Intraoperative Laparoscopic Hyperspectral Imaging during Esophagectomy—A Pilot Study Evaluating Esophagogastric Perfusion at the Anastomotic Sites
Abstract
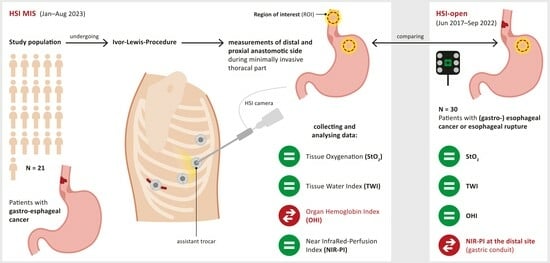
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Surgical Procedure and Hyperspectral Imaging
2.3. Postoperative Findings and Follow-Up
2.4. Data Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Comparison of Intraoperative Perfusion Imaging of the Gastroesophageal Sites (Group 1)
3.3. Perfusion Evaluation of the Gastric Conduit by HSI-MIS (Group 1) and HSI-Open (Group 2)
3.4. Intra- and Postoperative Findings and Follow-Up
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, F.; Siegel, B.A. Neoplasms of the esophagus and stomach. Semin. Nucl. Med. 2004, 34, 198–208. [Google Scholar] [CrossRef]
- Kato, H.; Fukuchi, M.; Miyazaki, T.; Nakajima, M.; Tanaka, N.; Inose, T.; Kimura, H.; Faried, A.; Saito, K.; Sohda, M.; et al. Surgical treatment for esophageal cancer. Current issues. Dig. Surg. 2007, 24, 88–95. [Google Scholar] [CrossRef]
- Kato, H.; Nakajima, M. Treatments for esophageal cancer: A review. Gen. Thorac. Cardiovasc. Surg. 2013, 61, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Pu, S.; Chen, H.; Zhou, C.; Yu, S.; Liao, X.; Zhu, L.; He, J.; Wang, B. Major Postoperative Complications in Esophageal Cancer After Minimally Invasive Esophagectomy Compared with Open Esophagectomy: An Updated Meta-analysis. J. Surg. Res. 2021, 257, 554–571. [Google Scholar] [CrossRef] [PubMed]
- Biere, S.S.A.Y.; van Berge Henegouwen, M.I.; Maas, K.W.; Bonavina, L.; Rosman, C.; Garcia, J.R.; Gisbertz, S.S.; Klinkenbijl, J.H.G.; Hollmann, M.W.; de Lange, E.S.M.; et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicentre, open-label, randomised controlled trial. Lancet 2012, 379, 1887–1892. [Google Scholar] [CrossRef]
- Fabbi, M.; Hagens, E.R.C.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Anastomotic leakage after esophagectomy for esophageal cancer: Definitions, diagnostics, and treatment. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2021, 34, doaa039. [Google Scholar] [CrossRef]
- Mariette, C.; Markar, S.R.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D’Journo, X.B.; Brigand, C.; Perniceni, T.; Carrère, N.; et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N. Engl. J. Med. 2019, 380, 152–162. [Google Scholar] [CrossRef]
- Boyle, N.H.; Manifold, D.; Jordan, M.H.; Mason, R.C. Intraoperative assessment of colonic perfusion using scanning laser Doppler flowmetry during colonic resection. J. Am. Coll. Surg. 2000, 191, 504–510. [Google Scholar] [CrossRef]
- Jones, C.E.; Watson, T.J. Anastomotic Leakage Following Esophagectomy. Thorac. Surg. Clin. 2015, 25, 449–459. [Google Scholar] [CrossRef]
- Low, D.E.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.E.; D’Journo, X.B.; Griffin, S.M.; Hölscher, A.H.; Hofstetter, W.L.; Jobe, B.A.; et al. International Consensus on Standardization of Data Collection for Complications Associated with Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann. Surg. 2015, 262, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Hagens, E.R.C.; Reijntjes, M.A.; Anderegg, M.C.J.; Eshuis, W.J.; van Berge Henegouwen, M.I.; Gisbertz, S.S. Risk Factors and Consequences of Anastomotic Leakage After Esophagectomy for Cancer. Ann. Thorac. Surg. 2021, 112, 255–263. [Google Scholar] [CrossRef]
