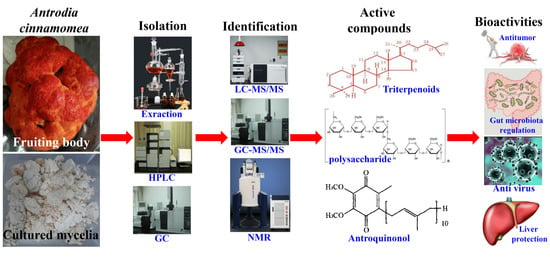
Review of Bioactivity, Isolation, and Identification of Active Compounds from Antrodia cinnamomea
Abstract
:1. Introduction
2. Isolation and Purification of Bioactive Compounds from A. cinnamomea
2.1. Polysaccharides
2.2. Triterpenoids
2.3. Ubiquinone Derivatives
2.4. Maleic and Succinic Acid Derivatives
2.5. Benzene Derivatives
2.6. Gycoproteins
3. Identification and Quantification of Bioactive Compounds from A. cinnamomea
4. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, M.C.; El-Shazly, M.; Wu, T.Y.; Du, Y.C.; Chang, T.T.; Chen, C.F.; Hsu, Y.M.; Lai, K.H.; Chiu, C.P.; Chang, F.R.; et al. Recent research and development of Antrodia cinnamomea. Pharmacol Ther. 2013, 139, 124–156. [Google Scholar] [CrossRef]
- Chang, T.T.; Chou, W.N. Antrodia cinnamomea sp. nov. on Cinnamomum kanehirai in Taiwan. Mycol. Res. 1995, 99, 756–758. [Google Scholar] [CrossRef]
- Liu, Y.K.; Chen, K.H.; Leu, Y.L.; Way, T.D.; Wang, L.W.; Chen, Y.J.; Liu, Y.M. Ethanol extracts of Cinnamomum kanehirai Hayata leaves induce apoptosis in human hepatoma cell through caspase-3 cascade. Onco Targets Ther. 2015, 8, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Liu, H.; Liu, J.; Xing, H.; Wang, F.; Deng, L. Efficient production of 4-Acetylantroquinonol B from Antrodia cinnamomea through two-stage carbon source coordination optimization. Bioresour. Technol. Rep. 2021, 15, 100732. [Google Scholar] [CrossRef]
- Ao, Z.H.; Xu, Z.H.; Lu, Z.M.; Xu, H.Y.; Zhang, X.M.; Dou, W.F. Niuchangchih (Antrodia camphorata) and its potential in treating liver diseases. J. Ethnopharmacol. 2009, 121, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Ning, X.; Ye, M.; Yin, Y. Antrodia camphorata extract (ACE)-induced apoptosis is associated with BMP4 expression and p53-dependent ROS generation in human colon cancer cells. J. Ethnopharmacol. 2021, 268, 113570. [Google Scholar] [CrossRef]
- Cheng, J.J.; Chao, C.H.; Chang, P.C.; Lu, M.K. Studies on anti-inflammatory activity of sulfated polysaccharides from cultivated fungi Antrodia cinnamomea. Food Hydrocoll. 2016, 53, 37–45. [Google Scholar] [CrossRef]
- Lin, I.Y.; Pan, M.H.; Lai, C.S.; Lin, T.T.; Chen, C.T.; Chung, T.S.; Chen, C.L.; Lin, C.H.; Chuang, W.C.; Lee, M.C.; et al. CCM111, the water extract of Antrodia cinnamomea, regulates immune-related activity through STAT3 and NF-kappaB pathways. Sci. Rep. 2017, 7, 4862. [Google Scholar] [CrossRef]
- Chao, T.Y.; Hsieh, C.C.; Hsu, S.M.; Wan, C.H.; Lian, G.T.; Tseng, Y.H.; Kuo, Y.H.; Hsieh, S.C. Ergostatrien-3beta-ol (EK100) from Antrodia camphorata attenuates oxidative stress, inflammation, and liver injury in nitro and in vivo. Prev. Nutr. Food Sci. 2021, 26, 58–66. [Google Scholar] [CrossRef]
- Zhang, B.B.; Guan, Y.Y.; Hu, P.F.; Chen, L.; Xu, G.R.; Liu, L.; Cheung, P.C.K. Production of bioactive metabolites by submerged fermentation of the medicinal mushroom Antrodia cinnamomea: Recent advances and future development. Crit. Rev. Biotechnol. 2019, 39, 541–554. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Z.; Zhang, B.; Ge, M.; Ng, H.; Niu, Y.; Liu, L. Production, structure and morphology of exopolysaccharides yielded by submerged fermentation of Antrodia cinnamomea. Carbohydr. Polym. 2019, 205, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.W.; Chen, T.C.; Liu, C.H.; Wang, S.Y.; Shaw, J.F.; Chen, Y.T. Identification and isolation of an intermediate metabolite with dual antioxidant and anti-proliferative activity present in the fungus Antrodia cinnamomea cultured on an alternative medium with Cinnamomum kanehirai leaf extract. Plants 2021, 10, 737. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, N.; Baskaran, R.; Velmurugan, B.K.; Thanh, N.C. Antrodia cinnamomea-An updated minireview of its bioactive components and biological activity. J. Food Biochem. 2019, 43, e12936. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Tzeng, Y.M. Review of Pharmacological Effects of Antrodia camphorata and Its Bioactive Compounds. Evid. Based Complement. Altern. Med. 2011, 2011, 212641. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ding, Y.; Ye, M.; Zhu, T.; Tian, D.; Ding, K. A Novel Heterogalactan from Antrodia camphorata and anti-angiogenic activity of its sulfated derivative. Polymers 2017, 9, 228. [Google Scholar] [CrossRef]
