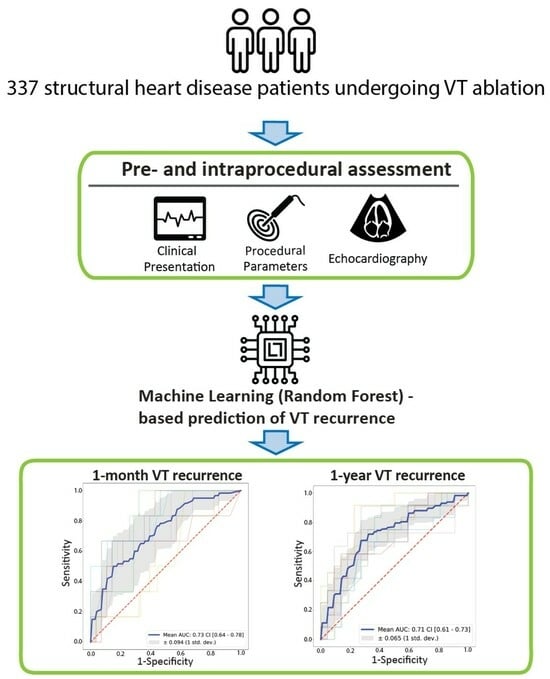
Machine-Learning-Based Prediction of 1-Year Arrhythmia Recurrence after Ventricular Tachycardia Ablation in Patients with Structural Heart Disease
Highlights
- Catheter ablation is an established treatment for ventricular tachycardia. However, procedural success varies and recurrence of the arrhythmia is not uncommon.
- Machine learning can be used to determine the key factors contributing to ventricular tachycardia recurrence.
- We designed a machine-learning pipeline capable of predicting arrhythmia recurrence within one month and one year following the procedure.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Ablation Procedure
2.3. Collected Data
2.4. Statistical Study
2.5. Machine Learning Pipeline
2.5.1. Software and Hardware
2.5.2. Establishment of Input Features
2.5.3. Data Pre-Processing
2.5.4. Feature Selection
Feature Ranking: MLP—Permutation Importance
Feature Ranking: RF—Permutation Importance
Feature Ranking: XGB—Permutation Importance
Feature Ranking: XGB—Recursive Feature Elimination (RFE)
Feature Ranking: Average (AVG)
Defining and Ranking Feature Groups
2.5.5. Model Selection
2.5.6. Model Evaluation
2.5.7. Comparison with the I-VT Score
- Age, LVEF, CRT/ICD device: as recorded in our database.
- Previous ablation: “No” in all cases, since our cohort consisted of first ablations.
- Clinical VT inducible at the end of the ablation: “Yes”, if clinical VT was inducible before ablation and it was not successfully eliminated.
- Non-clinical VT inducible at the end of ablation: “Yes”, if non-clinical VT was inducible before ablation and it was not successfully eliminated.
- No VT inducible at the end of ablation: as recorded in our database.
- Regarding the findings of the final programmed extrastimulation, the input option “Not tested” in the I-VT score refers to cases where no programmed extrastimulation (PES) was performed. Since final PES was part of our protocol, this branch of the decision tree was not used in our cohort.
