Primary Hepatocyte Isolation and Cultures: Technical Aspects, Challenges and Advancements
Abstract
:1. Introduction
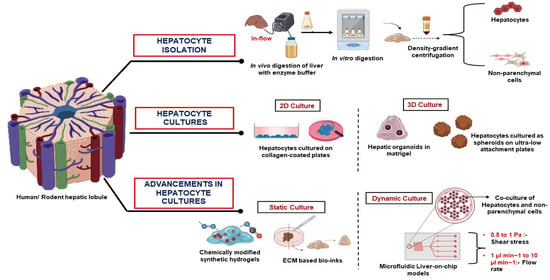
2. Isolation of Primary Hepatocytes
3. 2D Culture Models for Primary Hepatocytes
4. Strategies to Improve Hepatocyte Cultures
4.1. 3D Cultures of Hepatocytes
4.2. Co-Culture of Hepatocytes with NPCs
4.3. Liver-on-Chip Models for Hepatocyte Cultures
5. Media Components in Hepatocyte Cultures
6. Characterization of Primary Hepatocytes
7. Liver Zonation: Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersson, T.B.; Ingelman-Sundberg, M.; Heins, N. Hepatocyte-like cells derived from human embryonic stem cells specifically via definitive endoderm and a progenitor stage. J. Biotechnol. 2010, 145, 284–294. [Google Scholar]
- Duan, Y.Y.; Catana, A.; Meng, Y.; Yamamoto, N.; He, S.Q.; Gupta, S.; Gambhir, S.S.; Zerna, M.A. Differentiation and enrichment of hepatocyte-like cells from human embryonic stem cells in vitro and in vivo. Stem Cells 2007, 25, 3058–3068. [Google Scholar] [CrossRef] [PubMed]
- Lavon, N.; Yanuka, O.; Benvenisty, N. Differentiation and isolation of hepatic-like cells from human embryonic stem cells. Differentiation 2004, 72, 230–238. [Google Scholar] [CrossRef]
- McGill, M.R.K.; Yan, H.M.; Ramachandran, A.; Murray, G.J.; Rollins, D.E.; Jaeschke, H. HepaRG cells: A human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology 2011, 53, 974–982. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, Y.; Sasaki, Y.; Terasaki, N.; Kawataki, T.; Takekawa, K.; Iwase, Y.; Shimizu, T.; Sanoh, S.; Ohta, S. Comparison of Drug Metabolism and Its Related Hepatotoxic Effects in HepaRG, Cryopreserved Human Hepatocytes, and HepG2 Cell Cultures. Biol Pharm. Bull. 2018, 41, 722–732. [Google Scholar] [CrossRef] [Green Version]
- Bell, C.C.; Dankers, A.C.A.; Lauschke, V.M.; Sison-Young, R.; Jenkins, R.; Rowe, C.; Goldring, C.E.; Park, K.; Regan, S.L.; Walker, T.; et al. Comparison of Hepatic 2D Sandwich Cultures and 3D Spheroids for Long-term Toxicity Applications: A Multicenter Study. Toxicol. Sci. 2018, 162, 655–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, K.N.; Lim, W.S.; Zhang, P.; Lu, H.; Wen, J.; Ramakrishna, S.; Mao, H.Q. Stable immobilization of rat hepatocyte spheroids on galactosylated nanofiber scaffold. Biomaterials 2005, 26, 2537–2547. [Google Scholar] [CrossRef]
- Chien, H.W.; Lai, J.Y.; Tsai, W.B. Galactosylated electrospun membranes for hepatocyte sandwich culture. Coll. Surf. B Biointerfaces 2014, 116, 576–581. [Google Scholar] [CrossRef]
- Ghahremanzadeh, F.; Alihosseini, F.; Semnani, D. Investigation and comparison of new galactosylation methods on PCL/chitosan scaffolds for enhanced liver tissue engineering. Int. J. Biol. Macromol. 2021, 174, 278–288. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Shang, Y.; Yuan, Y.; Yang, J. Preparation and characterization of chitosan/galactosylated hyaluronic acid scaffolds for primary hepatocytes culture. J. Mater. Sci. Mater. Med. 2010, 21, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.N.; Tang, Y.N.; Quek, C.H.; Ramakrishna, S.; Leong, K.W.; Mao, H.Q. A dual-functional fibrous scaffold enhances P450 activity of cultured primary rat hepatocytes. Acta Biomater. 2007, 3, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Berry, M. Metabolic properties of cells isolated from adult mouse liver. J. Cell Biol. 1962, 15, 1–8. [Google Scholar] [CrossRef]
- Berry, M.; Friend, D. High-yield preparation of isolated rat liver parenchymal cells: A biochemical and fine structural study. J. Cell Biol. 1969, 43, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Howard, R.B.; Pesch, L.A. Respiratory activity of intact, isolated parenchymal cells from rat liver. J. Biol. Chem. 1968, 243, 3105–3109. [Google Scholar] [CrossRef]
