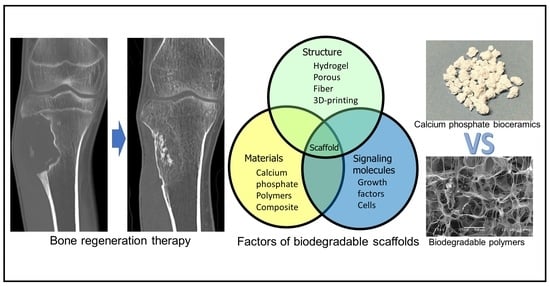
Bone-Regeneration Therapy Using Biodegradable Scaffolds: Calcium Phosphate Bioceramics and Biodegradable Polymers
Abstract
:1. Introduction
2. Clinical Application and New Basic Research on Synthetic Bone Composed of Calcium Phosphate Bioceramics
3. Research and Development of Biodegradable Polymers
3.1. Material of the Polymer
3.2. Biodegradable Polymer and Calcium Phosphate Bioceramics Composites
3.3. Three-Dimensional Structure of Synthetic Bonessc

3.4. Cells and Signaling Molecules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takahashi, K.; Okita, K.; Nakagawa, M.; Yamanaka, S. Induction of pluripotent stem cells from fibroblast cultures. Nat. Protoc. 2007, 2, 3081–3089. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater. Res. 2018, 22, 36. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.A.; El-Rosasy, M.A. Hybrid grafting of post-traumatic bone defects using beta-tricalcium phosphate and demineralized bone matrix. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Mataliotakis, G.I.; Angoules, A.G.; Kanakaris, N.K.; Giannoudis, P.V. Complications following autologous bone graft harvesting from the iliac crest and using the RIA: A systematic review. Injury 2011, 42 (Suppl 2), S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Goulet, J.A.; Senunas, L.E.; DeSilva, G.L.; Greenfield, M.L. Autogenous iliac crest bone graft. Complications and functional assessment. Clin. Orthop. Relat. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef]
- Kong, C.B.; Lee, S.Y.; Jeon, D.G. Staged lengthening arthroplasty for pediatric osteosarcoma around the knee. Clin. Orthop. Relat. Res. 2010, 468, 1660–1668. [Google Scholar] [CrossRef]
- Manfrini, M.; Bindiganavile, S.; Say, F.; Colangeli, M.; Campanacci, L.; Depaolis, M.; Ceruso, M.; Donati, D. Is There Benefit to Free Over Pedicled Vascularized Grafts in Augmenting Tibial Intercalary Allograft Constructs? Clin. Orthop. Relat. Res. 2017, 475, 1322–1337. [Google Scholar] [CrossRef]
- Muscolo, D.L.; Ayerza, M.A.; Aponte-Tinao, L.A. Massive allograft use in orthopedic oncology. Orthop. Clin. North Am. 2006, 37, 65–74. [Google Scholar] [CrossRef]
- Rabitsch, K.; Maurer-Ertl, W.; Pirker-Fruhauf, U.; Wibmer, C.; Leithner, A. Intercalary reconstructions with vascularised fibula and allograft after tumour resection in the lower limb. Sarcoma 2013, 2013, 160295. [Google Scholar] [CrossRef]
- Sotome, S.; Ae, K.; Okawa, A.; Ishizuki, M.; Morioka, H.; Matsumoto, S.; Nakamura, T.; Abe, S.; Beppu, Y.; Shinomiya, K. Efficacy and safety of porous hydroxyapatite/type 1 collagen composite implantation for bone regeneration: A randomized controlled study. J. Orthop. Sci. 2016, 21, 373–380. [Google Scholar] [CrossRef]
- Tamai, N.; Myoui, A.; Tomita, T.; Nakase, T.; Tanaka, J.; Ochi, T.; Yoshikawa, H. Novel hydroxyapatite ceramics with an interconnective porous structure exhibit superior osteoconduction in vivo. J. Biomed. Mater. Res. 2002, 59, 110–117. [Google Scholar] [CrossRef]
- Tanaka, T.; Kumagae, Y.; Saito, M.; Chazono, M.; Komaki, H.; Kikuchi, T.; Kitasato, S.; Marumo, K. Bone formation and resorption in patients after implantation of beta-tricalcium phosphate blocks with 60% and 75% porosity in opening-wedge high tibial osteotomy. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 86, 453–459. [Google Scholar] [CrossRef]