- Lin, C.C.; Pan, I.H.; Li, Y.R.; Pan, Y.G.; Lin, M.K.; Lu, Y.H.; Wu, H.C.; Chu, C.L. The adjuvant effects of high-molecule-weight polysaccharides purified from Antrodia cinnamomea on dendritic cell function and DNA vaccines. PLoS ONE 2015, 10, e0116191. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Guo, L.; Yang, Y.; Li, W.; Sheng, Y.; Wang, J.; Guan, Q.; Zhang, X. Study on antrodia camphorata polysaccharide in alleviating the neuroethology of PD mice by decreasing the expression of NLRP3 inflammasome. Phytother. Res. 2019, 33, 2288–2297. [Google Scholar] [CrossRef]
- Han, C.; Shen, H.; Yang, Y.; Sheng, Y.; Wang, J.; Li, W.; Zhou, X.; Guo, L.; Zhai, L.; Guan, Q. Antrodia camphorata polysaccharide resists 6-OHDA-induced dopaminergic neuronal damage by inhibiting ROS-NLRP3 activation. Brain Behav. 2020, 10, e01824. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Li, D.; Zang, W.; Zhu, H.; Wu, P.; Mei, Y.; Liang, Y. A polysaccharide from Antrodia cinnamomea mycelia exerts antitumor activity through blocking of TOP1/TDP1-mediated DNA repair pathway. Int. J. Biol. Macromol. 2018, 120, 1551–1560. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, A.; Qu, Y.; Wang, Z.; Zhang, Y.; Liu, Y.; Wang, N.; Teng, L.; Wang, D. Ameliorative effects of Antrodia cinnamomea polysaccharides against cyclophosphamide-induced immunosuppression related to Nrf2/HO-1 signaling in BALB/c mice. Int. J. Biol. Macromol. 2018, 116, 8–15. [Google Scholar] [CrossRef]
- Perera, N.; Yang, F.L.; Chang, C.M.; Lu, Y.T.; Zhan, S.H.; Tsai, Y.T.; Hsieh, J.F.; Li, L.H.; Hua, K.F.; Wu, S.H. Galactomannan from Antrodia cinnamomea Enhances the Phagocytic Activity of Macrophages. Org. Lett. 2017, 19, 3486–3489. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Tang, H.; Zha, Z.; Yin, H.; Wang, Y.; Wang, Y.; Li, H.; Yue, L. beta-d-glucan from Antrodia Camphorata ameliorates LPS-induced inflammation and ROS production in human hepatocytes. Int. J. Biol. Macromol. 2017, 104, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Perera, N.; Yang, F.L.; Lu, Y.T.; Li, L.H.; Hua, K.F.; Wu, S.H. Antrodia cinnamomea galactomannan elicits immuno-stimulatory activity through toll-like receptor 4. Int. J. Biol. Sci. 2018, 14, 1378–1388. [Google Scholar] [CrossRef]
- Tang, H.; Nie, W.; Xiao, J.; Zha, Z.; Chen, Q.; Yin, H. Structural characterization and anti-inflammatory effect in hepatocytes of a galactoglucan from Antrodia camphorata mycelium. RSC Adv. 2019, 9, 7664–7672. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.J.; Chao, C.H.; Lu, M.K. Large-scale preparation of sulfated polysaccharides with anti-angionenic and anti-inflammatory properties from Antrodia cinnamomia. Int. J. Biol. Macromol. 2018, 113, 1198–1205. [Google Scholar] [CrossRef]
- Lu, M.K.; Lin, T.Y.; Chang, C.C. Chemical identification of a sulfated glucan from Antrodia cinnamomea and its anti-cancer functions via inhibition of EGFR and mTOR activity. Carbohydr. Polym. 2018, 202, 536–544. [Google Scholar] [CrossRef]
- Lin, T.Y.; Tseng, A.J.; Chao, C.H.; Lu, M.K. Microelements induce changes in characterization of sulfated polysaccharides from Antrodia cinnamomea. Int. J. Biol. Macromol. 2018, 120, 952–958. [Google Scholar] [CrossRef]
- Lee, M.H.; Chao, C.H.; Hsu, Y.C.; Lu, M.K. Production, characterization, and functions of sulfated polysaccharides from zinc sulfate enriched cultivation of Antrodia cinnamomea. Int. J. Biol. Macromol. 2020, 159, 1013–1021. [Google Scholar] [CrossRef]
- Lu, M.K.; Lee, M.H.; Chao, C.H.; Hsu, Y.C. Physiochemical changes and mechanisms of anti-inflammation effect of sulfated polysaccharides from ammonium sulfate feeding of Antrodia cinnamomea. Int. J. Biol. Macromol. 2020, 148, 715–721. [Google Scholar] [CrossRef]