- Howard, R.B.; Christensen, A.K.; Gibbs, F.A.; Pesch, L.A. The enzymatic preparation of isolated intact parenchymal cells from rat liver. J. Cell Biol. 1967, 35, 675–684. [Google Scholar] [CrossRef] [Green Version]
- Seglen, P.O. Preparation of rat liver cells. I. Effect of Ca2+ on enzymatic dispersion of isolated, perfused liver. Exp. Cell Res. 1972, 74, 450–454. [Google Scholar] [CrossRef]
- Bojar, H.; Basler, M.; Fuchs, F.; Dreyfurst, R.; Staib, W.; Broelsch, C. Preparation of parenchymal and non-parenchymal cells from adult human liver--morphological and biochemical characteristics. J. Clin. Chem. Clin. Biochem. 1976, 14, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Guguen-Guillouzo, C.; Campion, J.P.; Brissot, P.; Glaise, D.; Launois, B.; Bourel, M.; Guillouzo, A. High yield preparation of isolated human adult hepatocytes by enzymatic perfusion of the liver. Cell Biol. Int. Rep. 1982, 6, 625–628. [Google Scholar] [CrossRef]
- Strom, S.C.; Jirtle, R.L.; Jones, R.S.; Novicki, D.L.; Rosenberg, M.R.; Novotny, A.; Irons, G.; McLain, J.R.; Michalopoulos, G. Isolation, culture, and transplantation of human hepatocytes. J. Natl. Cancer Inst. 1982, 68, 771–778. [Google Scholar]
- Reese, J.A.; Byard, J.L. Isolation and culture of adult hepatocytes from liver biopsies. In Vitro 1981, 17, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Schelcher, C.; Demmel, M.; Hauner, M.; Thasler, W.E. Isolation of human hepatocytes by a two-step collagenase perfusion procedure. J. Vis. Exp. 2013, 79, 50615. [Google Scholar]
- Green, C.J.; Charlton, C.A.; Wang, L.M.; Silva, M.; Morten, K.J.; Hodson, L. The isolation of primary hepatocytes from human tissue: Optimising the use of small non-encapsulated liver resection surplus. Cell Tissue Bank 2017, 18, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Kin, T.; Johnson, P.R.; Shapiro, A.M.; Lakey, J.R. Factors influencing the collagenase digestion phase of human islet isolation. Transplantation 2007, 83, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.R.; White, S.A.; London, N.J. Collagenase and human islet isolation. Cell Transpl. 1996, 5, 437–452. [Google Scholar] [CrossRef]
- Bale, S.S.; Geerts, S.; Jindal, R.; Yarmush, M.L. Isolation and co-culture of rat parenchymal and non-parenchymal liver cells to evaluate cellular interactions and response. Sci. Rep. 2016, 6, 25329. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; He, Y.; Bharadwaj, S.; Hammam, N.; Carnagey, K.; Myers, R.; Atala, A.; Van Dyke, M. Tissue-specific extracellular matrix coatings for the promotion of cell proliferation and maintenance of cell phenotype. Biomaterials 2009, 30, 4021–4028. [Google Scholar] [CrossRef]
- Rajendran, D.; Hussain, A.; Yip, D.; Parekh, A.; Shrirao, A.; Cho, C.H. Long-term liver-specific functions of hepatocytes in electrospun chitosan nanofiber scaffolds coated with fibronectin. J. Biomed. Mater. Res. Part A 2017, 105, 2119–2128. [Google Scholar] [CrossRef]
- Hirata, K.; Yoshida, Y.; Shiramatsu, K.; Freeman, A.E.; Hayasaka, H. Effects of laminin, fibronectin and type IV collagen on liver cell cultures. Pathobiology 1983, 51, 121–129. [Google Scholar] [CrossRef]
- Dunn, J.C.; Tompkins, R.G.; Yarmush, M.L. Hepatocytes in collagen sandwich: Evidence for transcriptional and translational regulation. J. Cell Biol. 1992, 116, 1043–1053. [Google Scholar] [CrossRef]