- Taylor, B.C.; French, B.G.; Fowler, T.T.; Russell, J.; Poka, A. Induced membrane technique for reconstruction to manage bone loss. J. Am. Acad. Orthop. Surg. 2012, 20, 142–150. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, J.; Zhu, K.; Cai, T.; Ma, X. Survival, complications and functional outcomes of cemented megaprostheses for high-grade osteosarcoma around the knee. Int. Orthop. 2018, 42, 927–938. [Google Scholar] [CrossRef]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Pfeiffer, R.; Murphy, G.; Daw, N.C.; Patiño-Garcia, A.; Troisi, R.J.; Hoover, R.N.; Douglass, C.; Schüz, J.; Craft, A.W.; et al. Height at diagnosis and birth-weight as risk factors for osteosarcoma. Cancer Causes Control. 2011, 22, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Tsagozis, P.; Parry, M.; Grimer, R. High complication rate after extendible endoprosthetic replacement of the proximal tibia: A retrospective study of 42 consecutive children. Acta. Orthop. 2018, 89, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Dimar, J.R., 2nd; Glassman, S.D.; Burkus, J.K.; Pryor, P.W.; Hardacker, J.W.; Carreon, L.Y. Two-year fusion and clinical outcomes in 224 patients treated with a single-level instrumented posterolateral fusion with iliac crest bone graft. Spine J. 2009, 9, 880–885. [Google Scholar] [CrossRef]
- Gore, D.R. The arthrodesis rate in multilevel anterior cervical fusions using autogenous fibula. Spine 2001, 26, 1259–1263. [Google Scholar] [CrossRef]
- Jakoi, A.M.; Iorio, J.A.; Cahill, P.J. Autologous bone graft harvesting: A review of grafts and surgical techniques. Musculoskelet. Surg. 2015, 99, 171–178. [Google Scholar] [CrossRef]
- Schwartz, C.E.; Martha, J.F.; Kowalski, P.; Wang, D.A.; Bode, R.; Li, L.; Kim, D.H. Prospective evaluation of chronic pain associated with posterior autologous iliac crest bone graft harvest and its effect on postoperative outcome. Health Qual. Life Outcomes 2009, 7, 49. [Google Scholar] [CrossRef]
- de Alencar, P.G.; Vieira, I.F. BONE BANKS. Rev. Bras. Ortop. 2010, 45, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Vikas, R.; Agrawal, H.S. Allogenic bone grafts in post-traumatic juxta-articular defects: Need for allogenic bone banking. Med. J. Armed Forces India 2017, 73, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Mohr, J.; Germain, M.; Winters, M.; Fraser, S.; Duong, A.; Garibaldi, A.; Simunovic, N.; Alsop, D.; Dao, D.; Bessemer, R.; et al. Bioburden Steering Committee and Musculoskeletal Tissue Working group. Disinfection of human musculoskeletal allografts in tissue banking: A systematic review. Cell Tissue Bank. 2016, 17, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Warnock, J.M.; Rowan, C.H.; Davidson, H.; Millar, C.; McAlinden, M.G. Improving efficiency of a regional stand alone bone bank. Cell Tissue Bank. 2016, 17, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zwitser, E.W.; Jiya, T.U.; Licher, H.G.; van Royen, B.J. Design and management of an orthopaedic bone bank in The Netherlands. Cell Tissue Bank. 2012, 13, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, K.; Inoue, G.; Takahira, N.; Takaso, M. Revision total hip arthroplasty-Salvage procedures using bone allografts in Japan. J. Orthop. Sci. 2017, 22, 593–600. [Google Scholar] [CrossRef]
- Zimmermann, G.; Moghaddam, A. Allograft bone matrix versus synthetic bone graft substitutes. Injury 2011, 42, S16–S21. [Google Scholar] [CrossRef]
- Kowalczewski, C.J.; Saul, J.M. Biomaterials for the Delivery of Growth Factors and Other Therapeutic Agents in Tissue Engineering Approaches to Bone Regeneration. Front Pharmacol. 2018, 9, 15. [Google Scholar] [CrossRef]
- Smith, C.A.; Board, T.N.; Rooney, P.; Eagle, M.J.; Richardson, S.M.; Hoyland, J.A. Human decellularized bone scaffolds from aged donors show improved osteoinductive capacity compared to young donor bone. PLoS ONE 2017, 12, 15. [Google Scholar] [CrossRef]
- Gitelis, S.; Virkus, W.; Anderson, D.; Piasecki, P.; Yao, T.K. Functional outcomes of bone graft substitutes for benign bone tumors. Orthopedics 2004, 27 (Suppl 1), S141–S144. [Google Scholar] [CrossRef] [PubMed]
- Kwok, T.Y.; Wong, H.K. Evolving Treatment Modality of Hand Enchondroma in a Local Hospital: From Autograft to Artificial Bone Substitutes. J. Orthop. Traumatol. Rehabil. 2016, 20, 19–23. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar] [CrossRef] [PubMed]
