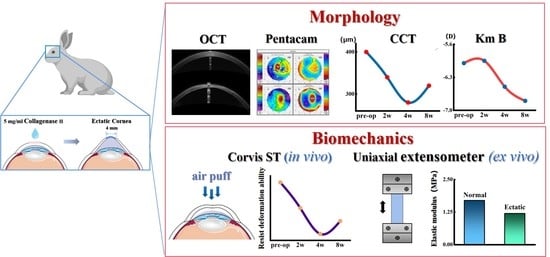
The Corneal Ectasia Model of Rabbit: A Validity and Stability Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model of Corneal Ectasia Establishment
2.2. Measurement of Corneal Morphology In Vivo
2.3. Measurement of Corneal Biomechanical In Vivo
2.4. Uniaxial Testing
2.5. Histology
2.6. Statistical Analyses
3. Results
3.1. Changes in Corneal Morphology with Time
3.1.1. Morphologic Parameters from Pentacam
3.1.2. Morphologic Parameters from SD–OCT
3.2. Changes in Corneal Mechanical Properties with Time
3.2.1. Corneal Mechanical Behaviors Determined by Corvis ST In Vivo
3.2.2. Corneal Elastic Modulus Determined by Uniaxial Tension Ex Vivo
3.3. H&E Staining and Picrosirius-Red Staining
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giolio, F.; Paolo, R. The keratoconus enigma: A review with emphasis on pathogenesis. Ocul. Surf. 2020, 18, 363–373. [Google Scholar]
- Kapasi, M.; Rocha, G. Comparison of visual and refractive outcomes following Intacs implantation in keratoconus eyes with central and eccentric cones. Can. J. Ophthalmol. 2012, 47, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Miguel, R.J.; Jacinto, S.R.; James, S.W. Keratoconus: A review. Contact Lens Anterior Eye 2010, 33, 157–166. [Google Scholar]
- Seitz, B.; Daas, L.; Hamon, L.; Xanthopoulou, K.; Goebels, S.; Spira-Eppig, C.; Razafimino, S.; Szentmáry, N.; Langenbucher, A.; Flockerzi, E. Stage-appropriate treatment of keratoconus. Ophthalmologe 2021, 118, 1069–1088. [Google Scholar] [CrossRef]
- Najmi, H.; Mobarki, Y.; Mania, K.; Altowairqi, B.; Basehi, M.; Mahfouz, M.S.; Elmahdy, M. The correlation between keratoconus and eye rubbing: A review. Int. J. Ophthalmol. 2019, 11, 1775–1781. [Google Scholar] [CrossRef] [PubMed]
- Veronica, M.T.; Cheryl, M.G.; Rakesh, J.; David, O.; Nicholas, M. A review of keratoconus: Diagnosis, pathophysiology, and genetics. Surv. Ophthalmol. 2017, 62, 770–783. [Google Scholar]
- Nielsen, K.; Hjortdal, J.; Pihlmann, M.; Corydon, T.J. Update on the genetics of keratoconus. Acta Ophthalmol. 2020, 2, 106–113. [Google Scholar] [CrossRef]
- Sorkin, N.; Varssano, D. Corneal collagen crosslinking: A systematic review. Ophthalmologica 2014, 232, 10–27. [Google Scholar] [CrossRef]
- Moghadam, F.A.; Jahromy, M.H.; Fazelipour, S.; Khakpour, S.; Younesian, M.S. Induction of experimental keratoconus in mice using collagenase. J. Physiol. Pharmacol. 2009, 13, 209–215. [Google Scholar]
- Qiao, J.; Li, H.; Song, W.J.; Tang, Y.; Rong, B.; Yang, S.L.; Wu, Y.; Yan, X.M. Creating a rabbit model for corneal ectasia in vitro with collagenase type II. Chin. J. Optom. Ophthalmol. Vis. Sci. 2016, 18, 275–279. [Google Scholar]
- Qiao, J.; Li, H.; Song, W.J.; Tang, Y.; Rong, B.; Yang, S.L.; Wu, Y.; Yan, X.M. Feasibility of constructing keratectasia animal model using collagenase type II. Chin. J. Exp. Ophthalmol. 2017, 35, 984–989. [Google Scholar]
- Qiao, J.; Li, H.L.; Tang, Y.; Song, W.J.; Rong, B.; Yang, S.L.; Wu, Y.; Yan, X.M. A rabbit model of corneal Ectasia generated by treatment with collagenase type II. BMC Ophthalmol. 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.X.; Yan, X.M. Sulforaphane protects rabbit corneas against oxidative stress injury in keratoconus through activation of the Nrf-2/HO-1 antioxidant pathway. Int. J. Mol. Med. 2018, 42, 2315–2328. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.B.; Huang, Y.; Chen, Y.; Ye, C.; Wei, W.; Feng, Y.; Mi, S.L. Study on patterned photodynamic cross-linking for keratoconus. Exp. Eye Res. 2021, 204, 108450. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Qin, X.; Zhang, H.X.; Li, L. Effect of collagenase type II on the biomechanical properties of rabbit cornea. Chin. J. Tissue Eng. Res. 2019, 23, 3556–3561. [Google Scholar]