- Kim, Y.; Lasher, C.D.; Milford, L.M.; Murali, T.M.; Rajagopalan, P. A comparative study of genome-wide transcriptional profiles of primary hepatocytes in collagen sandwich and monolayer cultures. Tissue Eng. Part C Methods 2010, 16, 1449–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deharde, D.; Schneider, C.; Hiller, T.; Fischer, N.; Kegel, V.; Lübberstedt, M.; Freyer, N.; Hengstler, J.G.; Andersson, T.B.; Seehofer, D.; et al. Bile canaliculi formation and biliary transport in 3D sandwich-cultured hepatocytes in dependence of the extracellular matrix composition. Arch. Toxicol. 2016, 90, 2497–2511. [Google Scholar] [CrossRef]
- Foster, E.; You, J.; Siltanen, C.; Patel, D.; Haque, A.; Anderson, L.; Revzin, A. Heparin hydrogel sandwich cultures of primary hepatocytes. Eur. Polym. J. 2015, 72, 726–735. [Google Scholar] [CrossRef]
- Chang, T.T.; Hughes-Fulford, M. Molecular mechanisms underlying the enhanced functions of three-dimensional hepatocyte aggregates. Biomaterials 2014, 35, 2162–2171. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.F.; Choi, Y.Y.; Kim, D.S.; Chung, B.G.; Lee, S.H. Concave microwell based size-controllable hepatosphere as a three-dimensional liver tissue model. Biomaterials 2011, 32, 8087–8096. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Ezan, F.; Cuvellier, M.; Bruyère, A.; Legagneux, V.; Langouët, S.; Baffet, G. Generation of proliferating human adult hepatocytes using optimized 3D culture conditions. Sci. Rep. 2021, 11, 515. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, S.P.; Costa, R.; Estrada, M.; Shevchenko, V.; Brito, C.; Alves, P.M. HepaRG microencapsulated spheroids in DMSO-free culture: Novel culturing approaches for enhanced xenobiotic and biosynthetic metabolism. Arch. Toxicol. 2015, 89, 1347–1358. [Google Scholar] [CrossRef]
- MacPherson, D.; Bram, Y.; Park, J.; Schwartz, R.E. Peptide-based scaffolds for the culture and maintenance of primary human hepatocytes. Sci. Rep. 2021, 11, 6772. [Google Scholar] [CrossRef]
- Biswas, S.; Vasudevan, A.; Yadav, N.; Yadav, S.; Rawal, P.; Kaur, I.; Tripathi, D.M.; Kaur, S.; Chauhan, V.S. Chemically Modified Dipeptide Based Hydrogel Supports Three-Dimensional Growth and Functions of Primary Hepatocytes. ACS Appl. Bio Mater. 2022, 5, 4354–4365. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Xue, R.; Li, T.; Leng, L.; Wang, Y.; Wang, J.; Ma, J.; Yan, J.; Yan, F.; et al. Liver extracellular matrices bioactivated hepatic spheroids as a model system for drug hepatotoxicity evaluations. Adv. Biosyst. 2018, 2, 1870091. [Google Scholar] [CrossRef] [Green Version]
- Messina, A.; Luce, E.; Hussein, M.; Dubart-Kupperschmitt, A. Pluripotent-stem-cell-derived hepatic cells: Hepatocytes and organoids for liver therapy and regeneration. Cells 2020, 9, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pettinato, G.; Lehoux, S.; Ramanathan, R.; Salem, M.M.; He, L.X.; Muse, O.; Flaumenhaft, R.; Thompson, M.T.; Rouse, E.A.; Cummings, R.D.; et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with Endothelial Cells. Sci. Rep. 2019, 9, 8920. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.C.; Kraaier, L.J.; Kluiver, T.A. Hepatocyte organoids and cell transplantation: What the future holds. Exp. Mol. Med. 2021, 53, 1512–1528. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gehart, H.; Artegiani, B.; LÖpez-Iglesias, C.; Dekkers, F.; Basak, O.; van Es, J.; de Sousa Lopes, S.M.; Begthel, H.; Korving, J.; et al. Long-term expansion of functional mouse and human hepatocytes as 3D organoids. Cell 2018, 175, 1591–1606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huch, M.; Dorrell, C.; Boj, S.F.; Van Es, J.H.; Li, V.S.; Van De Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef] [Green Version]
- Huch, M.; Gehart, H.; Van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.; Ellis, E.; Van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-term culture of genome-stable bipotent stem cells from adult human liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef] [Green Version]