- Albano, R.M.; Mourão, P.A. Isolation, fractionation, and preliminary characterization of a novel class of sulfated glycans from the tunic of Styela plicata (Chordata Tunicata). Int. J. Biol. Sci. 1986, 261, 758–765. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.B.; Zhao, J.W.; Lu, C.J.; Zhu, W. Structural characterization and macrophage immunomodulatory activity of a novel polysaccharide from Smilax glabra Roxb. Carbohydr. Polym. 2017, 156, 390–402. [Google Scholar] [CrossRef]
- Lu, M.K.; Chao, C.H.; Hsu, Y.C.; Chang, C.C. Structural sequencing and anti-inflammatory, anti-lung cancer activities of 1,4-alpha/beta-sulfomalonoglucan in Antrodia cinnamomea. Int. J. Biol. Macromol. 2021, 170, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Wang, Q.; Ji, S.; Huang, Y.; Liu, K.D.; Zhang, Z.X.; Bo, T.; Tzeng, Y.M.; Guo, D.A.; Ye, M. Metabolites identification and multi-component pharmacokinetics of ergostane and lanostane triterpenoids in the anticancer mushroom Antrodia cinnamomea. J. Pharm. Biomed. Anal. 2015, 111, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Li, B.; Wang, Z.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2021, 38, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Y.; Fan, W.L.; Wang, W.F.; Chen, T.; Tang, Y.C.; Chu, F.H.; Chang, T.T.; Wang, S.Y.; Li, M.Y.; Chen, Y.H.; et al. Genomic and transcriptomic analyses of the medicinal fungus Antrodia cinnamomea for its metabolite biosynthesis and sexual development. Proc. Natl. Acad. Sci. USA 2014, 111, E4743–E4752. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, R.; Li, L.; Dong, R.; Yin, H.; Wang, Y.; Yang, A.; Wang, J.; Li, C.; Wang, D. The triterpenoids-enriched extracts from Antrodia cinnamomea mycelia attenuate alcohol-induced chronic liver injury via suppression lipid accumulation in C57BL/6 mice. Food Sci. Hum. Wellness 2021, 10, 497–507. [Google Scholar] [CrossRef]
- Wang, Y.J.; Lee, S.C.; Hsu, C.H.; Kuo, Y.H.; Yang, C.C.; Lin, F.J. Antcins, triterpenoids from Antrodia cinnamomea, as new agonists for peroxisome proliferator-activated receptor α. J. Food Drug Anal. 2019, 27, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Liu, Y.L.; Li, F.Y.; Chang, C.I.; Wang, S.Y.; Lee, K.Y.; Li, S.L.; Chen, Y.P.; Jinn, T.R.; Tzen, J.T. Antcin A, a steroid-like compound from Antrodia camphorata, exerts anti-inflammatory effect via mimicking glucocorticoids. Acta Pharmacol. Sin. 2011, 32, 904–911. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C. Dehydroeburicoic acid from Antrodia camphorata prevents the diabetic and dyslipidemic state via modulation of glucose transporter 4, peroxisome proliferator-activated receptor alpha expression and amp-activated protein kinase phosphorylation in high-fat-fed mice. Int. J. Mol. Sci. 2016, 17, 872. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-H.; Kuo, Y.H.; Shih, C.C. Antidiabetic and hypolipidemic activities of eburicoic acid, a triterpenoid compound from Antrodia camphorata, by regulation of Akt phosphorylation, gluconeogenesis, and PPARα in streptozotocin-induced diabetic mice. RSC Adv. 2018, 8, 20462–20476. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Hsiao, L.W.; Kuo, Y.H.; Shih, C.C. Antidiabetic and antihyperlipidemic effects of sulphurenic acid, a triterpenoid compound from Antrodia camphorata, in streptozotocin-induced diabetic mice. Int. J. Mol. Sci. 2019, 20, 4897. [Google Scholar] [CrossRef]
- Qiao, X.; Song, W.; Wang, Q.; Liu, K.D.; Zhang, Z.X.; Bo, T.; Li, R.Y.; Liang, L.N.; Tzeng, Y.M.; Guo, D.A.; et al. Comprehensive chemical analysis of triterpenoids and polysaccharides in the medicinal mushroom Antrodia cinnamomea. RSC Adv. 2015, 5, 47040–47052. [Google Scholar] [CrossRef]
- Zhang, W.; Li, B.; Dong, X.; Wang, B.; Wu, Z.-Y. Enzyme-assisted extraction of cordycepin and adenosine from cultured Cordyceps militaris and purification by macroporous resin column chromatography. Sep. Sci. Technol. 2017, 52, 1350–1358. [Google Scholar] [CrossRef]
