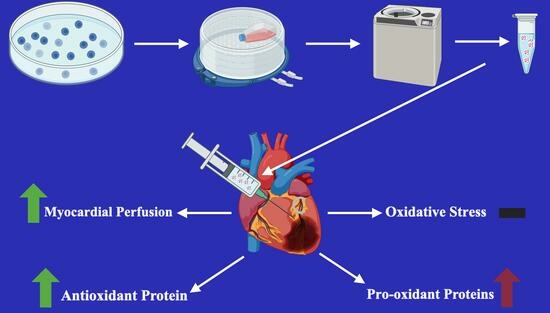
Intramyocardial Injection of Hypoxia-Conditioned Extracellular Vesicles Modulates Response to Oxidative Stress in the Chronically Ischemic Myocardium
Abstract
:1. Introduction
2. Methods
2.1. Large Animal Swine Model
2.2. Humane Animal Care
2.3. Ameroid Placement
2.4. Extracellular Vesicle Culture
2.5. Extracellular Vesicle Proteomics
2.6. Extracellular Vesicle Injection
2.7. Harvest and Perfusion
2.8. Lysate Production
2.9. Immunoblotting
2.10. Antibodies
2.11. Oxyblot
2.12. Nitrotyrosine Staining
2.13. Statistics
3. Results
3.1. Extracellular Vesicle Proteomics
3.2. Functional Data
3.3. Myocardial Protein Expression
3.4. Myocardial Oxyblot and Nitrotyrosine Staining
3.5. Fibrosis
3.6. Myocardial Perfusion Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lindstrom, M.; DeCleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; LeGrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Varieur Turco, J.; et al. Global Burden of Cardiovascular Diseases and Risks Collaboration, 1990–2021. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Laterza Ribeiro, M.; Correia, V.M.; Herling de Oliveira, L.L.; Soares, P.R.; Scudeler, T.L. Evolving Diagnostic and Management Advances in Coronary Heart Disease. Life 2023, 13, 951. [Google Scholar] [CrossRef] [PubMed]
- Kandaswamy, E.; Zuo, L. Recent Advances in Treatment of Coronary Artery Disease: Role of Science and Technology. Int. J. Mol. Sci. 2018, 19, 424. [Google Scholar] [CrossRef] [PubMed]
- Aïdoud, A.; Gana, W.; Poitau, F.; Debacq, C.; Leroy, V.; Nkodo, J.; Poupin, P.; Angoulvant, D.; Fougère, B. High Prevalence of Geriatric Conditions Among Older Adults With Cardiovascular Disease. J. Am. Heart Assoc. 2023, 12, e026850. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhang, Y.; Li, Y.; Luo, L.; Zhao, Y.; Yao, Y. Extracellular vesicles in cardiovascular diseases. Cell Death Discov. 2020, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Karbasiafshar, C.; Sellke, F.W.; Abid, M.R. Mesenchymal stem cell-derived extracellular vesicles in the failing heart: Past, present, and future. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H1999–H2010. [Google Scholar] [CrossRef]
- Alibhai, F.J.; Tobin, S.W.; Yeganeh, A.; Weisel, R.D.; Li, R.-K. Emerging roles of extracellular vesicles in cardiac repair and rejuvenation. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H733–H744. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortty, S.K.; Prakash, A.; Nechooshtan, G.; Hearn, S.; Gingeras, T.R. Extracellular vesicle-mediated transfer of processed and functional RNY5 RNA. RNA 2015, 21, 1966–1979. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Martin-Jaular, L.; Lavieu, G.; Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 2019, 21, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lei, Y.; Zhong, Y.; Chung, C.; Wang, M.; Hu, M.; Deng, L. New Insights Into the Regulatory Roles of Extracellular Vesicles in Tumor Angiogenesis and Their Clinical Implications. Front. Cell Dev. Biol. 2021, 9, 791882. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, Z.; Liu, X.; Tyler, B.M. Biogenesis and Biological Functions of Extracellular Vesicles in Cellular and Organismal Communication With Microbes. Front. Microbiol. 2022, 13, 817844. [Google Scholar] [CrossRef] [PubMed]
- Alsaadi, N.; Srinivasan, A.J.; Seshadri, A.; Shiel, M.; Neal, M.D.; Scott, M.J. The emerging therapeutic potential of extracellular vesicles in trauma. J. Leukoc. Biol. 2022, 111, 93–111. [Google Scholar] [CrossRef]