- Peng, W.C.; Logan, C.Y.; Fish, M.; Anbarchian, T.; Aguisanda, F.; Álvarez-Varela, A.; Wu, P.; Jin, Y.; Zhu, J.; Li, B.; et al. Inflammatory cytokine TNFα promotes the long-term expansion of primary hepatocytes in 3D culture. Cell 2018, 175, 1607–1619. [Google Scholar] [CrossRef] [Green Version]
- Gamboa, C.M.; Wang, Y.; Xu, H.; Kalemba, K.; Wondisford, F.E.; Sabaawy, H.E. Optimized 3D culture of hepatic cells for liver organoid metabolic assays. Cells 2021, 10, 3280. [Google Scholar] [CrossRef]
- Clement, B.; Guguen-Guillouzo, C.; Campion, J.P.; Glaise, D.; Bourel, M.; Guillouzo, A. Long-term co-cultures of adult human hepatocytes with rat liver epithelial cells: Modulation of albumin secretion and accumulation of extracellular material. Hepatology 1984, 4, 373–380. [Google Scholar] [CrossRef]
- Sengupta, S.; Johnson, B.; Seirup, M.; Ardalani, H.; Duffin, B.; Barrett-Wilt, G.A.; Stewart, R.; Thomson, J.A. Co-culture with mouse embryonic fibroblasts improves maintenance of metabolic function of human small hepatocyte progenitor cells. Curr. Res. Toxicol. 2020, 1, 70–84. [Google Scholar] [CrossRef]
- Guguen-Guillouzo, C.; Clément, B.; Baffet, G.; Beaumont, C.; Morel-Chany, E.; Glaise, D.; Guillouzo, A. Maintenance and reversibility of active albumin secretion by adult rat hepatocytes co-cultured with another liver epithelial cell type. Exp. Cell Res. 1983, 143, 47–54. [Google Scholar] [CrossRef]
- Bhandari, R.N.; Riccalton, L.A.; Lewis, A.L.; Fry, J.R.; Hammond, A.H.; Tendler, S.J.; Shakesheff, K.M. Liver tissue engineering: A role for co-culture systems in modifying hepatocyte function and viability. Tissue Eng. 2001, 7, 345–357. [Google Scholar] [CrossRef]
- Cho, C.H.; Berthiaume, F.; Tilles, A.W.; Yarmush, M.L. A new technique for primary hepatocyte expansion in vitro. Biotechnol. Bioeng. 2008, 101, 345–356. [Google Scholar] [CrossRef]
- Riccalton-Banks, L.; Liew, C.; Bhandari, R.; Fry, J.; Shakesheff, K. Long-term culture of functional liver tissue: Three-dimensional coculture of primary hepatocytes and stellate cells. Tissue Eng. 2003, 9, 401–410. [Google Scholar] [CrossRef]
- Krause, P.; Saghatolislam, F.; Koenig, S.; Unthan-Fechner, K.; Probst, I. Maintaining hepatocyte differentiation in vitro through co-culture with hepatic stellate cells. Vitr. Cell. Dev. Biol. Anim. 2009, 45, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Bhatia, S.N.; Yarmush, M.L.; Toner, M. Controlling cell interactions by micropatterning in co-cultures: Hepatocytes and 3T3 fibroblasts. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1997, 34, 189–199. [Google Scholar] [CrossRef]
- Bhatia, S.N.; Balis, U.J.; Yarmush, M.L.; Toner, M.J. Effect of cell–cell interactions in preservation of cellular phenotype: Cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999, 13, 1883–1900. [Google Scholar] [CrossRef] [Green Version]
- Bell, C.C.; Chouhan, B.; Andersson, L.C.; Andersson, H.; Dear, J.W.; Williams, D.P.; Söderberg, M. Functionality of primary hepatic non-parenchymal cells in a 3D spheroid model and contribution to acetaminophen hepatotoxicity. Arch. Toxicol. 2020, 94, 1251–1263. [Google Scholar] [CrossRef] [Green Version]
- Taymour, R.; Kilian, D.; Ahlfeld, T.; Gelinsky, M.; Lode, A. 3D bioprinting of hepatocytes: Core–shell structured co-cultures with fibroblasts for enhanced functionality. Sci. Rep. 2021, 11, 5130. [Google Scholar] [CrossRef]
- Shoemaker, J.T.; Zhang, W.; Atlas, S.I.; Bryan, R.A.; Inman, S.W.; Vukasinovic, J. A 3D cell culture organ-on-a-chip platform with a breathable hemoglobin analogue augments and extends primary human hepatocyte functions in vitro. Front. Mol. Biosci. 2020, 7, 296. [Google Scholar] [CrossRef]