- Li, B.; Kuang, Y.; Zhang, M.; He, J.B.; Xu, L.L.; Leung, C.H.; Ma, D.L.; Lo, J.Y.; Qiao, X.; Ye, M. Cytotoxic triterpenoids from Antrodia camphorata as sensitizers of paclitaxel. Org. Chem. Front. 2020, 7, 768–779. [Google Scholar] [CrossRef]
- Li, B.; Kuang, Y.; He, J.B.; Tang, R.; Xu, L.L.; Leung, C.H.; Ma, D.L.; Qiao, X.; Ye, M. Antcamphorols A-K, cytotoxic and ros scavenging triterpenoids from Antrodia camphorata. J. Nat. Prod. 2020, 83, 45–54. [Google Scholar] [CrossRef]
- Qiao, X.; An, R.; Huang, Y.; Ji, S.; Li, L.; Tzeng, Y.M.; Guo, D.A.; Ye, M. Separation of 25R/S-ergostane triterpenoids in the medicinal mushroom Antrodia camphorata using analytical supercritical-fluid chromatography. J. Chromatogr. A 2014, 1358, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.S.; Vani, M.G.; Hsieh, H.W.; Lin, C.C.; Wang, S.Y. Antcin-A modulates epithelial-to-mesenchymal transition and inhibits migratory and invasive potentials of human breast cancer cells via p53-mediated mir-200c activation. Planta Med. 2019, 85, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.I.; Chu, Y.L.; Ho, C.T.; Su, Y.C.; Kuo, Y.H.; Sheen, L.Y. Antcin K, an active triterpenoid from the fruiting bodies of basswood cultivated Antrodia cinnamomea, induces mitochondria and endoplasmic reticulum stress-mediated apoptosis in human hepatoma cells. J. Tradit. Complement Med. 2016, 6, 48–56. [Google Scholar] [CrossRef]
- Tsai, W.C.; Rao, Y.K.; Lin, S.S.; Chou, M.Y.; Shen, Y.T.; Wu, C.H.; Geethangili, M.; Yang, C.C.; Tzeng, Y.M. Methylantcinate A induces tumor specific growth inhibition in oral cancer cells via Bax-mediated mitochondrial apoptotic pathway. Bioorg. Med. Chem. Lett. 2010, 20, 6145–6148. [Google Scholar] [CrossRef]
- Zhang, B.B.; Hu, P.F.; Huang, J.; Hu, Y.D.; Chen, L.; Xu, G.R. Current advances on the structure, bioactivity, synthesis, and metabolic regulation of novel ubiquinone derivatives in the edible and medicinal mushroom Antrodia cinnamomea. J. Agric. Food Chem. 2017, 65, 10395–10405. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Wu, H.C.; Chang, J.M.; Ko, H.H.; Lin, C.H.; Chang, H.S. Chemical investigations and cytotoxic effects of metabolites from Antrodia camphorata against human hepatocellular carcinoma cells. Nat. Prod. Res. 2022, 1–11. [Google Scholar] [CrossRef]
- Lin, H.C.; Lin, M.H.; Liao, J.H.; Wu, T.H.; Lee, T.H.; Mi, F.L.; Wu, C.H.; Chen, K.C.; Cheng, C.H.; Lin, C.W. Antroquinonol, a ubiquinone derivative from the mushroom antrodia camphorata, inhibits colon cancer stem cell-like properties: Insights into the molecular mechanism and inhibitory targets. J. Agric. Food Chem. 2017, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Li, Q.; Song, X.; Xu, W.; Li, L.; Xu, A. Antroquinonol exerts immunosuppressive effect on cd8(+) t cell proliferation and activation to resist depigmentation induced by H2O2. Oxid. Med. Cell. Longev. 2017, 2017, 9303054. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Yeh, W.J.; Tan, H.Y.; Yang, H.Y. Antroquinonol attenuated abdominal and hepatic fat accumulation in rats fed an obesogenic diet. J. Food Sci. 2019, 84, 2682–2687. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, C.K.; Tsou, W.L.; Liu, S.Y.; Kuo, M.T.; Wen, W.C. A new cytotoxic agent from solid-state fermented mycelium of Antrodia camphorata. Planta Med. 2007, 73, 1412–1415. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, Y.; Liu, X.; Zhou, X.; Wang, Z.; Wang, G.; Xiong, Z.; Ai, L. Enhancement of antroquinonol production during batch fermentation using pH control coupled with an oxygen vector. J. Sci. Food Agric. 2019, 99, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhou, X.; Liang, L.; Liu, X.; Li, H.; Xiong, Z.; Wang, G.; Song, X.; Ai, L. Genetic evidence for the requirements of antroquinonol biosynthesis by Antrodia camphorata during liquid-state fermentation. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab086. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, S.; Wang, C.; Sun, W.; Jin, Y.; Gong, X.; Tong, S. Off-line comprehensive two-dimensional reversed-phase countercurrent chromatography with high-performance liquid chromatography: Orthogonality in separation of Polygonum cuspidatum Sieb. et Zucc. J. Sep. Sci. 2020, 43, 561–568. [Google Scholar] [CrossRef]