- van der Stok, J.; Hartholt, K.A.; Schoenmakers, D.A.L.; Arts, J.J.C. The available evidence on demineralised bone matrix in trauma and orthopaedic surgery: A systematic review. Bone Joint Res. 2017, 6, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Kombate, N.K.; Walla, A.; Ayouba, G.; Bakriga, B.M.; Dellanh, Y.Y.; Abalo, A.G.; Dossim, A.M. Reconstruction of traumatic bone loss using the induced membrane technique: Preliminary results about 11 cases. J. Orthop. 2017, 14, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Tamai, N.; Murase, T.; Myoui, A. Interconnected porous hydroxyapatite ceramics for bone tissue engineering. J. R. Soc. Interface. 2009, 6 (Suppl 3), S341–S348. [Google Scholar] [CrossRef] [PubMed]
- Masaoka, T.; Yamada, T.; Yuasa, M.; Yoshii, T.; Okawa, A.; Morita, S.; Kozaka, Y.; Hirano, M.; Sotome, S. Biomechanical evaluation of the rabbit tibia after implantation of porous hydroxyapatite/collagen in a rabbit model. J. Orthop. Sci. 2016, 21, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K.; Akita, K.; Fukuda, N.; Kamada, K.; Kudoh, T.; Ohe, G.; Mano, T.; Tsuru, K.; Ishikawa, K.; Miyamoto, Y. Compositional and histological comparison of carbonate apatite fabricated by dissolution-precipitation reaction and Bio-Oss®. J. Mater. Sci. Mater. Med. 2018, 29, 11. [Google Scholar] [CrossRef]
- Mano, T.; Akita, K.; Fukuda, N.; Kamada, K.; Kurio, N.; Ishikawa, K.; Miyamoto, Y. Histological comparison of three apatitic bone substitutes with different carbonate contents in alveolar bone defects in a beagle mandible with simultaneous implant installation. J. Biomed. Mater. Res. B. Appl. Biomater. 2020, 108, 1450–1459. [Google Scholar] [CrossRef]
- Ogino, Y.; Ayukawa, Y.; Tachikawa, N.; Shimogishi, M.; Miyamoto, Y.; Kudoh, K.; Fukuda, N.; Ishikawa, K.; Koyano, K. Staged Sinus Floor Elevation Using Novel Low-Crystalline Carbonate Apatite Granules: Prospective Results after 3-Year Functional Loading. Materials 2021, 14, 11. [Google Scholar] [CrossRef] [PubMed]
- Fukuba, S.; Okada, M.; Iwata, T. Clinical outcomes of periodontal regenerative therapy with carbonate apatite granules for treatments of intrabony defects, Class II and Class III furcation involvements: A 9-month prospective pilot clinical study. Regen. Ther. 2023, 24, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Pruksakorn, D.; Kongthavonskul, J.; Teeyakasem, P.; Phanphaisarn, A.; Chaiyawat, P.; Klangjorhor, J.; Arpornchayanon, O. Surgical outcomes of extracorporeal irradiation and re-implantation in extremities for high grade osteosarcoma: A retrospective cohort study and a systematic review of the literature. J. Bone Oncol. 2019, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Hamai, R.; Sakai, S. The material design of octacalcium phosphate bone substitute: Increased dissolution and osteogenecity. Acta Biomater. 2023, 158, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O. Octacalcium phosphate: Osteoconductivity and crystal chemistry. Acta Biomater. 2010, 6, 3379–3387. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.L.; Vacanti, J.P.; Paige, K.T.; Upton, J.; Vacanti, C.A. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast. Reconstr. Surg. 1997, 100, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Aoki, K.; Saito, N. Biodegradable Polymers as Drug Delivery Systems for Bone Regeneration. Pharmaceutics 2020, 12, 20. [Google Scholar] [CrossRef]
- Ko, H.F.; Sfeir, C.; Kumta, P.N. Novel synthesis strategies for natural polymer and composite biomaterials as potential scaffolds for tissue engineering. Philos. Trans. A Math Phys. Eng. Sci. 2010, 368, 1981–1997. [Google Scholar] [CrossRef]
- Long, F. Building strong bones: Molecular regulation of the osteoblast lineage. Nat. Rev. Mol. Cell Biol. 2012, 13, 27–38. [Google Scholar] [CrossRef]