- Schneider, W.C.; Potter, V.R. The assay of animal tissues for respiratory enzymes: II. Succinic dehydrogenase and cytochrome oxidase. J. Biol. Chem. 1943, 149, 217–227. [Google Scholar] [CrossRef]
- Seglen, P.O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976, 13, 29–83. [Google Scholar]
- Selden, C.; Casbard, A.; Themis, M.; Hodgson, H.J. Characterization of long-term survival of syngeneic hepatocytes in rat peritoneum. Cell Transplant. 2003, 12, 569–578. [Google Scholar] [CrossRef] [Green Version]
- Abu-Absi, S.F.; Hansen, L.K.; Hu, W.S. Three-dimensional co-culture of hepatocytes and stellate cells. Cytotechnology 2004, 45, 125–140. [Google Scholar] [CrossRef]
- Allen, J.W.; Bhatia, S.N. Formation of steady-state oxygen gradients in vitro: Application to liver zonation. Biotechnol. Bioeng. 2003, 82, 253–262. [Google Scholar] [CrossRef]
- McCarty, W.J.; Usta, O.B.; Yarmush, M.L. A Microfabricated Platform for Generating Physiologically-Relevant Hepatocyte Zonation. Sci. Rep. 2016, 6, 26868. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Kim, D.; Lee, S.H.; Sung, J.H. Microtechnology-based in vitro models: Mimicking liver function and pathophysiology. APL Bioeng. 2021, 5, 041505. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yamato, M.; Okano, T.; Kitamori, T.; Sato, K. Evaluation of effects of shear stress on hepatocytes by a microchip-based system. Meas. Sci. Technol. 2006, 17, 3167. [Google Scholar] [CrossRef]
- Vinci, B.; Duret, C.; Klieber, S.; Gerbal-Chaloin, S.; Sa-Cunha, A.; Laporte, S.; Suc, B.; Maurel, P.; Ahluwalia, A.; Daujat-Chavanieu, M. Modular bioreactor for primary human hepatocyte culture: Medium flow stimulates expression and activity of detoxification genes. Biotechnol. J. 2011, 6, 554–564. [Google Scholar] [CrossRef] [Green Version]
- Rashidi, H.; Alhaque, S.; Szkolnicka, D.; Flint, O.; Hay, D.C. Fluid shear stress modulation of hepatocyte-like cell function. Arch. Toxicol. 2016, 90, 1757–1761. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.J.; Hung, P.J.; Lee, L.P. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol. Bioeng. 2007, 97, 1340–1346. [Google Scholar] [CrossRef] [PubMed]
- Toh, Y.C.; Lim, T.C.; Tai, D.; Xiao, G.; van Noort, D.; Yu, H. A microfluidic 3D hepatocyte chip for drug toxicity testing. Lab Chip 2009, 9, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- Goral, V.N.; Hsieh, Y.C.; Petzold, O.N.; Clark, J.S.; Yuen, P.K.; Faris, R.A. Perfusion-based microfluidic device for three-dimensional dynamic primary human hepatocyte cell culture in the absence of biological or synthetic matrices or coagulants. Lab Chip 2010, 10, 3380–3386. [Google Scholar] [CrossRef]
- Banaeiyan, A.A.; Theobald, J.; Paukštyte, J.; Wölfl, S.; Adiels, C.B.; Goksör, M. Design and fabrication of a scalable liver-lobule-on-a-chip microphysiological platform. Biofabrication 2017, 9, 015014. [Google Scholar] [CrossRef] [PubMed]
- Esch, M.B.; Prot, J.M.; Wang, Y.I.; Miller, P.; Llamas-Vidales, J.R.; Naughton, B.A.; Applegate, D.R.; Shuler, M.L. Multi-cellular 3D human primary liver cell culture elevates metabolic activity under fluidic flow. Lab Chip 2015, 15, 2269–2277. [Google Scholar] [CrossRef] [Green Version]
- Prodanov, L.; Jindal, R.; Bale, S.S.; Hegde, M.; McCarty, W.J.; Golberg, I.; Bhushan, A.; Yarmush, M.L.; Usta, O.B. Long-term maintenance of a microfluidic 3D human liver sinusoid. Biotechnol. Bioeng. 2016, 113, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Jang, K.J.; Otieno, M.A.; Ronxhi, J.; Lim, H.K.; Ewart, L.; Kodella, K.R.; Petropolis, D.B.; Kulkarni, G.; Rubins, J.E.; Conegliano, D.; et al. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci. Transl. Med. 2019, 11, eaax5516. [Google Scholar] [CrossRef] [PubMed]