- Hoffmann, H.; Delko, T.; Kirchhoff, P.; Rosenthal, R.; Schäfer, J.; Kraljević, M.; Kettelhack, C. Colon Perfusion Patterns During Colorectal Resection Using Visible Light Spectroscopy. World J. Surg. 2017, 41, 2923–2932. [Google Scholar] [CrossRef]
- Kassis, E.S.; Kosinski, A.S.; Ross, P.; Koppes, K.E.; Donahue, J.M.; Daniel, V.C. Predictors of anastomotic leak after esophagectomy: An analysis of the society of thoracic surgeons general thoracic database. Ann. Thorac. Surg. 2013, 96, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Goense, L.; van Rossum, P.S.N.; Weijs, T.J.; van Det, M.J.; Nieuwenhuijzen, G.A.; Luyer, M.D.; van Leeuwen, M.S.; van Hillegersberg, R.; Ruurda, J.P.; Kouwenhoven, E.A. Aortic Calcification Increases the Risk of Anastomotic Leakage After Ivor-Lewis Esophagectomy. Ann. Thorac. Surg. 2016, 102, 247–252. [Google Scholar] [CrossRef] [PubMed]
- van Kooten, R.T.; Voeten, D.M.; Steyerberg, E.W.; Hartgrink, H.H.; van Berge Henegouwen, M.I.; van Hillegersberg, R.; Tollenaar, R.A.E.M.; Wouters, M.W.J.M. Patient-Related Prognostic Factors for Anastomotic Leakage, Major Complications, and Short-Term Mortality Following Esophagectomy for Cancer: A Systematic Review and Meta-Analyses. Ann. Surg. Oncol. 2022, 29, 1358–1373. [Google Scholar] [CrossRef]
- Finks, J.F.; Osborne, N.H.; Birkmeyer, J.D. Trends in hospital volume and operative mortality for high-risk surgery. N. Engl. J. Med. 2011, 364, 2128–2137. [Google Scholar] [CrossRef]
- Goense, L.; van Rossum, P.S.N.; Ruurda, J.P.; van Vulpen, M.; Mook, S.; Meijer, G.J.; van Hillegersberg, R. Radiation to the Gastric Fundus Increases the Risk of Anastomotic Leakage After Esophagectomy. Ann. Thorac. Surg. 2016, 102, 1798–1804. [Google Scholar] [CrossRef]
- Gronnier, C.; Tréchot, B.; Duhamel, A.; Mabrut, J.-Y.; Bail, J.-P.; Carrere, N.; Lefevre, J.H.; Brigand, C.; Vaillant, J.-C.; Adham, M.; et al. Impact of neoadjuvant chemoradiotherapy on postoperative outcomes after esophageal cancer resection: Results of a European multicenter study. Ann. Surg. 2014, 260, 764–770. [Google Scholar] [CrossRef]
- Karliczek, A.; Benaron, D.A.; Zeebregts, C.J.; Wiggers, T.; van Dam, G.M. Intraoperative ischemia of the distal end of colon anastomoses as detected with visible light spectroscopy causes reduction of anastomotic strength. J. Surg. Res. 2009, 152, 288–295. [Google Scholar] [CrossRef]
- Ris, F.; Hompes, R.; Cunningham, C.; Lindsey, I.; Guy, R.; Jones, O.; George, B.; Cahill, R.A.; Mortensen, N.J. Near-infrared (NIR) perfusion angiography in minimally invasive colorectal surgery. Surg. Endosc. 2014, 28, 2221–2226. [Google Scholar] [CrossRef]
- Casas, M.A.; Angeramo, C.A.; Bras Harriott, C.; Dreifuss, N.H.; Schlottmann, F. Indocyanine green (ICG) fluorescence imaging for prevention of anastomotic leak in totally minimally invasive Ivor Lewis esophagectomy: A systematic review and meta-analysis. Dis. Esophagus Off. J. Int. Soc. Dis. Esophagus 2022, 35, doab056. [Google Scholar] [CrossRef] [PubMed]