- Rashedi, I.; Talele, N.; Wang, X.H.; Hinz, B.; Radisic, M.; Keating, A. Collagen scaffold enhances the regenerative properties of mesenchymal stromal cells. PLoS ONE 2017, 12, e0187348. [Google Scholar] [CrossRef] [PubMed]
- Hoogenkamp, H.R.; Pot, M.W.; Hafmans, T.G.; Tiemessen, D.M.; Sun, Y.; Oosterwijk, E.; Feitz, W.F.; Daamen, W.F.; van Kuppevelt, T.H. Scaffolds for whole organ tissue engineering: Construction and in vitro evaluation of a seamless, spherical and hollow collagen bladder construct with appendices. Acta Biomater. 2016, 43, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, J.C.; Mehr, M.; Buxton, P.G.; Best, S.M.; Cameron, R.E. Optimising collagen scaffold architecture for enhanced periodontal ligament fibroblast migration. J. Mater. Sci. Mater. Med. 2018, 29, 166. [Google Scholar] [CrossRef] [PubMed]
- Carstens, M.H.; Chin, M.; Li, X.J. In situ osteogenesis: Regeneration of 10-cm mandibular defect in porcine model using recombinant human bone morphogenetic protein-2 (rhBMP-2) and helistat absorbable collagen sponge. J. Craniofac. Surg. 2005, 16, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Abada, H.T.; Golzarian, J. Gelatine sponge particles: Handling characteristics for endovascular use. Tech. Vasc. Interv. Radiol. 2007, 10, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Sangro, B.; Bilbao, I.; Herrero, I.; Corella, C.; Longo, J.; Beloqui, O.; Ruiz, J.; Zozaya, J.M.; Quiroga, J.; Prieto, J. Partial splenic embolization for the treatment of hypersplenism in cirrhosis. Hepatology 1993, 18, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Sonohara, S.; Yoshida, M.; Murai, M.; Shimokawa, S.; Fujimoto, R.; Fukushima, S.; Kokubo, S.; Nozaki, K.; Takahashi, K.; et al. A new recombinant human bone morphogenetic protein-2 carrier for bone regeneration. Int. J. Pharm. 2001, 223, 69–79. [Google Scholar] [CrossRef]
- Rohanizadeh, R.; Swain, M.V.; Mason, R.S. Gelatin sponges (Gelfoam) as a scaffold for osteoblasts. J. Mater. Sci. Mater. Med. 2008, 19, 1173–1182. [Google Scholar] [CrossRef]
- Chakraborty, P.K.; Adhikari, J.; Saha, P. Facile fabrication of electrospun regenerated cellulose nanofiber scaffold for potential bone-tissue engineering application. Int J Biol Macromol. 2019, 122, 644–652. [Google Scholar] [CrossRef]
- Sharifi, F.; Atyabi, S.M.; Norouzian, D.; Zandi, M.; Irani, S.; Bakhshi, H. Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int J Biol Macromol. 2018, 115, 243–248. [Google Scholar] [CrossRef]
- Liu, H.; Peng, H.; Wu, Y.; Zhang, C.; Cai, Y.; Xu, G.; Li, Q.; Chen, X.; Ji, J.; Zhang, Y.; et al. The promotion of bone regeneration by nanofibrous hydroxyapatite/chitosan scaffolds by effects on integrin-BMP/Smad signaling pathway in BMSCs. Biomaterials 2013, 34, 4404–4417. [Google Scholar] [CrossRef]
- Yan, H.J.; Casalini, T.; Hulsart-Billström, G.; Wang, S.; Oommen, O.P.; Salvalaglio, M.; Larsson, S.; Hilborn, J.; Varghese, O.P. Synthetic design of growth factor sequestering extracellular matrix mimetic hydrogel for promoting in vivo bone formation. Biomaterials 2018, 161, 190–202. [Google Scholar] [CrossRef]
- Paidikondala, M.; Wang, S.J.; Hilborn, J.; Larsson, S.; Varghese, O.P. Impact of Hydrogel Cross-Linking Chemistry on the in Vitro and in Vivo Bioactivity of Recombinant Human Bone Morphogenetic Protein-2. ACS Appl. Bio Mater. 2019, 2, 2006–2012. [Google Scholar] [CrossRef]
- Bach, F.H.; Fishman, J.A.; Daniels, N.; Proimos, J.; Anderson, B.; Carpenter, C.B.; Forrow, L.; Robson, S.C.; Fineberg, H.V. Uncertainty in xenotransplantation: Individual benefit versus collective risk. Nat. Med. 1998, 4, 141–144. [Google Scholar] [CrossRef]
- Butler, D. Last chance to stop acid think on risks of xenotransplants. Nature 1998, 391, 320–324. [Google Scholar] [CrossRef]
- Bhojani-Lynch, T. Late-Onset Inflammatory Response to Hyaluronic Acid Dermal Fillers. Plast. Reconstr. Surg. Glob. Open. 2017, 5, e1532. [Google Scholar] [CrossRef]