- Schepers, A.; Li, C.; Chhabra, A.; Seney, B.T.; Bhatia, S. Engineering a perfusable 3D human liver platform from iPS cells. Lab Chip 2016, 16, 2644–2653. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Zhang, X.; Chen, Z.; Luo, Y.; Lu, Y.; Liu, T.; Wu, Z.; Jin, Y.; Zhao, W.; Lin, B. A cell lines derived microfluidic liver model for investigation of hepatotoxicity induced by drug-drug interaction. Biomicrofluidics 2019, 13, 024101. [Google Scholar] [CrossRef]
- Gröger, M.; Rennert, K.; Giszas, B.; Weiß, E.; Dinger, J.; Funke, H.; Kiehntopf, M.; Peters, F.T.; Lupp, A.; Bauer, M.; et al. Monocyte-induced recovery of inflammation-associated hepatocellular dysfunction in a biochip-based human liver model. Sci. Rep. 2016, 6, 21868. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Li, N.; Yang, H.; Luo, C.; Gong, Y.; Tong, C.; Gao, Y.; Lü, S.; Long, M. Mimicking liver sinusoidal structures and functions using a 3D-configured microfluidic chip. Lab Chip 2017, 17, 782–794. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Choi, B.; No, D.Y.; Lee, G.; Lee, S.R.; Oh, H.; Lee, S.H. A 3D alcoholic liver disease model on a chip. Integr. Biol. 2016, 8, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewski, T.; Cornforth, T.; Snow, S.A.; Ouro-Gnao, L.; Rowe, C.; Large, E.M.; Hughes, D.J. Three-dimensional perfused human in vitro model of non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 204. [Google Scholar] [CrossRef]
- Lee, J.B.; Park, J.S.; Shin, Y.M.; Lee, D.H.; Yoon, J.K.; Kim, D.H.; Ko, U.H.; Kim, Y.; Bae, S.H.; Sung, H.J. Implantable vascularized liver chip for cross-validation of disease treatment with animal model. Adv. Funct. Mater. 2019, 29, 1900075. [Google Scholar] [CrossRef]
- Chandra, P.; Lecluyse, E.L.; Brouwer, K.L. Optimization of culture conditions for determining hepatobiliary disposition of taurocholate in sandwich-cultured rat hepatocytes. Vitr. Cell. Dev. Biol.-Anim. 2001, 37, 380–385. [Google Scholar] [CrossRef]
- Block, G.D.; Locker, J.; Bowen, W.C.; Petersen, B.E.; Katyal, S.; Strom, S.C.; Riley, T.; Howard, T.A.; Michalopoulos, G.K. Population expansion, clonal growth, and specific differentiation patterns in primary cultures of hepatocytes induced by HGF/SF, EGF and TGF alpha in a chemically defined (HGM) medium. J. Cell Biol. 1996, 132, 1133–1149. [Google Scholar] [CrossRef] [Green Version]
- Rose, T.M.; Weiford, D.M.; Gunderson, N.L.; Bruce, A.G. Oncostatin M (OSM) inhibits the differentiation of pluripotent embryonic stem cells in vitro. Cytokine 1994, 6, 48–54. [Google Scholar] [CrossRef]
- Laishes, B.A.; Williams, G.M. Conditions affecting primary cell cultures of functional adult rat hepatocytes. II. Dexamethasone enhanced longevity and maintenance of morphology. In Vitro 1976, 12, 821–832. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Bowen, W.C.; Mulé, K.; Luo, J. HGF-, EGF-, and dexamethasone-induced gene expression patterns during formation of tissue in hepatic organoid cultures. Gene Expr. J. Liver Res. 2003, 11, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, L.; Liu, W.; Ma, X.; Cen, J.; Sun, Z.; Wang, C.; Feng, S.; Zhang, Z.; Yue, L.; et al. In vitro expansion of primary human hepatocytes with efficient liver repopulation capacity. Cell Stem Cell 2018, 23, 806–819. [Google Scholar] [CrossRef] [Green Version]
- Knobeloch, D.; Ehnert, S.; Schyschka, L.; Büchler, P.; Schoenberg, M.; Kleeff, J.; Thasler, W.E.; Nussler, N.C.; Godoy, P.; Hengstler, J.; et al. Human hepatocytes: Isolation, culture, and quality procedures. Methods Mol. Biol. 2012, 806, 99–120. [Google Scholar]