- Wang, C.; Zuo, G.; Wang, X.; Kim, H.Y.; Zhao, S.; Sun, W.; Tong, S.; Lim, S.S. Retention mechanism of pH-peak-focusing in countercurrent chromatographic separation of baicalin and wogonoside from Scutellaria baicalensis Georgi. J. Sep. Sci. 2020, 43, 3806–3815. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, L.; Pu, F.; Xie, Z.; Tong, S.; Yan, J. An efficient high-speed countercurrent chromatography method for preparative isolation of highly potent anti-cancer compound antroquinonol from Antrodia camphorata after experimental design optimized extraction. J. Sep. Sci. 2021, 44, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Pan, J.H.; Liu, R.H.; Kuo, Y.H.; Sheen, L.Y.; Chiang, B.H. The 4-acetylantroquinonol B isolated from mycelium of Antrodia cinnamomea inhibits proliferation of hepatoma cells. J. Sci. Food Agric. 2010, 90, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.F.; Wang, S.W.; Lai, Y.W.; Liu, S.C.; Chen, Y.J.; Hsueh, T.Y.; Lin, C.C.; Lin, C.H.; Chung, C.H. 4-Acetylantroquinonol B suppresses prostate cancer growth and angiogenesis via a VEGF/PI3K/ERK/mTOR-dependent signaling pathway in subcutaneous xenograft and in vivo angiogenesis models. Int. J. Mol. Sci. 2022, 23, 1446. [Google Scholar] [CrossRef] [PubMed]
- Yen, I.C.; Yao, C.W.; Kuo, M.T.; Chao, C.L.; Pai, C.Y.; Chang, W.L. Anti-cancer agents derived from solid-state fermented Antrodia camphorata mycelium. Fitoterapia 2015, 102, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Yen, I.C.; Lee, S.Y.; Lin, K.T.; Lai, F.Y.; Kuo, M.T.; Chang, W.L. In vitro anticancer activity and structural characterization of ubiquinones from Antrodia cinnamomea mycelium. Molecules 2017, 22, 747. [Google Scholar] [CrossRef]
- Chen, Y.L.; Yen, I.C.; Lin, K.T.; Lai, F.Y.; Lee, S.Y. 4-Acetylantrocamol LT3, a New ubiquinone from Antrodia cinnamomea, inhibits hepatocellular carcinoma hepg2 cell growth by targeting YAP/TAZ, mTOR, and WNT/beta-catenin signaling. Am. J. Chin. Med. 2020, 48, 1243–1261. [Google Scholar] [CrossRef]
- Yang, S.S.; Wang, G.J.; Wang, S.Y.; Lin, Y.Y.; Kuo, Y.H.; Lee, T.H. New constituents with iNOS inhibitory activity from mycelium of Antrodia camphorata. Planta Med. 2009, 75, 512–516. [Google Scholar] [CrossRef]
- Speybrouck, D.; Lipka, E. Preparative supercritical fluid chromatography: A powerful tool for chiral separations. J. Chromatogr. A 2016, 1467, 33–55. [Google Scholar] [CrossRef]
- Gokila Vani, M.; Kumar, K.J.; Liao, J.W.; Chien, S.C.; Mau, J.L.; Chiang, S.S.; Lin, C.C.; Kuo, Y.H.; Wang, S.Y. Antcin C from Antrodia cinnamomea protects liver cells against free radical-induced oxidative stress and apoptosis in vitro and in vivo through Nrf2-dependent mechanism. Evid. Based Complement. Altern. Med. 2013, 2013, 296082. [Google Scholar] [CrossRef]
- Nakamura, N.; Hirakawa, A.; Gao, J.-J.; Kakuda, H.; Shiro, M.; Komatsu, Y.; Sheu, C.-C.; Hattori, M. Five new maleic and succinic acid derivatives from the mycelium of Antrodia camphorata and their cytotoxic effects on LLC tumor cell line. J. Nat. Prod. 2004, 67, 46–48. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.W.; Xia, Y.J.; Liu, X.F.; Wang, G.Q.; Xiong, Z.Q.; Ai, L.Z. Antrodin A from mycelium of Antrodia camphorata alleviates acute alcoholic liver injury and modulates intestinal flora dysbiosis in mice. J. Ethnopharmacol. 2020, 254, 112681. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Wang, J.; Sun, Q.; Xie, M.; Lu, Z.M.; Xu, H.Y.; Shi, J.S.; Xu, Z.H. Identification of antrodin B from Antrodia camphorata as a new anti-hepatofibrotic compound using a rapid cell screening method and biological evaluation. Hepatol. Res. 2016, 46, E15–E25. [Google Scholar] [CrossRef]