3. Results
3.1. Patient Population
3.2. Machine Learning Analysis
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al-Khatib, S.M.; Granger, C.B.; Huang, Y.; Lee, K.L.; Califf, R.M.; Simoons, M.L.; Armstrong, P.W.; Van de Werf, F.; White, H.D.; Simes, R.J.; et al. Sustained Ventricular Arrhythmias among Patients with Acute Coronary Syndromes with No ST-Segment Elevation: Incidence, Predictors, and Outcomes. Circulation 2002, 106, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Klein, H.; Levine, J.H.; Saksena, S.; Waldo, A.L.; Wilber, D.; et al. Improved Survival with an Implanted Defibrillator in Patients with Coronary Disease at High Risk for Ventricular Arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N. Engl. J. Med. 1996, 335, 1933–1940. [Google Scholar] [CrossRef] [PubMed]
- Poole, J.E.; Johnson, G.W.; Hellkamp, A.S.; Anderson, J.; Callans, D.J.; Raitt, M.H.; Reddy, R.K.; Marchlinski, F.E.; Yee, R.; Guarnieri, T.; et al. Prognostic Importance of Defibrillator Shocks in Patients with Heart Failure. N. Engl. J. Med. 2008, 359, 1009–1017. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Hammash, M.H.; Kim, J.H.; Kang, K.-W.; Miller, J.; McEvedy, S.M.; Hwang, S.Y.; Moser, D.K. Implantable Cardioverter Defibrillator Shocks and Psychological Distress: Examining the Mediating Roles of Implantable Cardioverter Defibrillator-Related Concerns and Perceived Control. J. Cardiovasc. Nurs. 2020, 35, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Cairns, J.A.; Connolly, S.J.; Roberts, R.; Gent, M. Randomised Trial of Outcome after Myocardial Infarction in Patients with Frequent or Repetitive Ventricular Premature Depolarisations: CAMIAT. Lancet 1997, 349, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Sapp, J.L.; Wells, G.A.; Parkash, R.; Stevenson, W.G.; Blier, L.; Sarrazin, J.-F.; Thibault, B.; Rivard, L.; Gula, L.; Leong-Sit, P.; et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N. Engl. J. Med. 2016, 375, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Santangeli, P.; Muser, D.; Maeda, S.; Filtz, A.; Zado, E.S.; Frankel, D.S.; Dixit, S.; Epstein, A.E.; Callans, D.J.; Marchlinski, F.E. Comparative Effectiveness of Antiarrhythmic Drugs and Catheter Ablation for the Prevention of Recurrent Ventricular Tachycardia in Patients with Implantable Cardioverter-Defibrillators: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Heart Rhythm 2016, 13, 1552–1559. [Google Scholar] [CrossRef]
- Ahmed, M.; Nudy, M.; Bussa, R.; Gonzalez, M.D.; Naccarelli, G.V.; Maheshwari, A. Treatment of Ventricular Tachycardia with Catheter Ablation versus Anti-Arrhythmic Drug Titration: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Interv. Card. Electrophysiol. 2023, 66, 843–845. [Google Scholar] [CrossRef]
- Betensky, B.P.; Marchlinski, F.E. Outcomes of Catheter Ablation of Ventricular Tachycardia in the Setting of Structural Heart Disease. Curr. Cardiol. Rep. 2016, 18, 68. [Google Scholar] [CrossRef]
- Szegedi, N.; Szeplaki, G.; Merkely, B.; Geller, L. Successful Emergency Ablation of Ventricular Tachycardia in the Early Postoperative Period after Left Ventricular Assist Device Implantation. Clin. Res. Cardiol. 2015, 104, 450–452. [Google Scholar] [CrossRef]