- Han, S.W.; Park, M.J.; Lee, S.H. Hyaluronic acid-induced diffuse alveolar hemorrhage: Unknown complication induced by a well-known injectable agent. Ann. Transl. Med. 2019, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Mullins, R.J.; Richards, C.; Walker, T. Allergic reactions to oral, surgical and topical bovine collagen. Anaphylactic risk for surgeons. Aust. N. Z. J. Ophthalmol. 1996, 24, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Okada, T.; Horiuchi, H.; Murakami, N.; Takahashi, J.; Nawata, M.; Ota, H.; Nozaki, K.; Takaoka, K. A biodegradable polymer as a cytokine delivery system for inducing bone formation. Nat. Biotechnol. 2001, 19, 332–335. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Jiang, Y.; Cai, Y.; Xu, G.; Tong, T.; Zhang, W.; Wang, L.; Ji, J.; Shi, P.; et al. Bi-layer collagen/microporous electrospun nanofiber scaffold improves the osteochondral regeneration. Acta Biomater. 2013, 9, 7236–7247. [Google Scholar] [CrossRef] [PubMed]
- van den Borne, M.P.; Raijmakers, N.J.; Vanlauwe, J.; Victor, J.; de Jong, S.N.; Bellemans, J.; Saris, D.B.; International Cartilage Repair Society. International Cartilage Repair Society (ICRS) and Oswestry macroscopic cartilage evaluation scores validated for use in Autologous Chondrocyte Implantation (ACI) and microfracture. Osteoarthr. Cartil. 2007, 15, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhu, W.; Song, J.; Wei, P.; Yang, F.; Liu, N.; Feng, R. Linear-dendrimer type methoxy-poly (ethylene glycol)-b-poly (epsilon-caprolactone) copolymer micelles for the delivery of curcumin. Drug Deliv. 2015, 22, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Z. Short-peptide-based molecular hydrogels: Novel gelation strategies and applications for tissue engineering and drug delivery. Nanoscale 2012, 4, 5259–5267. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, W.; Chou, J.; Wen, S.; Sun, Y.; Zhang, H. Electrospun nanosilicates-based organic/inorganic nanofibers for potential bone tissue engineering. Colloids Surf. B Biointerfaces 2018, 172, 90–97. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, X.; Huang, Q.; Zhang, R.; Feng, Q. Incorporation of silica nanoparticles to PLGA electrospun fibers for osteogenic differentiation of human osteoblast-like cells. Regen Biomater. 2018, 5, 229–238. [Google Scholar] [CrossRef]
- Timofejeva, A.; D’Este, M.; Loca, D. Calcium phosphate/polyvinyl alcohol composite hydrogels: A review on the freeze-thawing synthesis approach and applications in regenerative medicine. Eur. Polym. J. 2017, 95, 547–565. [Google Scholar] [CrossRef]
- Kim, H.; Yang, G.H.; Choi, C.H.; Cho, Y.S.; Kim, G. Gelatin/PVA scaffolds fabricated using a 3D-printing process employed with a low-temperature plate for hard tissue regeneration: Fabrication and characterizations. Int. J. Biol. Macromol. 2018, 120, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, W.C.; Hsu, C.C.; Shiu, L.Y.; Zeng, Y.J. Biocompatible testing and physical properties of curdlan-grafted poly(vinyl alcohol) scaffold for bone tissue engineering. Carbohydr. Polym. 2017, 157, 1341–1348. [Google Scholar] [CrossRef]
- Venugopal, J.; Low, S.; Choon, A.T.; Sampath Kumar, T.S.; Ramakrishna, S. Mineralization of osteoblasts with electrospun collagen/hydroxyapatite nanofibers. J. Mater. Sci. Mater. Med. 2008, 19, 2039–2046. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Yeo, M.; Lee, H.; Kim, G. Three-dimensional hierarchical composite scaffolds consisting of polycaprolactone, beta-tricalcium phosphate, and collagen nanofibers: Fabrication, physical properties, and in vitro cell activity for bone tissue regeneration. Biomacromolecules 2011, 12, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kane, R.J.; Weiss-Bilka, H.E.; Meagher, M.J.; Liu, Y.X.; Gargac, J.A.; Niebur, G.L.; Wagner, D.R.; Roeder, R.K. Hydroxyapatite reinforced collagen scaffolds with improved architecture and mechanical properties. Acta Biomater. 