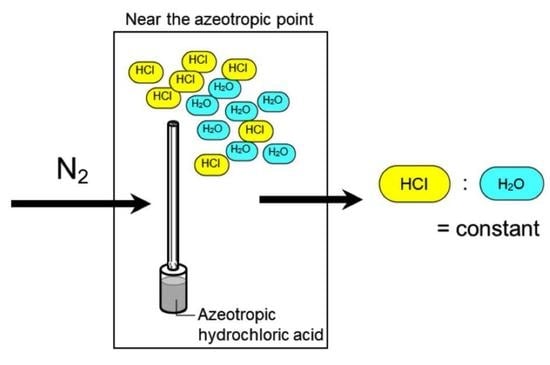
2.3. System for HCl Gas Generation
2.4. Measurements of Gaseous HCl Produced from the Generation System
2.5. Measuring the Changes in the Masses of the Diffusion Materials
3. Results and Discussion
3.1. Stability of Gaseous HCl
3.2. Evaluation of the Azeotrope System for HCl Gas Generation
3.3. Preparation of Gaseous HCl with Different Carrier Gas Flow Rates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perry, W.G.; Smith, F.A.; Kent, M.B. The halogens. In Patty’s Industrial Hygiene and Toxicology, 4th ed.; Clayton, G.D., Clayton, F.E., Eds.; John Wiley & Sons: New York, NY, USA, 1994; Volume 2, pp. 4487–4490. [Google Scholar]
- Sebacher, D.I.; Bendura, R.J.; Wornom, D.E. Hydrochloric acid aerosol and gaseous hydrogen chloride partitioning in a cloud contaminated by solid rocket exhaust. Atmos. Environ. 1980, 14, 543–547. [Google Scholar] [CrossRef]
- Hisham, M.W.M.; Bommaraju, T.V. Hydrogen chloride. In Kirk-Othmer Encyclopedia of Chemical Technology, 4th ed.; Kroschwitz, J.I., Howe-Grant, M., Eds.; John Wiley & Sons: New York, NY, USA, 1995; Volume 13, p. 894925. [Google Scholar]
- McCulloch, A.; Aucott, M.L.; Benkovitz, C.M.; Graedel, T.E.; Kleiman, G.; Midgley, P.M.; Li, Y.-F. Global emissions of hydrogen chloride and chloromethane from coal combustion, incineration and industrial activities: Reactive Chlorine Emissions Inventory. J. Geophys. Res. 1999, 104, 8391–8403. [Google Scholar] [CrossRef] [Green Version]
- Keene, W.C.; Khalil, M.A.K.; Erickson, D.J., III; McCulloch, A.; Graedel, T.E.; Loberr, J.M.; Aucott, M.L.; Gong, S.L.; Harper, D.B.; Kleiman, G.; et al. Composite global emissions of reactive chlorine from anthropogenic and natural sources: Reactive Chlorine Emissions Inventory. J. Geophys. Res. Atmos. 1999, 104, 8429–8440. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.; Sun, R.; Chi, H.-H.; Meng, X.; Li, Y.; Levendis, Y. Hydrogen chloride emissions from combustion of raw and torrefied biomass. Fuel 2017, 200, 37–46. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chapter 7—Chemistry of Inorganic Nitrogen Compounds. In Chemistry of the Upper and Lower Atmosphere, 1st ed.; Academic Press: San Diego, CA, USA, 1999; pp. 264–293. [Google Scholar]
- Mahieu, E.; Chipperfield, M.P.; Notholt, J.; Reddmann, T.; Anderson, J.; Bernath, P.F.; Blumenstock, T.; Coffey, M.T.; Dhomse, S.S.; Feng, W.; et al. Recent Northern Hemisphere stratospheric HCl increase due to atmospheric circulation changes. Nature 2014, 515, 104–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prather, M.J.; McElroy, M.B.; Wolfsy, S.C. Reductions in ozone at high concentrations of stratospheric halogens. Nature 1984, 312, 227–231. [Google Scholar] [CrossRef] [PubMed]
- EPA. Hydrochloric Acid (Hydrogen Chloride) Hazard Summary. 2000. Available online: https://www.epa.gov/sites/production/files/2016-09/documents/hydrochloric-acid.pdf (accessed on 6 May 2021).