- Zeineddin, A.; Wu, F.; Dong, J.-F.; Huang, H.; Zou, L.; Chao, W.; Dorman, B.; Kozar, R.A. Trauma-Derived Extracellular Vesicles Are Sufficient to Induce Endothelial Dysfunction and Coagulopathy. Shock. 2022, 58, 38–44. [Google Scholar] [CrossRef]
- Cavallari, C.; Ranghino, A.; Tapparo, M.; Cedrino, M.; Figliolini, F.; Grange, C.; Giannachi, V.; Garneri, P.; Deregibus, M.C.; Collino, F.; et al. Serum-derived extracellular vesicles (EVs) impact on vascular remodeling and prevent muscle damage in acute hind limb ischemia. Sci. Rep. 2017, 7, 8180. [Google Scholar] [CrossRef]
- Reed, S.L.; Escayg, A. Extracellular vesicles in the treatment of neurological disorders. Neurobiol. Dis. 2021, 157, 105445. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, H.; Sun, S.; Wang, L.; Sun, S. Extracellular vesicles and immunogenic stress in cancer. Cell Death Dis. 2021, 12, 894. [Google Scholar] [CrossRef]
- Sanwlani, R.; Gangoda, L. Role of Extracellular Vesicles in Cell Death and Inflammation. Cells 2021, 10, 2663. [Google Scholar] [CrossRef]
- Murphy, D.E.; de Jong, O.G.; Brouwer, M.; Wood, M.J.; Lavieu, G.; Schiffelers, R.M.; Vader, P. Extracellular vesicle-based therapeutics: Natural versus engineered targeting and trafficking. Exp. Mol. Med. 2019, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Danilushkina, A.A.; Emene, C.C.; Barlev, N.A.; Gomzikova, M.O. Strategies for Engineering of Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 13247. [Google Scholar] [CrossRef]
- You, B.; Yang, Y.; Zhou, Z.; Yan, Y.; Zhang, L.; Jin, J.; Qian, H. Extracellular Vesicles: A New Frontier for Cardiac Repair. Pharmaceutics 2022, 14, 1848. [Google Scholar] [CrossRef] [PubMed]
- Sabe, S.A.; Scrimgeour, L.A.; Karbasiafshar, C.; Sabra, M.; Xu, C.M.; Aboulgheit, A.; Abid, M.R.; Sellke, F.W. Extracellular vesicles modulate inflammatory signaling in chronically ischemic myocardium of swine with metabolic syndrome. J. Thorac. Cardiovasc. Surg. 2023, 165, e225–e236. [Google Scholar] [CrossRef] [PubMed]
- Potz, B.A.; Scrimgeour, L.A.; Pavlov, V.I.; Sodha, N.R.; Abid, M.R.; Sellke, F.W. Extracellular Vesicle Injection Improves Myocardial Function and Increases Angiogenesis in a Swine Model of Chronic Ischemia. J. Am. Heart Assoc. 2018, 7, e008344. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.M.; Karbasiafshar, C.; Brinck Teixeira, R.; Ahsan, N.; Blume Corssac, G.; Sellke, F.W.; Abid, M.R. Proteomic Assessment of Hypoxia-Pre-Conditioned Human Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicles Demonstrates Promise in the Treatment of Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 1674. [Google Scholar] [CrossRef] [PubMed]
- Pulido-Escribano, V.; Torrecillas-Baena, B.; Camacho-Cardenosa, M.; Dorado, G.; Gálvez-Moreno, M.Á.; Casado-Díaz, A. Role of hypoxia preconditioning in therapeutic potential of mesenchymal stem-cell-derived extracellular vesicles. World J. Stem Cells 2022, 14, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.-Y.; Zhang, T.-T.; Li, D.-J.; Zhou, E.; Fan, Y.-Q.; He, Q.; Wang, C.-Q.; Zhang, J.-F. Extracellular vesicles from hypoxia-preconditioned mesenchymal stem cells alleviates myocardial injury by targeting thioredoxin-interacting protein-mediated hypoxia-inducible factor-1α pathway. World J. Stem Cells 2022, 14, 183–199. [Google Scholar] [CrossRef]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [CrossRef]
- Sabe, S.A.; Xu, C.M.; Potz, B.A.; Malhotra, A.; Sabra, M.; Harris, D.D.; Broadwin, M.; Abid, M.R.; Sellke, F.W. Comparative Analysis of Normoxia- and Hypoxia-Modified Extracellular Vesicle Therapy in Function, Perfusion, and Collateralization in Chronically Ischemic Myocardium. Int. J. Mol. Sci. 2023, 24, 2076. [Google Scholar] [CrossRef]