- Toens, C.; Krones, C.J.; Blum, U.; Fernandez, V.; Grommes, J.; Hoelzl, F.; Stumpf, M.; Klinge, U.; Schumpelick, V. Validation of IC-VIEW fluorescence videography in a rabbit model of mesenteric ischaemia and reperfusion. Int. J. Color. Dis. 2006, 21, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Hennig, S.; Jansen-Winkeln, B.; Köhler, H.; Knospe, L.; Chalopin, C.; Maktabi, M.; Pfahl, A.; Hoffmann, J.; Kwast, S.; Gockel, I.; et al. Novel Intraoperative Imaging of Gastric Tube Perfusion during Oncologic Esophagectomy-A Pilot Study Comparing Hyperspectral Imaging (HSI) and Fluorescence Imaging (FI) with Indocyanine Green (ICG). Cancers 2021, 14, 97. [Google Scholar] [CrossRef]
- Renna, M.S.; Grzeda, M.T.; Bailey, J.; Hainsworth, A.; Ourselin, S.; Ebner, M.; Vercauteren, T.; Schizas, A.; Shapey, J. Intraoperative bowel perfusion assessment methods and their effects on anastomotic leak rates: Meta-analysis. Br. J. Surg. 2023, 110, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Hajivassiliou, C.A.; Greer, K.; Fisher, A.; Finlay, I.G. Non-invasive measurement of colonic blood flow distribution using laser Doppler imaging. Br. J. Surg. 1998, 85, 52–55. [Google Scholar] [CrossRef]
- Halicek, M.; Fabelo, H.; Ortega, S.; Callico, G.M.; Fei, B. In-Vivo and Ex-Vivo Tissue Analysis through Hyperspectral Imaging Techniques: Revealing the Invisible Features of Cancer. Cancers 2019, 11, 756. [Google Scholar] [CrossRef]
- Köhler, H.; Jansen-Winkeln, B.; Maktabi, M.; Barberio, M.; Takoh, J.; Holfert, N.; Moulla, Y.; Niebisch, S.; Diana, M.; Neumuth, T.; et al. Evaluation of hyperspectral imaging (HSI) for the measurement of ischemic conditioning effects of the gastric conduit during esophagectomy. Surg. Endosc. 2019, 33, 3775–3782. [Google Scholar] [CrossRef]
- Köhler, H.; Kulcke, A.; Maktabi, M.; Moulla, Y.; Jansen-Winkeln, B.; Barberio, M.; Diana, M.; Gockel, I.; Neumuth, T.; Chalopin, C. Laparoscopic system for simultaneous high-resolution video and rapid hyperspectral imaging in the visible and near-infrared spectral range. J. Biomed. Opt. 2020, 25, 086004. [Google Scholar] [CrossRef]
- Nickel, F.; Studier-Fischer, A.; Özdemir, B.; Odenthal, J.; Müller, L.R.; Knoedler, S.; Kowalewski, K.F.; Camplisson, I.; Allers, M.M.; Dietrich, M.; et al. Optimization of anastomotic technique and gastric conduit perfusion with hyperspectral imaging and machine learning in an experimental model for minimally invasive esophagectomy. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2023. [Google Scholar] [CrossRef]
- Jansen-Winkeln, B.; Holfert, N.; Köhler, H.; Moulla, Y.; Takoh, J.P.; Rabe, S.M.; Mehdorn, M.; Barberio, M.; Chalopin, C.; Neumuth, T.; et al. Determination of the transection margin during colorectal resection with hyperspectral imaging (HSI). Int. J. Color. Dis. 2019, 34, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Thomaßen, M.T.; Köhler, H.; Pfahl, A.; Stelzner, S.; Mehdorn, M.; Thieme, R.; Jansen-Winkeln, B.; Gockel, I.; Chalopin, C.; Moulla, Y. In vivo evaluation of a hyperspectral imaging system for minimally invasive surgery (HSI-MIS). Surg. Endosc. 2023, 37, 3691–3700. [Google Scholar] [CrossRef] [PubMed]
- Seidlitz, S.; Sellner, J.; Odenthal, J.; Özdemir, B.; Studier-Fischer, A.; Knödler, S.; Ayala, L.; Adler, T.J.; Kenngott, H.G.; Tizabi, M.; et al. Robust deep learning-based semantic organ segmentation in hyperspectral images. Med. Image Anal. 2022, 80, 102488. [Google Scholar] [CrossRef] [PubMed]