- Xu, X.Y.; Geng, Y.; Xu, H.X.; Ren, Y.; Liu, D.Y.; Mao, Y. Antrodia camphorata-derived antrodin c inhibits liver fibrosis by blocking tgf-beta and pdgf signaling pathways. Front. Mol. Biosci. 2022, 9, 835508. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.J.; Vani, M.G.; Chueh, P.J.; Mau, J.L.; Wang, S.Y. Antrodin C inhibits epithelial-to-mesenchymal transition and metastasis of breast cancer cells via suppression of Smad2/3 and beta-catenin signaling pathways. PLoS ONE 2015, 10, e0117111. [Google Scholar] [CrossRef]
- MD, W.; MJ, C.; BC, W. Maleimide and maleic anhydride derivatives from the mycelia of Antrodia cinnamomea and their nitric oxide inhibitory activities in macrophages. J. Nat. Prod. 2008, 71, 1258–1261. [Google Scholar] [CrossRef]
- Phuong, D.T.; Ma, C.M.; Hattori, M.; Jin, J.S. Inhibitory effects of antrodins A-E from Antrodia cinnamomea and their metabolites on hepatitis C virus protease. Phytother. Res. 2009, 23, 582–584. [Google Scholar] [CrossRef]
- Tsay, H.J.; Liu, H.K.; Kuo, Y.H.; Chiu, C.S.; Liang, C.C.; Chung, C.W.; Chen, C.C.; Chen, Y.P.; Shiao, Y.J. EK100 and antrodin c improve brain amyloid pathology in APP/PS1 transgenic mice by promoting microglial and perivascular clearance pathways. Int. J. Mol. Sci. 2021, 22, 10413. [Google Scholar] [CrossRef]
- You, R.I.; Lee, Y.P.; Su, T.Y.; Lin, C.C.; Chen, C.S.; Chu, C.L. A Benzenoid 4,7-Dimethoxy-5-Methyl-L, 3-Benzodioxole from Antrodia cinnamomea attenuates dendritic cell-mediated th2 allergic responses. Am. J. Chin. Med. 2019, 47, 1271–1287. [Google Scholar] [CrossRef]
- Yen, I.C.; Shi, L.S.; Chung, M.C.; Ahmetaj-Shala, B.; Chang, T.C.; Lee, S.Y. Antrolone, a novel benzoid derived from Antrodia cinnamomea, inhibits the LPS-induced inflammatory response in RAW264.7 macrophage cells by balancing the NF-κB and Nrf2 pathways. Am. J. Chin. Med. 2018, 46, 1297–1313. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Tsai, T.J.; Korivi, M.; Liu, J.Y.; Chen, H.J.; Lin, C.M.; Shen, Y.C.; Yang, H.L. Antitumor properties of Coenzyme Q0 against human ovarian carcinoma cells via induction of ROS-mediated apoptosis and cytoprotective autophagy. Sci. Rep. 2017, 7, 8062. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.M.; Yang, H.L.; Thiyagarajan, V.; Huang, T.H.; Huang, P.J.; Chen, S.C.; Liu, J.Y.; Hsu, L.S.; Chang, H.W.; Hseu, Y.C. Coenzyme Q0 enhances ultraviolet b-induced apoptosis in human estrogen receptor-positive breast (MCF-7) cancer cells. Integr. Cancer Ther. 2017, 16, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.L.; Lee, T.H.; Huang, T.H.; Wang, P.W.; Chen, Y.P.; Chen, C.C.; Chang, Z.Y.; Fang, J.Y.; Yang, S.C. Coenzyme Q0 from Antrodia cinnamomea exhibits drug-resistant bacteria eradication and keratinocyte inflammation mitigation to ameliorate infected atopic dermatitis in mouse. Front. Pharmacol. 2019, 10, 1445. [Google Scholar] [CrossRef] [PubMed]
- Shie, P.H.; Wang, S.Y.; Lay, H.L.; Huang, G.J. 4,7-Dimethoxy-5-methyl-1,3-benzodioxole from Antrodia camphorata inhibits LPS-induced inflammation via suppression of NF-kappaB and induction HO-1 in RAW264.7 cells. Int. Immunopharmacol. 2016, 31, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Jeon, H.W.; Lee, Y.W.; Lee, Y.M.; Song, K.W.; Park, M.H.; Nam, Y.S.; Ahn, H.C. Bio-artificial skin composed of gelatin and (1→3), (1→6)-β-glucan. Biomaterials 2003, 24, 2503–2511. [Google Scholar] [CrossRef]
- Moradali, M.F.; Mostafavi, H.; Ghods, S.; Hedjaroude, G.A. Immunomodulating and anticancer agents in the realm of macromycetes fungi (macrofungi). Int. Immunopharmacol. 2007, 7, 701–724. [Google Scholar] [CrossRef]
- Chiu, C.H.; Peng, C.C.; Ker, Y.B.; Chen, C.C.; Lee, A.; Chang, W.L.; Chyau, C.C.; Peng, R.Y. Physicochemical characteristics and anti-inflammatory activities of antrodan, a novel glycoprotein isolated from Antrodia cinnamomea mycelia. Molecules 2013, 19, 22–40. [Google Scholar] [CrossRef]