- Tung, R.; Vaseghi, M.; Frankel, D.S.; Vergara, P.; Di Biase, L.; Nagashima, K.; Yu, R.; Vangala, S.; Tseng, C.-H.; Choi, E.-K.; et al. Freedom from Recurrent Ventricular Tachycardia after Catheter Ablation Is Associated with Improved Survival in Patients with Structural Heart Disease: An International VT Ablation Center Collaborative Group Study. Heart Rhythm 2015, 12, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Wijnmaalen, A.P.; Ebert, M.; Baldinger, S.H.; Berruezo, A.; Catto, V.; Vaseghi, M.; Arya, A.; Kumar, S.; de Riva, M.; et al. Clinical Outcomes in Patients with Dilated Cardiomyopathy and Ventricular Tachycardia. J. Am. Coll. Cardiol. 2022, 80, 1045–1056. [Google Scholar] [CrossRef]
- Cueva-Parra, A.; Neach-De La Vega, D.; Yañez-Guerrero, P.; Bustillos-García, G.; Gómez-Flores, J.; Levinstein, M.; Morales, J.L.; Iturralde-Torres, P.; Márquez, M.F.; Nava, S. Acute and long-term success of ventricular tachycardia ablation in patients with ischemic heart disease in a Mexican center. Arch. Peru Cardiol. Cirugía Cardiovasc. 2022, 3, 179–187. [Google Scholar] [CrossRef]
- Tóth, P.; Komlósi, F.; Vámosi, P.; Arnóth, B.; Szegedi, N.; Salló, Z.; Piros, K.; Perge, P.; Osztheimer, I.; Ábrahám, P.; et al. Sex Differences in One-Year Recurrence and All-Cause Mortality Following Catheter Ablation of Ventricular Tachycardia in Structural Heart Disease. Front. Cardiovasc. Med. 2022, 9, 1061471. [Google Scholar] [CrossRef] [PubMed]
- Vergara, P.; Tzou, W.S.; Tung, R.; Brombin, C.; Nonis, A.; Vaseghi, M.; Frankel, D.S.; Di Biase, L.; Tedrow, U.; Mathuria, N.; et al. Predictive Score for Identifying Survival and Recurrence Risk Profiles in Patients Undergoing Ventricular Tachycardia Ablation: The I-VT Score. Circ. Arrhythm. Electrophysiol. 2018, 11, e006730. [Google Scholar] [CrossRef] [PubMed]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. 2015 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace 2015, 17, 1601–1687. [Google Scholar] [PubMed]
- Cronin, E.M.; Bogun, F.M.; Maury, P.; Peichl, P.; Chen, M.; Namboodiri, N.; Aguinaga, L.; Leite, L.R.; Al-Khatib, S.M.; Anter, E.; et al. 2019 HRS/EHRA/APHRS/LAHRS Expert Consensus Statement on Catheter Ablation of Ventricular Arrhythmias. Europace 2019, 21, 1143–1144. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Davidson-Pilon, C. Lifelines: Survival Analysis in Python. J. Open Source Softw. 2019, 4, 1317. [Google Scholar] [CrossRef]
- van Rossum, G. Python Reference Manual; Centrum Voor Wiskunde en Informatica: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Lemaître, G.; Nogueira, F.; Aridas, C.K. Imbalanced-Learn: A Python Toolbox to Tackle the Curse of Imbalanced Datasets in Machine Learning. J. Mach. Learn. Res. 2017, 18, 559–563. [Google Scholar]
- Lundberg, S.M.; Lee, S.-I. A Unified Approach to Interpreting Model Predictions. Adv. Neural Inf. Process. Syst. 2017, 30, 4768–4777. [Google Scholar]
- Benhar, H.; Idri, A.; Fernández-Alemán, J.L. Data Preprocessing for Heart Disease Classification: A Systematic Literature Review. Comput. Methods Programs Biomed. 2020, 195, 105635. [Google Scholar] [CrossRef] [PubMed]
- Werner de Vargas, V.; Schneider Aranda, J.A.; Dos Santos Costa, R.; da Silva Pereira, P.R.; Victória Barbosa, J.L. Imbalanced Data Preprocessing Techniques for Machine Learning: A Systematic Mapping Study. Knowl. Inf. Syst. 2023, 65, 31–57. [Google Scholar] [CrossRef] [PubMed]
- Altmann, A.; Toloşi, L.; Sander, O.; Lengauer, T. Permutation Importance: A Corrected Feature Importance Measure. Bioinformatics 2010, 26, 1340–1347. [Google Scholar] [CrossRef]