- Studier-Fischer, A.; Seidlitz, S.; Sellner, J.; Özdemir, B.; Wiesenfarth, M.; Ayala, L.; Odenthal, J.; Knödler, S.; Kowalewski, K.F.; Haney, C.M.; et al. Spectral organ fingerprints for machine learning-based intraoperative tissue classification with hyperspectral imaging in a porcine model. Sci. Rep. 2022, 12, 11028. [Google Scholar] [CrossRef]






| HSI-Open (Jun. 2017–Sep. 2022) (n = 30) | HSI-MIS (Jan.–Aug. 2023) (n = 21) | |
|---|---|---|
| Sex | ||
| Male/Female | 26/4 | 18/3 |
| Age (Mean ± SD) | 60.9 (±11.8) | 60.8 (±7.6) |
| ASA classification | ||
| ASA I | 0 | 0 |
| ASA II | 21 | 12 |
| ASA III | 9 | 9 |
| Risk factors | ||
| BMI (Mean ± SD) | 26.5 (±5.3) | 27.0 (±5.4) |
| Diabetes Mellitus | 5 | 7 |
| Hypertension | 19 | 8 |
| COPD | 0 | 2 |
| Smoking | 8 | 5 |
| Neoadjuvant therapy | ||
| Chemotherapy | 10 | 12 |
| Radio-chemotherapy | 16 | 7 |
| None | 4 | 0 |
| Histopathological entity | ||
| Adenocarcinoma | 20 | 17 |
| Squamous cell carcinoma | 7 | 3 |
| others | 3 | 1 |
| HSI-Open (Jun. 2017–Sep. 2022) (n = 30) | HSI-MIS (Jan.–Aug. 2023) (n = 21) | |
|---|---|---|
| Type of Operation | ||
| Robotic | 2 | 10 |
| TMIE 1 | 18 | 8 |
| Hybrid | 2 | 3 |
| Open | 8 | 0 |
| Operation duration | ||
| <360 min | 14 | 18 |
| >360 min | 16 | 3 |
| UICC Classification | ||
| 0 | 10 | 4 |
| I | 3 | 5 |
| II | 8 | 2 |
| III | 8 | 8 |
| IV | 1 | 2 |
| Size of the circular stapler | ||
| 25 mm | 12 | 9 |
| 28 mm | 15 | 12 |
| Suture | 3 | 0 |
| HSI tissue parameters (Median [Q1, Q2]) | ||
| StO2 (p = 0.059) | 67.7 [57.8, 79.6] | 60.6 [45.1, 70.5] |
| NIR PI (p = 0.012) | 63.9 [57.7, 70.1] | 76.5 [62.1, 83.2] |
| TWI (p = 0.962) | 51.4 [46.6, 58.4] | 54.0 [41.1, 61.6] |
| OHI (p = 0.353) | 43.2 [32.8, 58.7] | 48.2 [40.5, 55.9] |
| Anastomotic leak | ||
| Grade I (%) | 1 (3%) | 0 |
| Grade II (%) | 4 (13%) | 3 (14%) |
| Grade III (%) | 1 (3%) | 0 |
| Postoperative pneumonia | 4 | 0 |
| Length of stay (Median {range}) | 15 {8;68} | 13 {9;60} |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilgen, A.; Köhler, H.; Pfahl, A.; Stelzner, S.; Mehdorn, M.; Jansen-Winkeln, B.; Gockel, I.; Moulla, Y. Intraoperative Laparoscopic Hyperspectral Imaging during Esophagectomy—A Pilot Study Evaluating Esophagogastric Perfusion at the Anastomotic Sites. Bioengineering 2024, 11, 69. https://doi.org/10.3390/bioengineering11010069
Ilgen A, Köhler H, Pfahl A, Stelzner S, Mehdorn M, Jansen-Winkeln B, Gockel I, Moulla Y. Intraoperative Laparoscopic Hyperspectral Imaging during Esophagectomy—A Pilot Study Evaluating Esophagogastric Perfusion at the Anastomotic Sites. Bioengineering. 2024; 11(1):69. https://doi.org/10.3390/bioengineering11010069
Chicago/Turabian StyleIlgen, Annalena, Hannes Köhler, Annekatrin Pfahl, Sigmar Stelzner, Matthias Mehdorn, Boris Jansen-Winkeln, Ines Gockel, and Yusef Moulla. 2024. "Intraoperative Laparoscopic Hyperspectral Imaging during Esophagectomy—A Pilot Study Evaluating Esophagogastric Perfusion at the Anastomotic Sites" Bioengineering 11, no. 1: 69. https://doi.org/10.3390/bioengineering11010069
APA StyleIlgen, A., Köhler, H., Pfahl, A., Stelzner, S., Mehdorn, M., Jansen-Winkeln, B., Gockel, I., & Moulla, Y. (2024). Intraoperative Laparoscopic Hyperspectral Imaging during Esophagectomy—A Pilot Study Evaluating Esophagogastric Perfusion at the Anastomotic Sites. Bioengineering, 11(1), 69. https://doi.org/10.3390/bioengineering11010069