- Ker, Y.B.; Peng, C.C.; Chang, W.L.; Chyau, C.C.; Peng, R.Y. Hepatoprotective bioactivity of the glycoprotein, antrodan, isolated from Antrodia cinnamomea mycelia. PLoS ONE 2014, 9, e93191. [Google Scholar] [CrossRef]
- Chen, P.C.; Chen, C.C.; Ker, Y.B.; Chang, C.H.; Chyau, C.C.; Hu, M.L. Anti-metastatic effects of Antrodan with and without cisplatin on lewis lung carcinomas in a mouse xenograft model. Int. J. Mol. Sci. 2018, 19, 1565. [Google Scholar] [CrossRef]
- Fa, K.N.; Yang, C.M.; Chen, P.C.; Lee, Y.Y.; Chyau, C.C.; Hu, M.L. Anti-metastatic effects of antrodan, the Antrodia cinnamomea mycelia glycoprotein, in lung carcinoma cells. Int. J. Biol. Macromol. 2015, 74, 476–482. [Google Scholar] [CrossRef]
- Chyau, C.C.; Wang, H.F.; Zhang, W.J.; Chen, C.C.; Huang, S.H.; Chang, C.C.; Peng, R.Y. Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARgamma pathway. Int. J. Mol. Sci. 2020, 21, 360. [Google Scholar] [CrossRef] [Green Version]
- Ocampos, F.M.M.; Menezes, L.R.A.; Dutra, L.M.; Santos, M.F.C.; Ali, S.; Barison, A. NMR in Chemical Ecology: An Overview Highlighting the Main NMR Approaches. eMagRes 2017, 6, 325–341. [Google Scholar] [CrossRef]
- Zou, X.G.; Xu, M.T.; Dong, X.L.; Ying, Y.M.; Guan, R.F.; Wu, W.C.; Yang, K.; Sun, P.L. Solid-state-cultured mycelium of Antrodia camphorata exerts potential neuroprotective activities against 6-hydroxydopamine-induced toxicity in PC12 cells. J. Food Biochem. 2022, 46, e14208. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Du, Y.C.; Hsu, Y.M.; Lu, C.Y.; Singab, A.N.B.; El-Shazly, M.; Hwang, T.L.; Lin, W.Y.; Lai, K.H.; Lu, M.C.; et al. New approach to the characterization and quantification of Antrodia cinnamomea benzenoid components utilizing HPLC-PDA, qNMR and HPLC-tandem MS: Comparing the wild fruiting bodies and its artificial cultivated commercial products. Food Res. Int. 2013, 51, 23–31. [Google Scholar] [CrossRef]
- Huang, H.S.; Hsu, J.L.; Yu, C.C.; Chang, L.C.; Chen, C.R.; Huang, T.C.; Chang, C.I. Qualitative and quantitative analysis of seven signature components in the fruiting body of Antrodia cinnamomea by HPLC—ESI—MS/MS. Acta Chromatogr. 2016, 28, 387–402. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, P.; Ma, J.; Chen, N.; Guo, H.; Chen, Y.; Gan, X.; Wang, R.; Liu, X.; Fan, S.; et al. Antrodia cinnamomea exerts an anti-hepatoma effect by targeting PI3K/AKT-mediated cell cycle progression in vitro and in vivo. Acta Pharm. Sin. B 2022, 12, 890–906. [Google Scholar] [CrossRef]
- Murador, D.C.; de Souza Mesquita, L.M.; Vannuchi, N.; Braga, A.R.C.; de Rosso, V.V. Bioavailability and biological effects of bioactive compounds extracted with natural deep eutectic solvents and ionic liquids: Advantages over conventional organic solvents. Curr. Opin. Food Sci. 2019, 26, 25–34. [Google Scholar] [CrossRef]
- Chang, C.C.; Lu, Y.C.; Wang, C.C.; Ko, T.L.; Chen, J.R.; Wang, W.; Chen, Y.L.; Wang, Y.W.; Chang, T.H.; Hsu, H.F.; et al. Antrodia cinnamomea extraction waste supplementation promotes thermal stress tolerance and tissue regeneration ability of zebrafish. Molecules 2020, 25, 4213. [Google Scholar] [CrossRef]


| Polysaccharides Component | Sources | Extraction Method | Isolation and Purification Method | Reference |
|---|---|---|---|---|
| ACP | Mycelia | Extracted with cold water | GFC HW65F column (Tosoh Bioscience, 90 cmH × 1.6 cm D); flow rate, 0.4 mL/min | [21] |
| ACP1 | Mycelia | Extracted twice with hot water | DEAE-52 column (300 × 26 mm2) and Sephadex G-100 chromatography (1.0 × 80 cm) | [22] |
| ACP2 | Mycelia | Extracted with boiling water for 3 h | DEAE-52 column (300 × 26 mm2) and Sephadex G-100 chromatography (1.0 × 80 cm2) | [25,32] |
| ACW0 | Mycelia | Extracted with boiling water four times (4 h for each extraction) | DEAE Sepharose Fast Flow and Sephacryl S-100HR systems | [15] |
| ACPS | Mycelia | Extracted twice in double-distilled water at 95 °C for 4 h | DEAE-52 cellulose anion-exchange column (2.6 cm × 35 cm) | [20] |
| ACPS-1 | Mycelia | Extracted with boiled water for 3 h | DEAE-52 cellulose (2.6 × 20 cm2) and Sephadex G-100 column chromatography (1.1 × 100 cm2) | [19] |