- Kernbach, J.M.; Staartjes, V.E. Foundations of Machine Learning-Based Clinical Prediction Modeling: Part II-Generalization and Overfitting. Acta Neurochir. Suppl. 2022, 134, 15–21. [Google Scholar]
- Shreffler, J.; Huecker, M.R. Diagnostic Testing Accuracy: Sensitivity, Specificity, Predictive Values and Likelihood Ratios; StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Winter, E. Chapter 53 The Shapley Value. In Handbook of Game Theory with Economic Applications; Elsevier: Amsterdam, The Netherlands, 2002; Volume 3, pp. 2025–2054. [Google Scholar]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. PROC: An Open-Source Package for R and S+ to Analyze and Compare ROC Curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Yokokawa, M.; Desjardins, B.; Crawford, T.; Good, E.; Morady, F.; Bogun, F. Reasons for Recurrent Ventricular Tachycardia after Catheter Ablation of Post-Infarction Ventricular Tachycardia. J. Am. Coll. Cardiol. 2013, 61, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.Y.; Chan, Y.H.; Wang, Y.; Yeo, C.; Lim, E.T.S.; Tan, V.H. Catheter Ablation of Ventricular Arrhythmia in Patients with an Implantable Cardioverter-Defibrillator: A Systematic Review and Meta-Analysis. Can. J. Cardiol. 2022, 39, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Arenal, Á.; Hernández, J.; Calvo, D.; Ceballos, C.; Atéa, L.; Datino, T.; Atienza, F.; González-Torrecilla, E.; Eídelman, G.; Miracle, Á.; et al. Safety, Long-Term Results, and Predictors of Recurrence after Complete Endocardial Ventricular Tachycardia Substrate Ablation in Patients with Previous Myocardial Infarction. Am. J. Cardiol. 2013, 111, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, K.; Choi, E.-K.; Tedrow, U.B.; Koplan, B.A.; Michaud, G.F.; John, R.M.; Epstein, L.M.; Tokuda, M.; Inada, K.; Kumar, S.; et al. Correlates and Prognosis of Early Recurrence after Catheter Ablation for Ventricular Tachycardia Due to Structural Heart Disease. Circ. Arrhythm. Electrophysiol. 2014, 7, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Tzou, W.S.; Tung, R.; Frankel, D.S.; Di Biase, L.; Santangeli, P.; Vaseghi, M.; Bunch, T.J.; Weiss, J.P.; Tholakanahalli, V.N.; Lakkireddy, D.; et al. Outcomes after Repeat Ablation of Ventricular Tachycardia in Structural Heart Disease: An Analysis from the International VT Ablation Center Collaborative Group. Heart Rhythm 2017, 14, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Prasitlumkum, N.; Navaravong, L.; Desai, A.; Desai, D.; Cheungpasitporn, W.; Rattanawong, P.; Bunch, T.J.; Jongnarangsin, K.; Chokesuwattanaskul, R. Impact of Early Ventricular Tachycardia Ablation in Patients with an Implantable Cardioverter-Defibrillator: An Updated Systematic Review and Meta-Analysis of Randomized Controlled Trials. Heart Rhythm 2022, 19, 2054–2061. [Google Scholar] [CrossRef] [PubMed]
- Willems, S.; Tilz, R.R.; Steven, D.; Kääb, S.; Wegscheider, K.; Gellér, L.; Meyer, C.; Heeger, C.-H.; Metzner, A.; Sinner, M.F.; et al. Preventive or Deferred Ablation of Ventricular Tachycardia in Patients With Ischemic Cardiomyopathy and Implantable Defibrillator (BERLIN VT): A Multicenter Randomized Trial. Circulation 2020, 141, 1057–1067. [Google Scholar] [CrossRef]