- Beerheide, W.; Von Mach, M.A.; Ringel, M.; Fleckenstein, C.; Schumann, S.; Renzing, N.; Hildebrandt, A.; Brenner, W.; Jensen, O.; Gebhard, S.; et al. Downregulation of β2-microglobulin in human cord blood somatic stem cells after transplantation into livers of SCID-mice: An escape mechanism of stem cells? Biochem. Biophys. Res. Commun. 2002, 294, 1052–1063. [Google Scholar] [CrossRef] [PubMed]
- Hengstler, J.G.; Brulport, M.; Schormann, W.; Bauer, A.; Hermes, M.; Nussler, A.K.; Fandrich, F.; Ruhnke, M.; Ungefroren, H.; Griffin, L.; et al. Generation of human hepatocytes by stem cell technology: Definition of the hepatocyte. Expert Opin. Drug Metab. Toxicol. 2005, 1, 61–74. [Google Scholar] [CrossRef]
- Sharma, A.D.; Cantz, T.; Richter, R.; Eckert, K.; Henschler, R.; Wilkens, L.; Jochheim-Richter, A.; Arseniev, L.; Ott, M. Human cord blood stem cells generate human cytokeratin 18-negative hepatocyte-like cells in injured mouse liver. Am. J. Pathol. 2005, 167, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerets, H.H.J.; Tilmant, K.; Gerin, B.; Chanteux, H.; Depelchin, B.; Dhalluin, S.; Atienzar, F.A. Characterization of primary human hepatocytes, HepG2 cells, and HepaRG cells at the mRNA level and CYP activity in response to inducers and their predictivity for the detection of human hepatotoxins. Cell Biol. Toxicol. 2012, 28, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Das, P.C.; Cao, Y.; Cherrington, N.; Hodgson, E.; Rose, R.L. Fipronil induces CYP isoforms and cytotoxicity in human hepatocytes. Chem.-Biol. Interact. 2006, 164, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Doshi, U.; Li, A.P. Luciferin IPA–based higher throughput human hepatocyte screening assays for CYP3A4 inhibition and induction. J. Biomol. Screen. 2011, 16, 903–909. [Google Scholar] [CrossRef] [Green Version]
- Westerink, W.M.; Schoonen, W.G. Cytochrome P450 enzyme levels in HepG2 cells and cryopreserved primary human hepatocytes and their induction in HepG2 cells. Toxicol. Vitr. 2007, 21, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.; Tremmel, R.; Winter, S.; Fehr, S.; Battke, F.; Scheurenbrand, T.; Schaeffeler, E.; Biskup, S.; Schwab, M.; Zanger, U.M. A new panel-based next-generation sequencing method for ADME genes reveals novel associations of common and rare variants with expression in a human liver cohort. Front. Genet. 2019, 10, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gramignoli, R.; Tahan, V.; Dorko, K.; Venkataramanan, R.; Fox, I.J.; Ellis, E.C.S.; Vosough, M.; Strom, S.C. Rapid and sensitive assessment of human hepatocyte functions. Cell Transplant. 2014, 23, 1545–1556. [Google Scholar] [CrossRef]
- Shi, C.X.; Lin, Y.X.; Liu, F.P.; Chang, Y.C.; Li, R.; Li, C.W.; Li, Y.; He, J.S.; Ma, X.; Li, Z. Hepatoprotective effects of ethanol extracts from Folium Syringae against acetaminophen-induced hepatotoxicity in vitro and in vivo. J. Chin. Med. Assoc. 2017, 80, 623–629. [Google Scholar] [CrossRef]
- Bell, C.C.; Hendriks, D.F.G.; Moro, S.M.L.; Ellis, E.; Walsh, J.; Renblom, A.; Puigvert, L.F.; Dankers, A.C.A.; Jacobs, F.; Snoeys, J.; et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 2016, 6, 25187. [Google Scholar] [CrossRef] [Green Version]
- Mueller, D.; Tascher, G.; Müller-Vieira, U.; Knobeloch, D.; Nuessler, A.K.; Zeilinger, K.; Heinzle, E.; Noor, F. In-depth physiological characterization of primary human hepatocytes in a 3D hollow-fiber bioreactor. J. Tissue Eng. Regen. Med. 2011, 5, e207–e218. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt, R. Metabolic zonation of the liver: Regulation and implications for liver function. Pharmacol. Ther. 1992, 53, 275–354. [Google Scholar] [PubMed]