- Harris, D.D.; Sabe, S.A.; Sabra, M.; Xu, C.M.; Malhotra, A.; Broadwin, M.; Banerjee, D.; Abid, M.R.; Sellke, F.W. Intramyocardial Injection of Hypoxia-conditioned Extracellular Vesicles Modulates Apoptotic Signaling in Chronically Ischemic Myocardium. JTCVS Open 2023, 15, 220–228. [Google Scholar] [CrossRef]
- Sabra, M.; Sabe, S.A.; Harris, D.D.; Xu, C.M.; Broadwin, M.; Bellam, K.G.; Banerjee, D.; Abid, M.R.; Sellke, F.W. Ischemic myocardial inflammatory signaling in starvation versus hypoxia-derived extracellular vesicles: A comparative analysis. JTCVS Open 2023, 16, 419–428. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Kurian, G.A.; Rajagopal, R.; Vedantham, S.; Rajesh, M. The Role of Oxidative Stress in Myocardial Ischemia and Reperfusion Injury and Remodeling: Revisited. Oxid. Med. Cell Longev. 2016, 2016, 1656450. [Google Scholar] [CrossRef]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovacic, J.C. Impact of Oxidative Stress on the Heart and Vasculature Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, H.; Kinugawa, S.; Matsushima, S. Oxidative stress and heart failure. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H2181–H2190. [Google Scholar] [CrossRef]
- Qin, F.; Lennon-Edwards, S.; Lancel, S.; Biolo, A.; Siwik, D.A.; Pimentel, D.R.; Dorn, G.W.; Kang, Y.J.; Colucci, W.S. Cardiac-specific overexpression of catalase identifies hydrogen peroxide-dependent and independent-phases of myocardial remodeling, and prevents the progression to overt heart failure in Gαq-overexpressing transgenic mice. Circ. Heart Fail. 2010, 3, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Reiner, Ž.; Ruscica, M.; Tedeschi-Reiner, E.; Radbakhsh, S.; Bagheri Ekta, M.; Sahebkar, A. Antioxidant Effects of Statins by Modulating Nrf2 and Nrf2/HO-1 Signaling in Different Diseases. J. Clin. Med. 2022, 11, 1313. [Google Scholar] [CrossRef]
- Wu, M.-L.; Ho, Y.-C.; Lin, C.-Y.; Yet, S.-F. Heme oxygenase-1 in inflammation and cardiovascular disease. Am. J. Cardiovasc. Dis. 2011, 1, 150–158. [Google Scholar]
- Ardanaz, N.; Yang, X.-P.; Cifuentes, M.E.; Haurani, M.J.; Jackson, K.W.; Liao, T.-D.; Carretero, O.A.; Pagano, P.J. Lack of glutathione peroxidase 1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin II hypertension. Hypertension 2010, 55, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Ye, Y.; Wang, X.; Ma, B.; Wu, J.; Li, L.; Wang, L.; Wang, D.W.; Zou, Y. Heat shock transcription factor 1 protects against pressure overload-induced cardiac fibrosis via Smad3. J. Mol. Med. 2017, 95, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.B.; Pfeiffer, M.; Zhang, P.; Shafique, E.; Rayta, B.; Karbasiafshar, C.; Ahsan, N.; Sellke, F.W.; Abid, M.R. Reduction in mitochondrial ROS improves oxidative phosphorylation and provides resilience to coronary endothelium in non-reperfused myocardial infarction. Basic. Res. Cardiol. 2023, 118, 3. [Google Scholar] [CrossRef]
- Abid, M.R.; Tsai, J.C.; Spokes, K.C.; Deshpande, S.S.; Irani, K.; Aird, W.C. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. FASEB J. 2001, 15, 2548–2550. [Google Scholar] [CrossRef]
- Matsushima, S.; Tsutsui, H.; Sadoshima, J. Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion. Trends Cardiovasc. Med. 2014, 24, 202–205. [Google Scholar] [CrossRef]
- Matsushima, S.; Sadoshima, J. Yin and Yang of NADPH Oxidases in Myocardial Ischemia-Reperfusion. Antioxidants 2022, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, T.; Dahlman, J.; Eniola-Adefeso, L.; Ghiran, I.C.; Kurre, P.; Lam, W.A.; Lang, J.K.; Marbán, E.; Martín, P.; et al. Current challenges and future directions for engineering extracellular vesicles for heart, lung, blood and sleep diseases. J. Extracell. Vesicles 2023, 12, 12305. [Google Scholar] [CrossRef] [PubMed]