- Kremer, A.M.; Pal, T.M.; Boleij, J.S.; Schouten, J.P.; Rijcken, B. Airway hyperresponsiveness, prevalence of chronic respiratory symptoms, and lung function in workers exposed to irritants. Occup. Environ. Med. 1994, 51, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamrin, M.A. Workshop on the health effects of HCl in ambient air. Regul. Toxicol. Pharmacol. 1992, 15, 73–82. [Google Scholar] [CrossRef]
- National Research Council. Subcommittee on Rocket-Emission Toxicants. In Assessment of Exposure-Response Functions for Rocket-Emission Toxicants; National Academies Press: Washington, DC, USA, 1998; pp. 105–146. [Google Scholar]
- Sayers, R.R.; D-Valle, J.M.; Yant, W.P. Industrial hygiene and sanitation surveys in chemical establishments. Ind. Eng. Chem. 1934, 26, 1251–1255. [Google Scholar] [CrossRef]
- Muneer, G.B.; Posta-Kelty, L.A.; Sinclair, J.D. Chloride Accumulation on Indoor Zinc and Aluminum Surfaces. J. Electrochem. Soc. 1983, 130, 1983–1987. [Google Scholar] [CrossRef]
- Namieśnik, J. Generation of standard gaseous mixtures. J. Chromatogr. A 1984, 300, 79–108. [Google Scholar] [CrossRef]
- Aoki, N. A survey on standard gases for measuring ambient air. AIST Bull. Metrol. 2007, 6, 27–38. [Google Scholar]
- Mitchell, G.D. A review of permeation tubes and permeators. Sep. Purif. Methods 2000, 29, 119–128. [Google Scholar] [CrossRef]
- Tumbiolo, S.; Vincent, L.; Gal, J.-F.; Maria, P.-C. Thermogravimetric calibration of permeation tubes used for the preparation of gas standards for air pollution analysis. Analyst 2005, 130, 1369–1374. [Google Scholar] [CrossRef]
- Maria, P.-C.; Gal, J.-F.; Balza, M.; Peré-Trepat, E.; Tumbiolo, S.; Couret, J.-M. Using thermogravimetry for weight loss monitoring of permeation tubes used for generation of trace concentration gas standards. Anal. Chem. 2002, 74, 305–307. [Google Scholar] [CrossRef]
- Fielden, P.R.; Greenway, G.M. Diffusion apparatus for trace level vapor generation of tetramethyllead. Anal. Chem. 1989, 61, 1993–1996. [Google Scholar] [CrossRef]
- Gautrois, M.; Koppmann, R. Diffusion technique for the production of gas standards for atmospheric measurements. J. Chromatogr. A 1999, 848, 239–249. [Google Scholar] [CrossRef]
- Possanzini, M.; Di Palo, V.; Brancaleoni, E.; Frattoni, M.; Ciccioli, P. Dynamic system for the calibration of semi-volatile carbonyl compounds in air. J. Chromatogr. A 2000, 883, 171–183. [Google Scholar] [CrossRef]
- Scarano, E.; Calcagno, C.; Cignoli, L. A reliable source of very small amounts of hydrogen chloride for analytical purposes. Anal. Chim. Acta 1979, 110, 95–106. [Google Scholar] [CrossRef]
- Norton, G.A.; Eckels, D.E.; Chriswell, C.D. Development of Mercury and Hydrogen Chloride Emission Monitors for Coal Gasifiers, Final Report to the National Energy Technology Laboratory; Ames Laboratory, Iowa State University: Ames, IA, USA, 2001; pp. 85–104. [Google Scholar]
- Conde, M.; Taxen, C. Hydrochloric acid and water permeability in fluoropolymer tubes. Presented at the CORROSION 2000, Orlando, FL, USA, 26–31 March 2000; p. NACE-00572. [Google Scholar]
- Japanese Industrial Standard, JIS K 0107; Methods for Determination of Hydrogen Chloride in Flue Gas. Japanese Standards Association: Tokyo, Japan, 2012.
- Aoyagi, R.; Watanabe, F.; Matsunobu, K. Stability for formalin vapor with diffusion tube method. J. Work Environ. 2010, 31, 51–57. [Google Scholar]






| Temperature | Concentration for Hydrochloric Acid | Drm | DrHCl | DrHCl/Drm |
|---|---|---|---|---|
| (°C) | (%) | (µgmin−1) | (µgmin−1) | (%) |
| 70.0 | 14.8 | 36.12 ± 0.96 | 0.48 ± 0.00 | 1.3 |
| 20.6 | 35.03 ± 0.96 | 1.61 ± 0.05 | 4.6 | |
| 26.3 | 43.09 ± 0.96 | 12.81 ± 0.23 | 29.7 | |
| 90.0 | 14.8 | 89.13 ± 0.86 | 3.17 ± 0.00 | 3.6 |
| 20.6 | 79.68 ± 0.86 | 8.72 ± 0.00 | 10.9 | |
| 26.3 | 101.05 ± 0.86 | 56.78 ± 2.29 | 56.2 | |
| 108.5 | 14.8 | 327.19 ± 0.80 | 15.85 ± 0.00 | 4.8 |
| 20.6 | 365.85 ± 0.80 | 82.40 ± 2.74 | 22.5 | |
| 26.3 | 386.62 ± 0.80 | 261.57 ± 3.65 | 67.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoyagi, R.; Sekine, Y.; Kaifuku, Y.; Matsunobu, K. Development of Continuous Gas Generation Method for Hydrogen Chloride Using Azeotropic Hydrochloric Acid System. ChemEngineering 2022, 6, 12. https://doi.org/10.3390/chemengineering6010012
Aoyagi R, Sekine Y, Kaifuku Y, Matsunobu K. Development of Continuous Gas Generation Method for Hydrogen Chloride Using Azeotropic Hydrochloric Acid System. ChemEngineering. 2022; 6(1):12. https://doi.org/10.3390/chemengineering6010012
Chicago/Turabian StyleAoyagi, Reiji, Yoshika Sekine, Yuichiro Kaifuku, and Kunitoshi Matsunobu. 2022. "Development of Continuous Gas Generation Method for Hydrogen Chloride Using Azeotropic Hydrochloric Acid System" ChemEngineering 6, no. 1: 12. https://doi.org/10.3390/chemengineering6010012
APA StyleAoyagi, R., Sekine, Y., Kaifuku, Y., & Matsunobu, K. (2022). Development of Continuous Gas Generation Method for Hydrogen Chloride Using Azeotropic Hydrochloric Acid System. ChemEngineering, 6(1), 12. https://doi.org/10.3390/chemengineering6010012