- Kuck, K.-H.; Tilz, R.R.; Deneke, T.; Hoffmann, B.A.; Ventura, R.; Hansen, P.S.; Zarse, M.; Hohnloser, S.H.; Kautzner, J.; Willems, S.; et al. Impact of Substrate Modification by Catheter Ablation on Implantable Cardioverter-Defibrillator Interventions in Patients with Unstable Ventricular Arrhythmias and Coronary Artery Disease: Results from the Multicenter Randomized Controlled SMS (Substrate Modification Study): Results from the Multicenter Randomized Controlled SMS (Substrate Modification Study). Circ. Arrhythm. Electrophysiol. 2017, 10, e004422. [Google Scholar] [CrossRef]
- Tung, R.; Josephson, M.E.; Reddy, V.; Reynolds, M.R. SMASH-VT Investigators Influence of Clinical and Procedural Predictors on Ventricular Tachycardia Ablation Outcomes: An Analysis from the Substrate Mapping and Ablation in Sinus Rhythm to Halt Ventricular Tachycardia Trial (SMASH-VT). J. Cardiovasc. Electrophysiol. 2010, 21, 799–803. [Google Scholar] [CrossRef]
- Frankel, D.S.; Mountantonakis, S.E.; Zado, E.S.; Anter, E.; Bala, R.; Cooper, J.M.; Deo, R.; Dixit, S.; Epstein, A.E.; Garcia, F.C.; et al. Noninvasive Programmed Ventricular Stimulation Early after Ventricular Tachycardia Ablation to Predict Risk of Late Recurrence. J. Am. Coll. Cardiol. 2012, 59, 1529–1535. [Google Scholar] [CrossRef]
- Haanschoten, D.M.; Smit, J.J.J.; Adiyaman, A.; Ramdat Misier, A.R.; Hm Delnoy, P.P.; Elvan, A. Long-Term Outcome of Catheter Ablation in Post-Infarction Recurrent Ventricular Tachycardia. Scand. Cardiovasc. J. 2019, 53, 62–70. [Google Scholar] [CrossRef]
- Kakadiaris, I.A.; Vrigkas, M.; Yen, A.A.; Kuznetsova, T.; Budoff, M.; Naghavi, M. Machine Learning Outperforms ACC/AHA CVD Risk Calculator in MESA. J. Am. Heart Assoc. 2018, 7, e009476. [Google Scholar] [CrossRef]
- Bazoukis, G.; Stavrakis, S.; Zhou, J.; Bollepalli, S.C.; Tse, G.; Zhang, Q.; Singh, J.P.; Armoundas, A.A. Machine Learning versus Conventional Clinical Methods in Guiding Management of Heart Failure Patients-a Systematic Review. Heart Fail. Rev. 2021, 26, 23–34. [Google Scholar] [CrossRef]







| Data Available N (%) | All Patients N = 337 (100%) | 1-Month VT Recurrence | 1-Year VT Recurrence | |||||
|---|---|---|---|---|---|---|---|---|
| 1-Month VT Recurrence N = 60 (18%) | No 1-Month VT Recurrence N = 277 (82%) | p | 1-Year VT Recurrence N = 117 (35%) | No 1-Year VT Recurrence N = 220 (65%) | p | |||
| Age | 337 (100%) | 68.7 (60.2–74.8) | 68.2 (61.6–76.1) | 68.7 (60.1–74.8) | 0.619 | 67.5 (61.1–74.7) | 68.9 (59.3–75.0) | 0.934 |
| Male | 337 (100%) | 295 (88%) | 51 (85%) | 244 (88%) | 0.659 | 102 (87%) | 192 (88%) | 1.000 |
| Atrial fibrillation | 337 (100%) | 109 (32%) | 25 (42%) | 84 (30%) | 0.126 | 45 (38%) | 64 (29%) | 0.109 |
| Hypertension | 337 (100%) | 252 (75%) | 43 (72%) | 209 (76%) | 0.622 | 83 (71%) | 169 (77%) | 0.261 |
| Diabetes | 337 (100%) | 113 (34%) | 18 (30%) | 95 (34%) | 0.613 | 43 (37%) | 70 (32%) | 0.445 |
| COPD | 337 (100%) | 42 (12%) | 8 (13%) | 34 (12%) | 1.000 | 13 (11%) | 29 (13%) | 0.697 |
| CAD | 337 (100%) | 283 (84%) | 48 (80%) | 235 (85%) | 0.426 | 95 (81%) | 188 (86%) | 0.339 |
| ICD | 337 (100%) | 249 (74%) | 47 (78%) | 202 (73%) | 0.508 | 95 (81%) | 154 (70%) | 0.042 * |
| CRT | 337 (100%) | 80 (24%) | 20 (33%) | 60 (22%) | 0.081 | 42 (36%) | 38 (17%) | <0.001 * |
| HF | 331 (98%) | 272 (82%) | 53 (88%) | 219 (81%) | 0.234 | 100 (85%) | 172 (80%) | 0.314 |
| NYHA | 306 (91%) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.120 | 2 (1–3) | 2 (1–3) | 0.027* |
| SCD | 337 (100%) | 61 (18%) | 12 (20%) | 49 (18%) | 0.823 | 19 (16%) | 42 (19%) | 0.605 |