2015, 17, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Enayati, M.S.; Behzad, T.; Sajkiewicz, P.; Rafienia, M.; Bagheri, R.; Ghasemi-Mobarakeh, L.; Kolbuk, D.; Pahlevanneshan, Z.; Bonakdar, S.H. Development of electrospun poly (vinyl alcohol)-based bionanocomposite scaffolds for bone tissue engineering. J. Biomed. Mater. Res. A. 2018, 106, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, J.S.; Alvarez, V.A. Mechanical properties of polyvinylalcohol/hydroxyapatite cryogel as potential artificial cartilage. J. Mech. Behav. Biomed. Mater. 2014, 34, 47–56. [Google Scholar] [CrossRef]
- Hamai, R.; Sakai, S.; Shiwaku, Y.; Anada, T.; Tsuchiya, K.; Ishimoto, T.; Nakano, T.; Suzuki, O. Octacalcium phosphate crystals including a higher density dislocation improve its materials osteogenecity. Appl. Mater. Today 2022, 26, 12. [Google Scholar] [CrossRef]
- Ruckh, T.T.; Carroll, D.A.; Weaver, J.R.; Popat, K.C. Mineralization Content Alters Osteogenic Responses of Bone Marrow Stromal Cells on Hydroxyapatite/Polycaprolactone Composite Nanofiber Scaffolds. J. Funct. Biomater. 2012, 3, 776–798. [Google Scholar] [CrossRef]
- Mohseni, M.; Jahandideh, A.; Abedi, G.; Akbarzadeh, A.; Hesaraki, S. Assessment of tricalcium phosphate/collagen (TCP/collagene) nanocomposite scaffold compared with hydroxyapatite (HA) on healing of segmental femur bone defect in rabbits. Artif. Cells Nanomed. Biotechnol. 2018, 46, 242–249. [Google Scholar] [CrossRef]
- Suzuki, O.; Shiwaku, Y.; Hamai, R. Octacalcium phosphate bone substitute materials: Comparison between properties of biomaterials and other calcium phosphate materials. Dent. Mater. J. 2020, 39, 187–199. [Google Scholar] [CrossRef]
- Li, Z.M.; Tang, S.; Shi, Z.; Li, B.; Feng, D.; Xie, D.L.; Han, T.; Li, C.Y. Multi-scale cellular PLA-based bionic scaffold to promote bone regrowth and repair. Int. J. Biol. Macromol. 2023, 245, 12. [Google Scholar] [CrossRef]
- Wang, W.Z.; Liu, P.; Zhang, B.Q.; Gui, X.Y.; Pei, X.; Song, P.; Yu, X.; Zhang, Z.D.; Zhou, C.C. Fused Deposition Modeling Printed PLA/Nano 0-TCP Composite Bone Tissue Engineering Scaffolds for Promoting Osteogenic Induction Function. Int. J. Nanomed. 2023, 18, 5815–5830. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Azar, M.H.; Sayedain, S.S.; Dargah, M.S.; Alizadeh, R.; Arab, M.; Askarinya, A.; Kaviani, A.; Beheshtizadeh, N.; Azami, M. Material extrusion additive manufacturing of poly(lactic acid)/Ti6Al4V@calcium phosphate core-shell nanocomposite scaffolds for bone tissue applications. Int. J. Biol. Macromol. 2024, 255, 15. [Google Scholar] [CrossRef] [PubMed]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C. 2019, 104, 13. [Google Scholar] [CrossRef] [PubMed]
- Hokugo, A.; Ozeki, M.; Kawakami, O.; Sugimoto, K.; Mushimoto, K.; Morita, S.; Tabata, Y. Augmented bone regeneration activity of platelet-rich plasma by biodegradable gelatin hydrogel. Tissue Eng. 2005, 11, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Re, F.; Sartore, L.; Moulisova, V.; Cantini, M.; Almici, C.; Bianchetti, A.; Chinello, C.; Dey, K.; Agnelli, S.; Manferdini, C.; et al. 3D gelatin-chitosan hybrid hydrogels combined with human platelet lysate highly support human mesenchymal stem cell proliferation and osteogenic differentiation. J. Tissue Eng. 2019, 10, 2041731419845852. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Tsujigiwa, H.; Nagatsuka, H.; Nagai, N.; Yoshinobu, J.; Okano, M.; Fukushima, K.; Takeuchi, A.; Yoshino, T.; Nishizaki, K. Regeneration of rat auditory ossicles using recombinant human BMP-2/collagen composites. J. Biomed. Mater. Res. A. 2005, 73, 133–141. [Google Scholar] [CrossRef]
- Takahashi, Y.; Yamamoto, M.; Tabata, Y. Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and beta-tricalcium phosphate. Biomaterials 2005, 26, 3587–3596. [Google Scholar] [CrossRef]