- Riau, A.K.; Tan, N.Y.; Angunawela, R.I.; Htoon, H.M.; Chaurasia, S.S.; Mehta, J.S. Reproducibility and age-related changes of ocular parametric measurements in rabbits. BMC Vet. Res. 2012, 8, 138. [Google Scholar] [CrossRef]
- Giuliano, S.; Sebastien, B.; Roberto, P.; Seok, H.Y. Biomechanical Characterization of Keratoconus Corneas ex-vivo with Brillouin Microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4490–4495. [Google Scholar]
- Naderan, M.; Shoar, S.; Kamaleddin, M.A.; Rajabi, M.T.; Naderan, M.; Khodadadi, M. Keratoconus Clinical Findings According to Different Classifications. Cornea 2015, 34, 1005–1011. [Google Scholar] [CrossRef]
- Li, Y.; Chamberlain, W.; Tan, O.; Brass, R.; Weiss, J.L.; Huang, D. Subclinical keratoconus detection by pattern analysis of corneal and epithelial thickness maps with optical coherence tomography. J. Cataract Refract. Surg. 2016, 42, 284–295. [Google Scholar] [CrossRef]
- Li, Y.; Tan, O.; Brass, R.; Weiss, J.L.; Huang, D. Corneal Epithelial Thickness Mapping by Fourier-domain Optical Coherence Tomography in Normal and Keratoconic Eyes. Ophthalmology 2012, 119, 2425–2433. [Google Scholar] [CrossRef]
- Rocha, K.M.; Perez-Straziota, C.E.; Stulting, R.D.; Randleman, J.B. Spectral-Domain SD-OCT Analysis of Regional Epithelial Thickness Profiles in Keratoconus, Postoperative Corneal Ectasia, and Normal Eyes. J. Refract. Surg. 2013, 29, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Jay, C.E.; Sanjay, V.P.; Jay, W.M.; Cherie, B.N.; David, O.H.; William, M.B. Keratocyte density in keratoconus: A confocal microscopy study. Am. J. Ophthalmol. 2002, 134, 689–695. [Google Scholar]
- Hollingsworth, J.G.; Efron, N.; Tullo, A.B. In vivo corneal confocal microscopy in keratoconus. Ophthalmic Physiol. Opt. 2005, 25, 254–260. [Google Scholar] [CrossRef]
- Uçakhan, O.O.; Kanpolat, A.; Ylmaz, N.; Ozkan, M. In vivo confocal microscopy findings in keratoconus. Eye Contact Lens 2006, 32, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sandali, O.; El Sanharawi, M.; Temstet, C.; Hamiche, T.; Galan, A.; Ghouali, W. Fourier-domain optical coherence tomography imaging in keratoconus: A corneal structural classifcation. Ophthalmology 2013, 120, 2403–2412. [Google Scholar] [CrossRef]
- Kamiya, K.; Kono, Y.; Takahashi, M.; Shoji, N. Comparison of Simulated Keratometry and Total Refractive Power for Keratoconus According to the Stage of Amsler-Krumeich Classification. Sci. Rep. 2018, 8, 12436. [Google Scholar] [CrossRef]
- Ganesh, S.; Patel, U.; Brar, S. Posterior corneal curvature changes following Refractive Small Incision Lenticule Extraction. Clin. Ophthalmol. 2015, 9, 1359–1364. [Google Scholar] [CrossRef]
- Tomidokoro, A.; Oshika, T.; Amano, S.; Higaki, S.; Maeda, N.; Miyata, K. Changes in anterior and posterior corneal curvatures in keratoconus. Ophthalmology 2000, 107, 1328–1332. [Google Scholar] [CrossRef]
- Edmund, C. Posterior corneal curvature and its influence on corneal dioptric power. Acta Ophthalmol. 2010, 72, 715–720. [Google Scholar] [CrossRef]
- Ahmadi Hosseini, S.M.; Abolbashari, F.; Niyazmand, H.; Sedaghat, M.R. Efficacy of corneal tomography parameters and biomechanical characteristic in keratoconus detection. Contact Lens Anterior Eye 2014, 37, 26–30. [Google Scholar] [CrossRef]