| EF | 305 (91%) | 34 (27–42) | 30 (25–35) | 35 (27.2–43) | 0.012 * | 33 (25–38) | 35 (28–44.2) | 0.008 * |
| LVESD | 283 (84%) | 50 (42–57) | 54 (47–60) | 48.5 (41–56) | 0.011 * | 53 (47–59) | 47 (41–54) | <0.001 * |
| TAPSE | 264 (78%) | 19 (15–23) | 17 (14–20) | 19 (16–23) | 0.019 * | 18 (14–20.2) | 19 (16–23) | 0.028 * |
| E wave DT | 262 (78%) | 162 (133–213) | 150 (123–193) | 165 (137–220) | 0.083 | 150 (127–200) | 170 (140–220) | 0.008 * |
| MR | 299 (89%) | 2 (1–2) | 2 (1–3) | 2 (1–2) | 0.036 * | 2 (1–3) | 2 (1–2) | 0.007 * |
| TR III-IV | 278 (82%) | 34 (12%) | 8 (15%) | 26 (12%) | 0.592 | 13 (13%) | 21 (12%) | 0.881 |
| Amiodarone | 331 (98%) | 233 (70%) | 47 (80%) | 186 (68%) | 0.118 | 88 (76%) | 145 (67%) | 0.140 |
| Beta blocker | 331 (98%) | 302 (91%) | 53 (90%) | 249 (92%) | 0.867 | 106 (91%) | 196 (91%) | 1.000 |
| ICD shock | 333 (99%) | 140 (42%) | 33 (57%) | 107 (39%) | 0.018 * | 60 (52%) | 80 (37%) | 0.009 * |
| HD instability | 337 (100%) | 132 (39%) | 36 (60%) | 96 (35%) | 0.001 * | 58 (50%) | 74 (34%) | 0.007 * |
| Incessant VT | 337 (100%) | 114 (34%) | 30 (50%) | 84 (30%) | 0.006 * | 52 (44%) | 62 (28%) | 0.004 * |
| Electrical storm | 336 (100%) | 135 (40%) | 35 (58%) | 100 (36%) | 0.003 * | 57 (49%) | 78 (36%) | 0.027 * |
| Inducible VT morphologies | 334 (99%) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.166 | 1 (1–2) | 1 (1–2) | 0.001 * |
| Clinical VT inducible | 333 (99%) | 261 (78%) | 49 (83%) | 212 (77%) | 0.431 | 104 (90%) | 157 (72%) | <0.001 * |
| Non-clinical VT(s) inducible | 332 (99%) | 106 (32%) | 21 (36%) | 85 (31%) | 0.609 | 38 (33%) | 68 (31%) | 0.909 |
| Clinical VT cycle length | 268 (80%) | 400 (340–460) | 400 (342–450) | 400 (340–460) | 0.828 | 400 (354–458) | 386 (333–458) | 0.114 |
| Clinical VT eliminated | 321 (95%) | 284 (88%) | 44 (79%) | 240 (91%) | 0.020 * | 100 (88%) | 184 (88%) | 1.000 |
| All VTs eliminated | 322 (96%) | 255 (79%) | 40 (70%) | 215 (81%) | 0.095 | 91 (80%) | 164 (79%) | 0.950 |
| Major complications | 334 (99%) | 32 (10%) | 10 (17%) | 22 (8%) | 0.061 | 15 (13%) | 17 (8%) | 0.186 |
| 1-Month VT Recurrence | |||||
| AVG | XGB, RFE | MLP, PI | RF, PI | XGB, PI | |
| HD instability | 1 | 0 | 0 | 0 | 6 |
| LVEF | 2 | 1 | 4 | 2 | 1 |
| TAPSE | 3 | 7 | 1 | 3 | 4 |
| Age | 4 | 3 | 13 | 4 | 0 |
| Clinical VT eliminated | 5 | 2 | 3 | 5 | 12 |
| LVESD | 6 | 4 | 12 | 1 | 5 |
| ICD shock | 7 | 9 | 2 | 7 | 9 |
| Clinical VT cycle length | 8 | 6 | 14 | 6 | 2 |
| E wave DT | 9 | 5 | 10 | 11 | 3 |
| Electrical storm | 10 | 8 | 6 | 9 | 8 |
| MR | 11 | 10 | 8 | 8 | 10 |
| Incessant VT | 12 | 11 | 5 | 13 | 11 |
| Inducible VT morphologies | 13 | 16 | 7 | 10 | 13 |
| NYHA | 14 | 13 | 15 | 15 | 7 |
| All VTs eliminated | 15 | 12 | 11 | 14 | 15 |
| Atrial fibrillation | 16 | 15 | 9 | 16 | 14 |
| TR III-IV | 17 | 14 | 16 | 12 | 16 |
| 1-year VT recurrence | |||||
| AVG | XGB, RFE | MLP, PI | RF, PI | XGB, PI | |
| LVESD | 1 | 1 | 4 | 0 | 0 |
| Inducible VT morphologies | 2 | 0 | 0 | 1 | 7 |
| MR | 3 | 4 | 1 | 2 | 6 |
| ICD shock | 4 | 6 | 2 | 3 | 9 |
| Age | 5 | 2 | 13 | 5 | 1 |
| E wave DT | 6 | 5 | 6 | 7 | 3 |
| LVEF | 7 | 3 | 12 | 8 | 2 |
| HD instability | 8 | 8 | 3 | 4 | 10 |
| TAPSE | 9 | 7 | 7 | 9 | 4 |
| Incessant VT | 10 | 9 | 5 | 11 | 11 |
| Clinical VT cycle length | 11 | 10 | 16 | 6 | 5 |
| NYHA | 12 | 11 | 8 | 10 | 8 |
| All VTs eliminated | 13 | 13 | 10 | 12 | 13 |
| TR III-IV | 14 | 12 | 14 | 13 | 12 |