| Antibody | Company | Catalog Number | Dilution |
|---|---|---|---|
| Primary Antibodies | |||
| 3-Nitrotyrosine | Novus | NB110-96877 | 1:100 |
| Catalase | Cell Signaling | 12980 | 1:1000 |
| Glutathione Peroxidase-1 | Cell Signaling | 3206 | 1:1000 |
| Heat Shock Factor 1 | Cell Signaling | 12972 | 1:1000 |
| Heme Oxygenase-1 | Cell Signaling | 43966 | 1:1000 |
| KEAP-1 | Proteibtech | 60027 | 1:1000 |
| NF-kB | Cell Signaling | 8242 | 1:1000 |
| NADPH Oxidase 1 | Abcam | ab121009 | 1:2000 |
| NADPH Oxidase 2 | Abcam | ab80897 | 1:1000 |
| NADPH Oxidase 3 | Proteibtech | 20065 | 1:1000 |
| p47phox | Abcam | ab795 | 1:2000 |
| p67phox | Proteibtech | 67594 | 1:1000 |
| Peroxiredoxin-1 | Cell Signaling | 8499 | 1:1000 |
| Superoxide Dismutase 2 | Cell Signaling | 13141 | 1:1000 |
| Mitochondrial Uncoupling Protein 2 | Proteibtech | 11081 | 1:1000 |
| Secondary Antibodies | |||
| Anti-goat HRP-linked Antibody | Abcam | ab97110 | 1:2000 |
| Anti-mouse HRP-linked Antibody | Cell Signaling | 7076S | 1:2000 |
| Anti-rabbit HRP-linked Antibody | Cell Signaling | 7074S | 1:2000 |
| Protein Name | Log10 (Abundance) |
|---|---|
| Annexin A1 | 6.2 |
| Catalase | 6.5 |
| Ferritin Heavy Chain 1 | 7.3 |
| Glutathione S-Transferase Pi 1 | 5.3 |
| Heat Shock Protein B1 | 6.4 |
| Hemoglobin Subunit Alpha 2 | 8.4 |
| Hemoglobin Subunit Epsilon 1 | 6.3 |
| Peroxiredoxin-1 | 6.6 |
| Peroxiredoxin-2 | 5.4 |
| Peroxiredoxin-5 | 4.8 |
| Peroxiredoxin-6 | 6.3 |
| Phosphoglycerate Kinase 1 | 5.6 |
| Protein Disulfide-Isomerase | 6.2 |
| Ras-Related Protein Rap-1b | 7.6 |
| Superoxide Dismutase 1 | 5.9 |
| Thioredoxin | 4.2 |
| Rest | Paced | |||
|---|---|---|---|---|
| r | p | r | p | |
| Catalase | 0.00 | 1.00 | −0.04 | 0.96 |
| GPX-1 | −0.61 | 0.17 | −0.14 | 0.78 |
| HSF-1 | −0.36 | 0.44 | −0.21 | 0.66 |
| HO-1 | −0.75 | 0.07 | 0.04 | 0.96 |
| NOX 1 | −0.50 | 0.27 | 0.46 | 0.30 |
| NOX 3 | 0.32 | 0.50 | 0.00 | 1.00 |
| p47phox | 0.00 | 1.00 | 0.21 | 0.66 |
| p67phox | −0.71 | 0.09 | 0.14 | 0.78 |
| SOD 2 | 0.64 | 0.14 | −0.07 | 0.91 |
| UCP 2 | 0.54 | 0.24 | 0.14 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, D.D.; Sabe, S.A.; Broadwin, M.; Xu, C.; Stone, C.; Kanuparthy, M.; Malhotra, A.; Abid, M.R.; Sellke, F.W. Intramyocardial Injection of Hypoxia-Conditioned Extracellular Vesicles Modulates Response to Oxidative Stress in the Chronically Ischemic Myocardium. Bioengineering 2024, 11, 125. https://doi.org/10.3390/bioengineering11020125
Harris DD, Sabe SA, Broadwin M, Xu C, Stone C, Kanuparthy M, Malhotra A, Abid MR, Sellke FW. Intramyocardial Injection of Hypoxia-Conditioned Extracellular Vesicles Modulates Response to Oxidative Stress in the Chronically Ischemic Myocardium. Bioengineering. 2024; 11(2):125. https://doi.org/10.3390/bioengineering11020125
Chicago/Turabian StyleHarris, Dwight D., Sharif A. Sabe, Mark Broadwin, Cynthia Xu, Christopher Stone, Meghamsh Kanuparthy, Akshay Malhotra, M. Ruhul Abid, and Frank W. Sellke. 2024. "Intramyocardial Injection of Hypoxia-Conditioned Extracellular Vesicles Modulates Response to Oxidative Stress in the Chronically Ischemic Myocardium" Bioengineering 11, no. 2: 125. https://doi.org/10.3390/bioengineering11020125
APA StyleHarris, D. D., Sabe, S. A., Broadwin, M., Xu, C., Stone, C., Kanuparthy, M., Malhotra, A., Abid, M. R., & Sellke, F. W. (2024). Intramyocardial Injection of Hypoxia-Conditioned Extracellular Vesicles Modulates Response to Oxidative Stress in the Chronically Ischemic Myocardium. Bioengineering, 11(2), 125. https://doi.org/10.3390/bioengineering11020125