- Mannion, L.S.; Tromans, C.; O’Donnell, C. Reduction in corneal volume with severity of keratoconus. Curr. Eye Res. 2011, 36, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xu, L.Y.; Fan, Q.; Gu, Y.W.; Song, P.; Zhang, B.; Zhao, D.Q.; Pang, C.J.; Wang, S.W. Evaluation of new corvis ST parameters in normal, post-LASiK, post-LASiK keratectasia and keratoconus eyes. Sci. Rep. 2020, 10, 5676. [Google Scholar] [CrossRef]
- Elias, F.; Larissa, H.; Kassandra, X.; Loay, D.; Cristian, M.; Achim, L.; Berthold, S. Reliability analysis of successive Corneal Visualization Scheimpflug Technology measurements in different keratoconus stages. Acta Ophthalmol. 2021, 100, e83–e90. [Google Scholar]
- Xiao, M.; Zhang, X.Y.; Wang, S.L. Assessment of Corneal Biomechanical Properties in Keratoconus Using the New Parameters of Corvis ST. Chin. J. Optom. Ophthal. Vis. Sci. 2021, 23, 641–646. [Google Scholar]
- Vellara, H.R.; Patel, D.V. Biomechanical properties of the keratoconic cornea: A review. Clin. Exp. Optom. 2015, 98, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Choksi, T.M.; Shetty, R.; Sahdev, S.I. To study corneal biomechanics using corvis ST in normal and keratoconus corneas. Inter. J. Sci. Res. 2020, 9, 34–37. [Google Scholar]
- Li, Y.; Xu, Z.Q.; Liu, Q.L.; Wang, Y.Z.; Lin, K.; Xia, J.H.; Chen, S.H.; Hu, L. Relationship between corneal biomechanical parameters and corneal sublayer thickness measured by Corvis ST and UHR-SD-OCT in keratoconus and normaleyes. Eye Vis. 2021, 8, 2. [Google Scholar] [CrossRef]
- Vinciguerra, R.; Romano, V.; Arbabi, E.M.; Brunner, M.; Willoughby, C.E.; Batterbury, M.; Kaye, S.B. In vivo Early Corneal Biomechanical Changes after Corneal Cross-linking in Patients with Progressive Keratoconus. J. Refract. Surg. 2017, 33, 840–846. [Google Scholar] [CrossRef]
- Edmund, C. Corneal elasticity and ocular rigidity in normal and keratoconic eyes. Acta Ophthalmol. 1988, 66, 134–140. [Google Scholar] [CrossRef]
- Eric, R.M.; James, V.J.; Tibor, J. Measurement of an elasticity map in the human cornea. Investig. Ophthalmol. Vis. Sci. 2016, 57, 32–36. [Google Scholar]
- Eric, M.; Moritz, W.; Tibor, J.; Donald, J.B.; Golroxan, S.; Stephanie, S.; Cristina, M.K.; James, V.J. Axial mechanical and structural characterization of keratoconus corneas. Exp. Eye Res. 2018, 175, 14–19. [Google Scholar]
- Galvis, V.; Tello, A.; Barrera, R.; Nino, C.A. Inflammation in Keratoconus. Cornea 2015, 34, e22–e23. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Ghosh, A.; Lim, R.R.; Subramani, M.; Mihir, K.; Reshma, A.R.; Ranganath, A.; Nagarai, S.; Beuerman, R.; Das, D.; et al. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Investig. Ophthalmol. Vis. Sci. 2015, 56, 738–750. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; He, R.; Wang, X.; Song, Y.; Yao, J.; Liu, X.; Yang, X.; Chen, W.; Li, X. The Corneal Ectasia Model of Rabbit: A Validity and Stability Study. Bioengineering 2023, 10, 479. https://doi.org/10.3390/bioengineering10040479
Wei J, He R, Wang X, Song Y, Yao J, Liu X, Yang X, Chen W, Li X. The Corneal Ectasia Model of Rabbit: A Validity and Stability Study. Bioengineering. 2023; 10(4):479. https://doi.org/10.3390/bioengineering10040479
Chicago/Turabian StyleWei, Junchao, Rui He, Xiaogang Wang, Yaowen Song, Jinhan Yao, Xiaona Liu, Xin Yang, Weiyi Chen, and Xiaona Li. 2023. "The Corneal Ectasia Model of Rabbit: A Validity and Stability Study" Bioengineering 10, no. 4: 479. https://doi.org/10.3390/bioengineering10040479
APA StyleWei, J., He, R., Wang, X., Song, Y., Yao, J., Liu, X., Yang, X., Chen, W., & Li, X. (2023). The Corneal Ectasia Model of Rabbit: A Validity and Stability Study. Bioengineering, 10(4), 479. https://doi.org/10.3390/bioengineering10040479