| Na10_SPS-F3 | Mycelia | 0.1 M sodium acetate (pH 5.5) containing 5 mM cysteine, 100 mg papain, and 5 mM EDTA at 60 °C for 24 h | GFC (103 × 1.5 cm2 Fractogel BioSeccolumn) | [33] |
| Triterpenoids Component | Sources | Extraction Method | Isolation and Purification Method | Reference |
|---|---|---|---|---|
| Antcin A | Fruiting bodies | Extracted with MeOH at room temperature for 7 days | Silica gel column and semi-preparative HPLC | [48] |
| Antcin K | Fruiting bodies | Extracted with ethyl acetate for 3 days | Silica gel column and HPLC | [49] |
| Methylantcinate A | Fruiting bodies | Extracted with n-hexane, chloroform, and methanol under reflux | Silica gel column chromatography and thin-layer chromatography | [50] |
| Sulphurenic acid | Mycelia | Extracted with methanol at room temperature for 4 days and then partitioned (three times) with ethyl acetate | Silica gel and HPLC | [42] |
| Dehydroeburicoic Acid | Mycelia | Extracted thrice with methanol at room temperature (4 days × 3) | Silica gel and HPLC | [40] |
| Eburicoic acid | Mycelia | Extracted thrice with methanol at room temperature (4 days × 3) | Silica gel and HPLC | [41] |
| Ubiquinone Derivatives Component | Sources | Extraction Method | Isolation and Purification Method | Reference |
|---|---|---|---|---|
| Antroquinonol | Mycelia (solid-state) | Extracted three time with n-hexane by stirring at room temperature for 6 h | Silica-gel gravity column (230–400 mesh, 5 × 45 cm2) and Sephadex LH-20 (5 × 70 cm2) | [56] |
| 4-Acetylantroquinonol B | Mycelia | Extracted with ethyl acetate | HPLC and silica gel column (4.6 × 250 mm2) | [62] |
| Antrocinnamone | Mycelia | Extracted with 95% alcohol | Column chromatography and HPLC | [65] |
| 4-Acetylantrocamol LT3 | Mycelia | Extracted with 95% alcohol | Column chromatography and HPLC | [65,66] |
| Maleic and Succinic Acid Derivatives | Sources | Extraction Method | Isolation and Purification Method | Reference |
|---|---|---|---|---|
| Antrodin A | Mycelia | Extracted with absolute ethanol at a ratio of 1:100 (g/mL), the ethanol extract was then extracted twice with ethyl acetate: water = 1:1 | Silica gel column chromatography in a Reveleris PREP purification system | [71] |
| Antrodin C | Mycelia | Extracted in methanol and then partitioned with n-hexane chloroform and ethyl acetate | Silica gel column and semipreparative HPLC | [73] |
| Antrodin B | Mycelia | extracted with methanol for 24 h at room temperature. | Silica gel column and semipreparative HPLC | [72] |
| Benzene Derivatives | Sources | Extraction Method | Isolation and Purification Method | Reference |
|---|---|---|---|---|
| DMB | Mycelia | Extracted with methanol | Silica gel column and Sephadex LH-20 column | [83] |
| Antrolone | Mycelia | Extracted with 95% ethanol | silica gel column chromatography and medium pressure liquid chromatography | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-X.; Wang, J.-J.; Lu, C.-L.; Gao, Y.-J.; Gao, L.; Yang, Z.-Q. Review of Bioactivity, Isolation, and Identification of Active Compounds from Antrodia cinnamomea. Bioengineering 2022, 9, 494. https://doi.org/10.3390/bioengineering9100494
Li H-X, Wang J-J, Lu C-L, Gao Y-J, Gao L, Yang Z-Q. Review of Bioactivity, Isolation, and Identification of Active Compounds from Antrodia cinnamomea. Bioengineering. 2022; 9(10):494. https://doi.org/10.3390/bioengineering9100494
Chicago/Turabian StyleLi, Hua-Xiang, Juan-Juan Wang, Chun-Lei Lu, Ya-Jun Gao, Lu Gao, and Zhen-Quan Yang. 2022. "Review of Bioactivity, Isolation, and Identification of Active Compounds from Antrodia cinnamomea" Bioengineering 9, no. 10: 494. https://doi.org/10.3390/bioengineering9100494
APA StyleLi, H. -X., Wang, J. -J., Lu, C. -L., Gao, Y. -J., Gao, L., & Yang, Z. -Q. (2022). Review of Bioactivity, Isolation, and Identification of Active Compounds from Antrodia cinnamomea. Bioengineering, 9(10), 494. https://doi.org/10.3390/bioengineering9100494