- Aoki, K.; Haniu, H.; Kim, Y.A.; Saito, N. The Use of Electrospun Organic and Carbon Nanofibers in Bone Regeneration. Nanomaterials 2020, 10, 14. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Lee, H.; Yeo, M.; Ahn, S.; Kang, D.O.; Jang, C.H.; Lee, H.; Park, G.M.; Kim, G.H. Designed hybrid scaffolds consisting of polycaprolactone microstrands and electrospun collagen-nanofibers for bone tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 263–270. [Google Scholar] [CrossRef]
- Deng, N.; Sun, J.; Li, Y.; Chen, L.; Chen, C.; Wu, Y.; Wang, Z.; Li, L. Experimental study of rhBMP-2 chitosan nano-sustained release carrier-loaded PLGA/nHA scaffolds to construct mandibular tissue-engineered bone. Arch. Oral. Biol. 2019, 102, 16–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Fu, L.; Ye, S.; Wang, M.; Zhou, Y. Fabrication and Application of Novel Porous Scaffold in Situ-Loaded Graphene Oxide and Osteogenic Peptide by Cryogenic 3D Printing for Repairing Critical-Sized Bone Defect. Molecules 2019, 24, 1669. [Google Scholar] [CrossRef]
- Das, A.; Fishero, B.A.; Christophel, J.J.; Li, C.J.; Kohli, N.; Lin, Y.; Dighe, A.S.; Cui, Q. Poly(lactic-co-glycolide) polymer constructs cross-linked with human BMP-6 and VEGF protein significantly enhance rat mandible defect repair. Cell Tissue Res. 2016, 364, 125–135. [Google Scholar] [CrossRef]
- Berner, A.; Boerckel, J.D.; Saifzadeh, S.; Steck, R.; Ren, J.; Vaquette, C.; Zhang, J.Q.; Nerlich, M.; Guldberg, R.E.; Hutmacher, D.W.; et al. Biomimetic tubular nanofiber mesh and platelet rich plasma-mediated delivery of BMP-7 for large bone defect regeneration. Cell Tissue Res. 2012, 347, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Asatrian, G.; Pham, D.; Hardy, W.R.; James, A.W.; Peault, B. Stem cell technology for bone regeneration: Current status and potential applications. Stem Cells Cloning. 2015, 8, 39–48. [Google Scholar] [PubMed]
- Jin, Y.Z.; Lee, J.H. Mesenchymal Stem Cell Therapy for Bone Regeneration. Clin. Orthop. Surg. 2018, 10, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario, C.; Rodriguez-Evora, M.; Reyes, R.; Delgado, A.; Evora, C. BMP-2, PDGF-BB, and bone marrow mesenchymal cells in a macroporous beta-TCP scaffold for critical-size bone defect repair in rats. Biomed. Mater. 2015, 10, 045008. [Google Scholar] [CrossRef] [PubMed]
- Roussy, Y.; Bertrand Duchesne, M.P.; Gagnon, G. Activation of human platelet-rich plasmas: Effect on growth factors release, cell division and in vivo bone formation. Clin. Oral Implants Res. 2007, 18, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Ma, X.; Li, J.; Cheng, Y.; Cao, Y.; Wang, Z.; Shi, X.; Du, Y.; Deng, H.; Li, Z. Incorporating platelet-rich plasma into coaxial electrospun nanofibers for bone tissue engineering. Int J Pharm. 2018, 547, 656–666. [Google Scholar] [CrossRef]






| Author, Year | Natural or Synthetic | Polymer | Characteristics |
|---|---|---|---|
| Castens et al., 2005 [54] | Natural | Collagen | Used to repair porcine mandibular bone defect |
| Yokota et al., 2001 [57] | Natural | Gelatin | Coated in poly(D,L-lactic-co-glycolic acid) |
| Rohanizadeh et al., 2008 [58] | Natural | Gelatin | Used to culture human osteoblast-like cells |
| Chakraborty et al., 2001 [59] | Natural | Cellulose | Non-woven nanofibrous scaffolds made by electrospinnig |
| Sharifi et al., 2018 [60] | Natural | Chitosan | Composite with PCL |
| Liu et al., 2018 [61] | Natural | Chitosan | Composite with HA |
| Yan et al., 2018 [62] | Natural | Hyaluronic acid | Used as carrier for BMP-2 to form ectopic bone in rat |
| Paidikondala et al., 2019 [63] | Natural | Hyaluronic acid | Composite with hydrazone |
| Saito et al., 2001 [69] | Synthetic | PLA-DX-PEG | Used to form ectopic bone in dorsum of mouse |
| Aoki et al., 2020 [48] | Synthetic | PLA-DX-PEG | Used to repair ulnar segmental bone defect of rabbit |