| Electrical storm | 15 | 14 | 9 | 16 | 14 |
| Atrial fibrillation | 16 | 15 | 11 | 14 | 15 |
| Clinical VT eliminated | 17 | 16 | 15 | 15 | 16 |
| 1-month VT recurrence | Model | Pre-Processing | Oversampling | Hyperparameters | Mean AUC (Test) |
| RF | Not used | Not used | RF__class_weight: None; RF__criterion: log_loss; RF__max_depth: 1; RF__max_features: 1; RF__min_samples_leaf: 2; RF__n_estimators: 300; RF__random_state: 0 | 0.730 | |
| MLP | SS | SMOTE | NN__activation: logistic; NN__alpha: 0.00225; NN__hidden_layer_sizes: 3; NN__max_iter: 161; NN__random_state: 0; NN__solver: adam | 0.729 | |
| XGB | Not used | Not used | XGB__alpha: 7.99; XGB__colsample_bytree: 1.0; XGB__gamma: 0.1; XGB__max_depth: 3; XGB__min_child_weight: 0.0; XGB__random_state: 0; XGB__scale_pos_weight: 2.0; XGB__subsample: 0.5 | 0.708 | |
| 1-year VT recurrence | Model | Pre-processing | Oversampling | Hyperparameters | mean AUC (test) |
| RF | SS | SMOTE | RF__class_weight: balanced_subsample; RF__criterion: gini; RF__max_depth: 2; RF__max_features: 7; RF__min_samples_leaf: 2; RF__n_estimators: 320; RF__random_state: 100 | 0.713 | |
| XGB | Not used | Not used | XGB__colsample_bytree: 1.0; XGB__gamma: 9.79; XGB__max_depth: 3; XGB__min_child_weight: 0.0; XGB__random_state: 0; XGB__scale_pos_weight: 3.14; XGB__subsample: 0.897 | 0.711 | |
| MLP | SS | SMOTE | NN__activation: logistic; NN__alpha: 0.000192; NN__hidden_layer_sizes: 50; NN__max_iter: 50; NN__random_state: 0; NN__solver: adam | 0.709 |
| Model Type | Scaling | Pre-Processing | AUC (Test) | AUC (Train) | Accuracy | Sensitivity | Specificity | LR+ | LR− | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1-month | Random forest | Not used | Not used | 0.730 | 0.758 | 0.68 | 0.63 | 0.7 | 3.2 | 0.53 |
| 1-year | Random forest | SS | SMOTE | 0.713 | 0.751 | 0.71 | 0.61 | 0.77 | 2.8 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komlósi, F.; Tóth, P.; Bohus, G.; Vámosi, P.; Tokodi, M.; Szegedi, N.; Salló, Z.; Piros, K.; Perge, P.; Osztheimer, I.; et al. Machine-Learning-Based Prediction of 1-Year Arrhythmia Recurrence after Ventricular Tachycardia Ablation in Patients with Structural Heart Disease. Bioengineering 2023, 10, 1386. https://doi.org/10.3390/bioengineering10121386
Komlósi F, Tóth P, Bohus G, Vámosi P, Tokodi M, Szegedi N, Salló Z, Piros K, Perge P, Osztheimer I, et al. Machine-Learning-Based Prediction of 1-Year Arrhythmia Recurrence after Ventricular Tachycardia Ablation in Patients with Structural Heart Disease. Bioengineering. 2023; 10(12):1386. https://doi.org/10.3390/bioengineering10121386
Chicago/Turabian StyleKomlósi, Ferenc, Patrik Tóth, Gyula Bohus, Péter Vámosi, Márton Tokodi, Nándor Szegedi, Zoltán Salló, Katalin Piros, Péter Perge, István Osztheimer, and et al. 2023. "Machine-Learning-Based Prediction of 1-Year Arrhythmia Recurrence after Ventricular Tachycardia Ablation in Patients with Structural Heart Disease" Bioengineering 10, no. 12: 1386. https://doi.org/10.3390/bioengineering10121386
APA StyleKomlósi, F., Tóth, P., Bohus, G., Vámosi, P., Tokodi, M., Szegedi, N., Salló, Z., Piros, K., Perge, P., Osztheimer, I., Ábrahám, P., Széplaki, G., Merkely, B., Gellér, L., & Nagy, K. V. (2023). Machine-Learning-Based Prediction of 1-Year Arrhythmia Recurrence after Ventricular Tachycardia Ablation in Patients with Structural Heart Disease. Bioengineering, 10(12), 1386. https://doi.org/10.3390/bioengineering10121386