- Strauss, O.; Phillips, A.; Ruggiero, K.; Bartlett, A.; Dunbar, P.R. Immunofluorescence identifies distinct subsets of endothelial cells in the human liver. Sci. Rep. 2017, 7, 44356. [Google Scholar] [CrossRef] [PubMed]
- Dobie, R.; Wilson-Kanamori, J.R.; Henderson, B.E.; Smith, J.R.; Matchett, K.P.; Portman, J.R.; Wallenborg, K.; Picelli, S.; Zagorska, A.; Pendem, S.V.; et al. Single-Cell Transcriptomics Uncovers Zonation of Function in the Mesenchyme during Liver Fibrosis. Cell Rep. 2019, 29, 1832–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.W.; Choi, Y.J.; Yong, W.J.; Pati, F.; Shim, J.H.; Kang, K.S.; Kang, I.H.; Park, J.; Cho, D.W. Development of a 3D cell printed construct considering angiogenesis for liver tissue engineering. Biofabrication 2016, 8, 015007. [Google Scholar] [CrossRef]
- Janani, G.; Priya, S.; Dey, S.; Mandal, B.B. Mimicking Native Liver Lobule Microarchitecture In Vitro with Parenchymal and Non-parenchymal Cells Using 3D Bioprinting for Drug Toxicity and Drug Screening Applications. ACS Appl. Mater. Interfaces 2022, 14, 10167–10186. [Google Scholar] [CrossRef]
- Tilles, A.W.; Baskaran, H.; Roy, P.; Yarmush, M.L.; Toner, M. Effects of oxygenation and flow on the viability and function of rat hepatocytes cocultured in a microchannel flat-plate bioreactor. Biotechnol. Bioeng. 2001, 73, 379–389. [Google Scholar] [CrossRef]





| S. No. | Group | Shear Stress | Cell Type Used | Ref |
|---|---|---|---|---|
| 1. | Tanaka et al. | 1.4 to 60 dyne/cm2 | HepG2 | [68] |
| 2. | Vinci et al. | 5 × 10−6 dyne/cm2 | Primary hepatocytes | [69] |
| 3. | Rashidi et al. | 2.9–4.7 × 10−6 dyne/cm2 | Hepatocyte-like cells derived from human embryonic stem cells and induced PSCs. | [70] |
| S.No. | Group | Microfluidic Chip Specifications | Cell Type Used | Ref |
|---|---|---|---|---|
| 1. | Lee et al. | 2 µm in width, 1 µm in height, and 30 µm in length | Primary hepatocytes | [71] |
| 2. | Toh et al. | 3D ECM was formed by injection of methylated collagen and terpolymer hydroxylethylmethacrylate–methylmethacrylate–methylacrylic acid. | 3D Culture of HepG2 and primary hepatocytes | [72] |
| 3. | Goral et al. | Patterened microstructure of PDMS added to the bottom of the cell culture chamber serves as independent perfusion microchannels. | Primary human hepatocytes | [73] |
| 4. | Banaeiyan et al. | Liver-lobule-like hexagonal tissue culture chambers having flow channels with flow rate 1 µL/min | HepG2 cells and human-induced pluripotent stem cell (hiPSC)-derived hepatocytes | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, I.; Vasudevan, A.; Rawal, P.; Tripathi, D.M.; Ramakrishna, S.; Kaur, S.; Sarin, S.K. Primary Hepatocyte Isolation and Cultures: Technical Aspects, Challenges and Advancements. Bioengineering 2023, 10, 131. https://doi.org/10.3390/bioengineering10020131
Kaur I, Vasudevan A, Rawal P, Tripathi DM, Ramakrishna S, Kaur S, Sarin SK. Primary Hepatocyte Isolation and Cultures: Technical Aspects, Challenges and Advancements. Bioengineering. 2023; 10(2):131. https://doi.org/10.3390/bioengineering10020131
Chicago/Turabian StyleKaur, Impreet, Ashwini Vasudevan, Preety Rawal, Dinesh M. Tripathi, Seeram Ramakrishna, Savneet Kaur, and Shiv K. Sarin. 2023. "Primary Hepatocyte Isolation and Cultures: Technical Aspects, Challenges and Advancements" Bioengineering 10, no. 2: 131. https://doi.org/10.3390/bioengineering10020131
APA StyleKaur, I., Vasudevan, A., Rawal, P., Tripathi, D. M., Ramakrishna, S., Kaur, S., & Sarin, S. K. (2023). Primary Hepatocyte Isolation and Cultures: Technical Aspects, Challenges and Advancements. Bioengineering, 10(2), 131. https://doi.org/10.3390/bioengineering10020131