| Zhang et al., 2013 [70] | Synthetic | PLA | Composite with collagen |
| Wang et al., 2018 [74] | Synthetic | PCL | Composite with nanosilicate |
| Yang et al., 2018 [75] | Synthetic | PLGA | Composite with nanosilicate |
| Kim et al., 2018 [77] | Synthetic | PVA | 3D-printed scaffold |
| Hsieh et al., 2018 [78] | Synthetic | PVA | Used to evaluate biodegradation of 3D scaffolds |
| Author, Year | Biodegradable Polymer | Calcium Phosphate | Characteristics |
|---|---|---|---|
| Venugopal et al., 2008 [79] | Collagen | HA | Used to evaluate calcification caused by human fetal osteoblast cells |
| Yeo et al., 2011 [81] | PCL, collagen | βTCP | Used to culture human osteoblast-like cells |
| Kane et al., 2015 [83] | Collagen | HA | Used to evaluate the compressive modulus of the scaffold |
| Enayati et al., 2018 [84] | PVA | HA | Used to culture human osteoblast-like cells |
| Hamai et al., 2022 [86] | Gelatin | OCP | Used to repair critical-sized calvarial defect of rat |
| Ruckh et al., 2012 [87] | PCL | HA | Used to evaluate osteogenic potential according to HA content |
| Mohseni et al., 2018 [88] | Collagen | βTCP | Used to repair ulnar segmental bone defect of rabbit |
| Suzuki et al., 2020 [89] | Collagen | OCP | Used to compare HA and βTCP |
| Li et al., 2023 [90] | PLA | HA | Used to evaluate osteogenic potential of rat BMSCs |
| Zarei et al., 2024 [92] | PLA | OCP | Composite with Ti6Al4V to evaluate compressive strength |
| Hassanajili et al., 2019 [93] | PLA, PCL | HA | Used to evaluate porosity and compressive modulus with blending ratio of each material |
| Author, Year | Materials | Structure |
|---|---|---|
| Saito et al., 2001 [69] | PLA-DX-PEG | Hydrogel |
| Aoki et al., 2020 [48] | PLA-DX-PEG | Hydrogel |
| Hokugo et al., 2005 [94] | Gelatin | Hydrogel |
| Re et al., 2019 [95] | Chitosan | Hydrogel |
| Takeda et al., 2005 [96] | Collagen | Sponge made by freeze-drying |
| Takahashi et al., 2005 [97] | Gelatin/βTCP | Sponge made by freeze-drying |
| Lee et al., 2011 [100] | Collagen | Collagen nanofiber made by electrospinning, hardened with PCL |
| Enayati et al., 2018 [84] | PVA/HA | Fiber made by electrospinning |
| Deng et al., 2019 [101] | PLGA/HA/chitosan | 3D printing |
| Zhang et al., 2019 [102] | PLGA/βTCP/GO | 3D printing |
| Author, Year | Materials | Structure |
|---|---|---|
| Deng et al., 2019 [101] | PLGA/HA/chitosan | BMP-2 |
| Das et al., 2016 [103] | PLAGA | BMP-6, VEGF |
| Berner et al., 2012 [104] | PCL | BMP-7, PRP |
| Liu et al., 2013 [61] | Chitosan/HA | BMSC |
| Cheng et al., 2018 [109] | Silk fibroin/PCL | PRP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoki, K.; Ideta, H.; Komatsu, Y.; Tanaka, A.; Kito, M.; Okamoto, M.; Takahashi, J.; Suzuki, S.; Saito, N. Bone-Regeneration Therapy Using Biodegradable Scaffolds: Calcium Phosphate Bioceramics and Biodegradable Polymers. Bioengineering 2024, 11, 180. https://doi.org/10.3390/bioengineering11020180
Aoki K, Ideta H, Komatsu Y, Tanaka A, Kito M, Okamoto M, Takahashi J, Suzuki S, Saito N. Bone-Regeneration Therapy Using Biodegradable Scaffolds: Calcium Phosphate Bioceramics and Biodegradable Polymers. Bioengineering. 2024; 11(2):180. https://doi.org/10.3390/bioengineering11020180
Chicago/Turabian StyleAoki, Kaoru, Hirokazu Ideta, Yukiko Komatsu, Atsushi Tanaka, Munehisa Kito, Masanori Okamoto, Jun Takahashi, Shuichiro Suzuki, and Naoto Saito. 2024. "Bone-Regeneration Therapy Using Biodegradable Scaffolds: Calcium Phosphate Bioceramics and Biodegradable Polymers" Bioengineering 11, no. 2: 180. https://doi.org/10.3390/bioengineering11020180
APA StyleAoki, K., Ideta, H., Komatsu, Y., Tanaka, A., Kito, M., Okamoto, M., Takahashi, J., Suzuki, S., & Saito, N. (2024). Bone-Regeneration Therapy Using Biodegradable Scaffolds: Calcium Phosphate Bioceramics and Biodegradable Polymers. Bioengineering, 11(2), 180. https://doi.org/10.3390/bioengineering11020180